Abstract
Aims
To evaluate the anti-anginal and anti-ischaemic efficacy of the selective If current inhibitor ivabradine in patients with chronic stable angina pectoris receiving beta-blocker therapy.
Methods and results
In this double-blinded trial, 889 patients with stable angina receiving atenolol 50 mg/day were randomized to receive ivabradine 5 mg b.i.d. for 2 months, increased to 7.5 mg b.i.d. for a further 2 months, or placebo. Patients underwent treadmill exercise tests at the trough of drug activity using the standard Bruce protocol for randomization and at 2 and 4 months. Total exercise duration at 4 months increased by 24.3 ± 65.3 s in the ivabradine group, compared with 7.7 ± 63.8 s with placebo (P < 0.001). Ivabradine was superior to placebo for all exercise test criteria at 4 months (P < 0.001 for all) and 2 months (P-values between <0.001 and 0.018). Ivabradine in combination with atenolol was well tolerated. Only 1.1% of patients withdrew owing to sinus bradycardia in the ivabradine group.
Conclusion
The combination of ivabradine 7.5 mg b.i.d. and atenolol at the commonly used dosage in clinical practice in patients with chronic stable angina pectoris produced additional efficacy with no untoward effect on safety or tolerability.
Keywords: Ivabradine, Myocardial ischaemia, Stable angina pectoris, Heart rate, If inhibition, Combination therapy
Introduction
Angina pectoris is typically caused by myocardial ischaemia owing to an imbalance between myocardial perfusion and oxygen demand. Elevated heart rate increases myocardial oxygen demand and limits tissue perfusion, the latter by reducing the duration of diastole during which most myocardial perfusion occurs. Long-term prognosis in patients with chronic stable angina pectoris is favourable1 and medical and interventional therapies show similar benefit.2,3 Thus, current treatment guidelines advocate an initial approach with medical therapy.4–6 Beta-blockers reduce myocardial ischaemia and prevent angina pectoris largely by lowering heart rate and are recommended as an initial therapy for stable angina pectoris, unless contraindicated.6–8
In modern clinical practice, however, many patients with stable angina pectoris require treatment with more than one anti-anginal drug, in addition to short-acting nitrates.6,9 Ivabradine is a pure heart rate-lowering agent that acts by inhibiting If, an important ionic current involved in the pacemaker activity in cells of the sino-atrial node.10 Ivabradine reduces the slope of spontaneous diastolic depolarization in these cells and lowers heart rate at rest and during exercise. Given as monotherapy (with short-acting nitrates allowed as required), ivabradine has demonstrated anti-ischaemic and anti-anginal efficacy in randomized trials in patients with chronic stable angina pectoris when compared with placebo11 and has been shown to be non-inferior to atenolol12 or amlodipine.13 The drug has been recommended for the medical management of patients with stable angina pectoris who are intolerant of beta-blockers or in whom these agents are contraindicated.6
The primary purpose of the present study was to evaluate the anti-ischaemic and anti-anginal efficacy of ivabradine, relative to placebo, when given to patients with chronic stable angina pectoris receiving beta-blocker therapy.
Methods
Study population
Eligible patients were male and female outpatients aged ≥18 and ≤75 years, with a history of chronic angina pectoris on effort for ≥3 months before study entry, and evidence of coronary artery disease documented by one or more of the following criteria: myocardial infarction ≥3 months before study entry; percutaneous coronary angioplasty ≥6 months or coronary artery bypass surgery ≥3 months before study entry; coronary angiography showing ≥50% diameter stenosis of one or more major coronary arteries; positive scintigraphic test showing exercise-induced reversible myocardial ischaemia; or a positive stress echocardiography showing regional wall motion abnormality and failure of normal rise in left ventricular ejection fraction with exercise. Other inclusion criteria included sinus rhythm at the pre-selection visit, current treatment with atenolol 50 mg o.d., or another beta-blocker at equivalent doses for at least 3 months. Patients had to show three positive symptom-limited exercise tolerance tests (ETTs) with the standard Bruce protocol during the run-in period and stability of ETT results between the second and third tests.
Exclusion criteria included: heart rate <60 b.p.m. on ECG at rest; significant heart disease other than coronary artery disease; angina pectoris at rest, unstable angina pectoris, Prinzmetal or microvascular angina; severe heart failure symptoms (New York Heart Association class III or IV); symptomatic hypotension or uncontrolled hypertension (resting systolic blood pressure >180 mmHg or diastolic blood pressure >100 mmHg); chronic or paroxysmal atrial fibrillation present at the pre-selection visit; atrial flutter; a pacemaker or implanted defibrillator; any condition that interferes with the ability to perform or interpret ETT (e.g. physical incapacity, Wolff–Parkinson–White syndrome, complete left bundle branch block, left ventricular hypertrophy); contraindication or intolerance to atenolol; previous treatment with atenolol at a dose >50 mg o.d., or another beta-blocker at a corresponding dose; recent treatment with amiodarone (<3 months) or bepridil (<7 days); known severe renal failure, liver function test abnormality, or known electrolyte disorder; anaemia (blood haemoglobin <110 g/L or 6.8 mmol/L); and thyroid disorders unless controlled by thyroxine for ≥3 months.
Written informed consent was obtained from all patients. The study was performed in accordance with the ethical principles stated in the Declaration of Helsinki, 1964, as revised in Washington, 2002. The study was approved by the institutional review board of each participating centre.
Study design
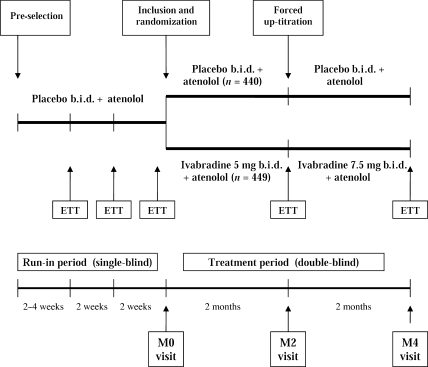
This was a randomized, double-blind, parallel-group study comparing the anti-anginal and anti-ischaemic effects of ivabradine with placebo when given to patients with chronic stable angina pectoris receiving the beta-blocker atenolol. The study consisted of a 6 to 8 week single-blind run-in period followed by a 4-month, double-blind treatment period (Figure 1). During the run-in period, all patients received atenolol 50 mg o.d. and placebo b.i.d. and underwent three ETTs. The first ETT took place 2 weeks after the pre-selection visit for patients already treated with atenolol 50 mg o.d., or 4 weeks after pre-selection for patients previously treated with another beta-blocker and switched to atenolol 50 mg o.d. on the day of pre-selection. The second ETT (2 weeks later) and the third ETT (10 days after the second ETT and designated as baseline, M0; Figure 1) were used to assess stability of patients’ ETT results. All three ETTs had to be positive (see details in what follows), and time to 1 mm ST-segment depression had to be within ± 20% or ± 1 min between the second and third ETTs for patients to be included.
Figure 1.
Summary of study design.
Included patients (i.e. those with positive and stable ETT results during the run-in period) were randomized to receive ivabradine or placebo given in combination with atenolol 50 mg o.d. for the entire double-blind treatment period. The ivabradine dose was 5 mg b.i.d. for 2 months, with a forced up-titration to 7.5 mg b.i.d. for the final 2 months, provided that resting heart rate was not <50 b.p.m. at the 2 month (M2) visit. The random allocation schedule was computer-generated using non-adaptive balanced randomization, stratified by the centre. An independent organization, Fisher Clinical Services, supervised randomization. Study treatment was allocated via an automated fax system. Ivabradine and placebo tablets were of similar appearance. Treatment efficacy was evaluated by further ETT at 2 and 4 months of study drug treatment (M2 and M4, respectively; Figure 1). Patients also kept an anginal symptoms diary, in which the occurrence of anginal attacks and the consumption of short-acting nitrates were recorded.
Short-acting nitrates could be taken as required, but not within 3 h before the ETT. Other drugs that could interfere with the natural course of angina pectoris (long-acting nitrates, calcium antagonists, other beta-blockers, potassium channel openers, molsidomine, trimetazidine) or the interpretation of ST-segment changes (anti-arrhythmic agents, digitalis, monoamine oxidase inhibitors) were not allowed during the trial. Drugs with known or suspected interactions with ivabradine (antifungal azole derivatives, macrolide antibiotics, cyclosporine, antiprotease agents) or atenolol (floctafenine, sultopride, clonidine, reserpine, guanethidine, mefloquine, anticholinesterase agents) were also not allowed.
The study was registered on the ClinicalTrials.gov website (identifier NCT00202566).
Exercise tolerance testing
Symptom-limited treadmill ETTs, using the standard Bruce protocol, were performed in the morning at approximately the same time of day on each visit. Patients did not take their morning dose of study treatment before ETT, so that tests were performed at the trough of drug activity for both the study drug and the background treatment, 12 h after the last intake of ivabradine and 24 h after the last intake of atenolol.
Time to onset of angina and time to limiting angina during ETT were determined by the investigator, whereas total exercise duration and time to 1 mm ST-segment depression were determined by central reading. Heart rate was obtained from the ECG recording, and rate–pressure product (heart rate × systolic blood pressure) at rest and at peak exercise was determined using blood pressure measurements made by the investigator.
During the run-in period, ETTs were considered positive if stopped owing to the occurrence of limiting angina pectoris accompanied by at least 1 mm ST-segment depression between 3 and 12 min of exercise. During the treatment period (i.e. at M2 and M4), ETT could continue beyond 12 min or stop before 3 min, and stopping criteria included limiting angina pectoris, dyspnoea, and extreme fatigue. The ST-segment was measured 80 ms after the J-point in three consecutive QRS complexes with a flat baseline. If ST-segment depression was present at rest, the change was calculated from the value at rest to the value during exercise. If ST-segment elevation was present at rest, ST depression during exercise was calculated from the ECG isoelectric line. Thus, time to 1 mm ST-segment depression was calculated as the time to 1 mm ST-segment depression in the case of an isoelectric or elevated ST-segment at rest, and as the time to a further 1 mm depression in the case of ST depression at rest. During study drug treatment, if 1 mm ST-segment depression or angina did not occur during the ETT, total exercise duration was used as the measure of time to 1 mm ST-segment depression and time to onset of angina.
ETT and ECG tracings were analysed centrally at a reading centre by cardiologists blinded to treatment allocation. When reading the M2 and M4 ETT tracings during study drug treatment, the cardiologist did not have access to previous ETT data.
Endpoints
The primary efficacy endpoint was the change in total exercise duration from baseline (M0) to end of treatment (M4), measured during ETT at the trough of ivabradine activity in the full analysis set, defined as all randomized patients who took one or more doses of study medication and had one or more evaluations of the main efficacy endpoint. Secondary efficacy endpoints included changes from baseline to M4 in other ETT criteria, heart rate and rate–pressure product at rest and at peak of exercise, and changes from baseline to M2 in all ETTs, heart rate, and rate–pressure product criteria. Changes in anginal attack frequency and short-acting nitrate consumption recorded in patients’ diaries were also analysed as secondary efficacy criteria.
Statistical analysis
The sample size was calculated to demonstrate the superiority of ivabradine compared with placebo in the primary efficacy endpoint. For a standard deviation of 110 s, 350 patients per group were necessary to detect a ≥30 s difference with 95% power and a one-sided type I error of 2.5%. Assuming withdrawal of 5% of patients after randomization, approximately 750 patients would have to be included in the study.
Data are presented as mean values ± SD, and 95% confidence intervals (two-sided) are given as appropriate. The main analysis of ETT criteria was performed on an intention-to-treat basis in the full analysis set, and the last non-missing value observed over a 4-month treatment period has been considered in the analyses. Groups were compared using a parametric covariance analysis adjusted for country factor and baseline value as covariate. The type I error was set at 2.5% (one-sided). Sensitivity analyses were also performed using a parametric analysis of variance without adjustment, and a non-parametric covariance analysis based on Wilcoxon rank norm14 with adjustment. For the frequency of anginal attacks and consumption of short-acting nitrates, 95% confidence intervals were calculated using a parametric analysis of variance without adjustment, and a non-parametric approach without adjustment based on the Hodges–Lehmann’s estimator15 was used as sensitivity analysis. Safety analyses were performed on all patients who received one or more doses of study drug.
Results
Patient characteristics
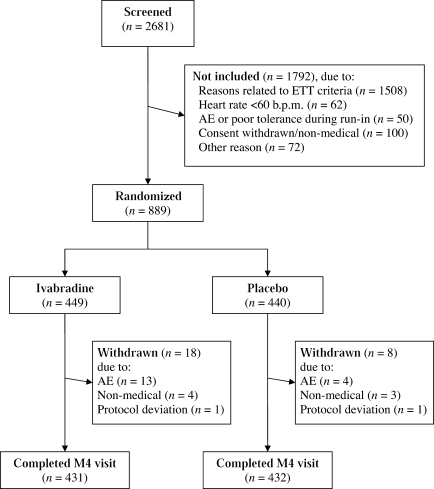
In all, 889 patients from 219 centres in 20 countries were randomized to the ivabradine (n = 449) and placebo (n = 440) groups between August 2005 and June 2007. The full analysis set consisted of 875 patients (98% of those randomized), and the disposition of patients throughout the study including the run-in period is shown in Figure 2. A total of 26 patients withdrew early from study medications, 18 (4%) in the ivabradine group and 8 (2%) in the placebo group. In the ivabradine group, 393 patients (90%) had their dose up-titrated from 5 to 7.5 mg b.i.d. after 2 months of treatment.
Figure 2.
Disposition of patients throughout the study. AE, adverse event; ETT, exercise tolerance test.
The mean age of patients was approximately 60 years, 84% were male, 94% were Caucasian, and the majority (69%) was in Canadian Cardiovascular Society angina class II at baseline (Table 1). Clinical and ETT characteristics at baseline were similar between patients randomized to the ivabradine and placebo groups. Randomized patients showed good stability of ETT results between the second and third (M0) ETTs during the run-in period: mean total exercise duration differed by only ∼5 s between the second (444 ± 107 s) and third (448 ± 106 s) run-in ETTs. All patients but three were in sinus rhythm at inclusion on centrally analysed ECG tracings.
Table 1.
Baseline clinical and exercise tolerance test characteristics of randomized patients
| Ivabradine (n = 449) | Placebo (n = 440) | P-value | |
|---|---|---|---|
| Age, years | 59.6 ± 7.6 | 60.1 ± 8.0 | 0.30 |
| Male, n (%) | 380 (84.6) | 370 (84.1) | 0.82 |
| Smoker (including ex-smoker), n (%) | 271 (60.4) | 250 (56.8) | 0.28 |
| CCS angina class, I/II/III, % | 21.2/67.0/11.8 | 17.7/70.2/12.0 | 0.43 |
| Previous MI, n (%) | 225 (50.1) | 226 (51.4) | 0.71 |
| Previous PCI, n (%) | 95 (21.2) | 89 (20.2) | 0.49 |
| Previous CABG, n (%) | 135 (30.1) | 123 (28.0) | 0.73 |
| Diabetes mellitus, n (%) | 97 (21.6) | 96 (21.8) | 0.94 |
| Concomitant treatments at inclusion | |||
| Acetylsalicylic acid, n (%) | 369 (82.2) | 373 (84.8) | 0.30 |
| Statins, n (%) | 341 (75.9) | 330 (75.0) | 0.74 |
| ACE-inhibitors, n (%) | 235 (52.3) | 252 (57.3) | 0.21 |
| Supine BP (mmHg) systolic | 127.3 ± 12.0 | 127.6 ± 12.6 | 0.67 |
| Supine BP (mmHg) diastolic | 78.6 ± 7.4 | 78.1 ± 7.2 | 0.27 |
| Heart rate at rest (b.p.m.) | |||
| Mean ± SD | 66.9 ± 6.9 | 67.2 ± 6.9 | 0.57 |
| Min–max | 41–99 | 53–107 | |
| Total exercise duration (s) | |||
| Mean ± SD | 445.1 ± 105.5 | 451.1 ± 107.4 | 0.40 |
| Min–max | 224–716 | 221–720 | |
| Time to limiting angina (s) | |||
| Mean ± SD | 441.4 ± 105.6 | 446.7 ± 107.2 | 0.46 |
| Min–max | 223–716 | 216–715 | |
| Time to angina onset (s) | |||
| Mean ± SD | 351.3 ± 104.5 | 357.0 ± 104.5 | 0.42 |
| Min–max | 129–659 | 81–674 | |
| Time to 1 mm ST-segment depression (s) | |||
| Mean ± SD | 338.1 ± 97.2 | 347.1 ± 103.4 | 0.18 |
| Min–max | 185–655 | 185–715 | |
| Heart rate at peak exercise (b.p.m.) | |||
| Mean ± SD | 128.6 ± 16.9 | 129.9 ± 18.0 | 0.29 |
| Min–max | 82–179 | 75–179 | |
| RPP at rest (b.p.m. × mmHg) | |||
| Mean ± SD | 9389 ± 1661 | 9427 ± 1830 | 0.75 |
| Min–max | 5800–15 200 | 4500–20 320 | |
| RPP at peak exercise (b.p.m. × mmHg) | |||
| Mean ± SD | 21 110 ± 4300 | 21 249 ± 4566 | 0.64 |
| Min–max | 10 660–36 800 | 10 500–34 500 | |
Mean values ± standard deviation unless otherwise stated. BP, blood pressure; CABG, coronary artery bypass graft; CCS, Canadian Cardiovascular Society classification; MI, myocardial infarction; PCI, percutaneous coronary intervention; RPP, rate–pressure product.
Efficacy
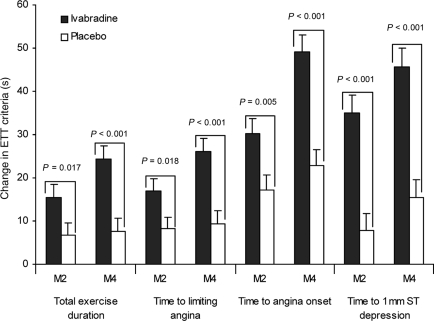
The primary efficacy criterion, change in total exercise duration at M4 in the full analysis set, increased by 24.3 ± 65.3 s in the ivabradine group compared with 7.7 ± 63.8 s in the placebo group (P < 0.001). More patients experienced an improvement in TED of >30 s in the ivabradine group (48%) than in the placebo group (34%, P < 0.001). There were also improvements with ivabradine treatment, relative to placebo, in all other ETT criteria at M4 (P < 0.001 for all; Table 2, Figure 3).
Table 2.
Changes in exercise tolerance test criteria between baseline and end of study (M4) in the full analysis set
| Ivabradine (n = 441) | Placebo (n = 434) | |
|---|---|---|
| Total exercise duration (s) | ||
| Baseline | 445.6 ± 105.6 | 450.7 ± 107.5 |
| End of study | 469.9 ± 119.2 | 458.4 ± 111.1 |
| Change | 24.3 ± 65.3 | 7.7 ± 63.8 |
| Differencea (SE) | 16.3 (4.3) | |
| 95% CI | 7.9–24.7 | |
| P-valueb | <0.001 | |
| Time to limiting angina (s) | ||
| Baseline | 441.9 ± 105.7 | 446.6 ± 107.4 |
| End of study | 467.9 ± 119.8 | 456.0 ± 111.1 |
| Change | 26.0 ± 65.7 | 9.4 ± 63.8 |
| Differencea (SE) | 16.3 (4.3) | |
| 95% CI | 7.9–24.7 | |
| P-valueb | <0.001 | |
| Time to angina onset (s) | ||
| Baseline | 352.5 ± 104.6 | 357.2 ± 104.8 |
| End of study | 401.6 ± 125.5 | 379.9 ± 115.8 |
| Change | 49.1 ± 83.3 | 22.7 ± 79.1 |
| Differencea (SE) | 25.5 (5.4) | |
| 95% CI | 15.0–36.0 | |
| P-valueb | <0.001 | |
| Time to 1 mm ST depression (s) | ||
| Baseline | 337.8 ± 97.2 | 347.2 ± 104.0 |
| End of study | 383.5 ± 123.2 | 362.6 ± 122.5 |
| Change | 45.7 ± 93.0 | 15.4 ± 86.6 |
| Differencea (SE) | 28.5 (6.0) | |
| 95% CI | 16.8–40.3 | |
| P-valueb | <0.001 | |
Mean values ± standard deviation unless otherwise stated. CI, confidence interval; SE, standard error.
aIvabradine minus placebo, estimate from parametric approach adjusted on baseline and country factors.
bStudent's t-test for superiority.
Figure 3.
Changes in exercise tolerance test criteria between baseline and M2 visit and between baseline and end of study (M4) in the full analysis set.
Improvements in all ETT criteria, relative to placebo, were also seen in the ivabradine group at 2 months of therapy with ivabradine 5 mg b.i.d., (M2; Table 3) although the changes were larger at the end of the study and with higher doses.
Table 3.
Changes in exercise tolerance test criteria between baseline and M2 visit in the full analysis set
| Ivabradine (n = 441) | Placebo (n = 434) | |
|---|---|---|
| Total exercise duration (s) | ||
| Baseline | 445.6 ± 105.6 | 450.7 ± 107.1 |
| Change | 15.5 ± 60.0 | 6.8 ± 56.5 |
| Differencea (SE) | 8.2 (3.9) | |
| 95% CI | 0.6–15.7 | |
| P-valueb | 0.017 | |
| Time to limiting angina (s) | ||
| Baseline | 441.9 ± 105.7 | 446.6 ± 107.4 |
| Change | 17.0 ± 60.7 | 8.2 ± 56.8 |
| Differencea (SE) | 8.2 (3.9) | |
| 95% CI | 0.6–15.8 | |
| P-valueb | 0.018 | |
| Time to angina onset (s) | ||
| Baseline | 352.5 ± 104.6 | 357.2 ± 104.8 |
| Change | 30.2 ± 72.2 | 17.2 ± 72.3 |
| Differencea (SE) | 12.3 (4.8) | |
| 95% CI | 2.9–21.7 | |
| P-valueb | 0.005 | |
| Time to 1 mm ST depression (s) | ||
| Baseline | 337.4 ± 97.6 | 347.3 ± 103.8 |
| Change | 35.0 ± 84.1 | 7.8 ± 82.6 |
| Differencea (SE) | 25.3 (5.6) | |
| 95% CI | 14.4–36.3 | |
| P-valueb | <0.001 | |
Mean values ± standard deviation unless otherwise stated. CI, confidence interval; SE, standard error.
aIvabradine minus placebo, estimate from parametric approach adjusted on baseline and country factors.
bStudent's t-test for superiority.
Ivabradine treatment produced dose-dependent reductions in heart rate and rate–pressure product, both at rest and at the peak of exercise at M4 (Table 4). Corresponding reductions were also seen at M2, with further reduction at M4 in each case (Table 5). The frequency of angina attacks decreased significantly from baseline to M4 in both treatment groups, from 1.8 ± 3.3 to 0.9 ± 2.4 attacks/week in the ivabradine group, and from 1.6 ± 2.4 to 0.9 ± 2.1 attacks/week with placebo (between-group difference not significant). Among symptomatic patients [here defined as those who experienced one or more angina attacks during the run-in period, n = 625 (70%)], angina attack frequency was reduced by 1.3 attacks/week (relative change −52%) with ivabradine and by 1.0 attack/week (relative change −46%) with placebo (between-group difference not significant).
Table 4.
Changes in heart rate and rate–pressure product between baseline and end of study (M4)
| Ivabradine (n = 431) | Placebo (n = 432) | |
|---|---|---|
| Heart rate at rest (b.p.m.) | ||
| Baseline | 67.0 ± 6.8 | 67.2 ± 6.9 |
| Change | −8.7 ± 9.8 | −1.4 ± 9.8 |
| Differencea (SE) | −7.4 (0.6) | |
| 95% CI | −8.7 to −6.2 | |
| Heart rate at peak exercise (b.p.m.) | ||
| Baseline | 128.6 ± 16.9 | 130.1 ± 17.9 |
| Change | −11.3 ± 13.2 | −0.9 ± 12.3 |
| Differencea (SE) | −10.8 (0.8) | |
| 95% CI | −12.4 to −9.1 | |
| RPP at rest (b.p.m. × mmHg) | ||
| Baseline | 9403 ± 1662 | 9429 ± 1830 |
| Change | −1269 ± 1655 | −360 ± 1622 |
| Differencea (SE) | −920 (99) | |
| 95% CI | −1115 to −725 | |
| RPP at peak exercise (b.p.m. × mmHg) | ||
| Baseline | 21 125 ± 4287 | 21 288 ± 4552 |
| Change | −1630 ± 3474 | −66 ± 3447 |
| Differencea (SE) | −1612 (219) | |
| 95% CI | −2041 to −1183 | |
Mean values ± standard deviation unless otherwise stated. CI, confidence interval; SE, standard error; RPP, rate–pressure product.
aIvabradine minus placebo, estimate from parametric approach adjusted on baseline and country factors.
Table 5.
Changes in heart rate and rate–pressure product between baseline and M2
| Ivabradine (n = 449) | Placebo (n = 440) | |
|---|---|---|
| Heart rate at rest (b.p.m.) | ||
| Baseline | 67.0 ± 6.9 | 67.2 ± 6.9 |
| Change | −6.9 ± 9.7 | −1.1 ± 10.2 |
| Differencea (SE) | −6.0 (0.7) | |
| 95% CI | −7.2 to −4.7 | |
| Heart rate at peak exercise (b.p.m.) | ||
| Baseline | 128.6 ± 16.9 | 130.1 ± 17.9 |
| Change | −8.9 ± 11.7 | 0.1 ± 11.0 |
| Differencea (SE) | −9.2 (0.7) | |
| 95% CI | −10.7 to −7.8 | |
| RPP at rest (b.p.m. × mmHg) | ||
| Baseline | 9403 ± 1662 | 9433 ± 1830 |
| Change | −1163 ± 1613 | −354 ± 1593 |
| Differencea (SE) | −822 (97) | |
| 95% CI | −1012 to −632 | |
| RPP at peak exercise (b.p.m. × mmHg) | ||
| Baseline | 21 125 ± 4287 | 21 288 ± 4552 |
| Change | −1439 ± 3436 | −10 ± 2972 |
| Differencea (SE) | −1482 (204) | |
| 95% CI | −1882 to −1082 | |
Mean values ± standard deviation unless otherwise stated. CI, confidence interval; SE, standard error; RPP, rate–pressure product.
aIvabradine minus placebo, estimate from parametric approach adjusted on baseline and country factors.
Safety
Ivabradine was well tolerated in the study: the numbers of patients withdrawn from treatment owing to emergent adverse events were 13 (2.9%) in the ivabradine group and 4 (0.9%) with placebo (difference not significant). Among these emergent adverse events, there were five serious in the ivabradine group (1.1%) and three in the placebo group (0.7%). The most frequent causes of withdrawal related to bradycardia [ivabradine five patients (1.1%), placebo none] and unstable or aggravated angina pectoris [ivabradine three patients (0.7%), placebo one (0.2%)]. The most frequent emergent adverse events were those related to bradycardia, reported by 19 patients (4.2%) in the ivabradine group (12 patients with ivabradine 5 mg b.i.d. and 7 with ivabradine 7.5 mg b.i.d.) and 2 patients (0.5%) with placebo. Only 1.1% of adverse events related to bradycardia were symptomatic. Phosphenes (luminous phenomena described as increases in brightness in limited areas of the visual field) and blurred vision, which have been associated with ivabradine treatment in previous studies,11–13 were reported by nine patients (2%) in the ivabradine group and four (0.9%) in the placebo group. There were small, non-significant changes in supine blood pressure from baseline to the last value on treatment (from 127.3 ± 12.0 to 128.3 ± 14.8 mmHg for systolic blood pressure and from 78.6 ± 7.4 to 78.1 ± 8.0 mmHg for diastolic blood pressure with ivabradine, and from 127.6 ± 12.6 to 126.1 ± 14.8 and 78.1 ± 7.2 to 78.1 ± 7.5 mmHg, respectively, with placebo). There was one death during the treatment period, a fatal suicide in the ivabradine group and two deaths after the last study drug intake in the placebo group.
Discussion
The main finding from this study is that long-term heart rate reduction by ivabradine produced a significant improvement relative to placebo in the primary efficacy criterion, total exercise duration at the trough of drug activity at 4 months of treatment, in patients with chronic stable angina pectoris receiving the beta-blocker atenolol. There were also significant improvements with ivabradine in all ETT criteria at 2 months of treatment, with further improvement at 4 months after forced up-titration of the ivabradine dose from 5 to 7.5 mg b.i.d. These improvements in exercise capacity were accompanied by reductions in heart rate and rate–pressure product at rest and at the peak of exercise, which also were significant at 2 months and larger at 4 months.
The primary efficacy endpoint used in the present study, total exercise duration in standardized ETT at the trough of drug activity, is the one specified in current European guidelines.16 The dose of atenolol used as background therapy throughout the study was 50 mg per day. This reflects general clinical practice, and was the median atenolol dose in a recent population study of beta-blocker use in patients after an acute myocardial infarction.17 In spite of recommendation to titrate beta-blockers to full dosages, data in clinical practice reveal substantial underdosing of all beta-blockers, with dosages generally ≤50% than the dosages that randomized trials have proved to be effective.17 This could in part be related to reduced tolerability of higher doses of beta-blockers because of fatigue, depression, bronchospasm, or erectile dysfunction. Thus in patients who cannot be given higher doses of beta-blockers or in whom sufficient heart rate reduction cannot be achieved, the combination with ivabradine appears to be an appropriate therapeutic option.
Combination therapy is widely used in clinical practice in order to achieve adequate control of angina. In the Euro Heart Survey of the initial management of stable angina pectoris, the majority (59%) of patients were on two or more anti-anginal drugs. Among patients scheduled to be managed by medical therapy alone, the proportion was even higher at 80%.8 However, clinical trials evaluating combination therapy have yielded inconsistent results. Most studies have been small and many have not shown significant benefits of combinations as opposed to single-drug therapy. For example, the TIBET study, one of the largest studies of combination therapy, compared atenolol with slow-release nifedipine given alone and in combination, and showed no significant benefit of combination therapy for any ETT criterion.18 Similarly, studies of amlodipine or diltiazem on top of atenolol19 and the combination of amlodipine and atenolol20 showed no additional improvement in ETT criteria by adding a second drug to atenolol. A meta-analysis of randomized studies comparing the addition of a calcium antagonist to beta-blocker therapy and vice versa found small but significant benefits of combination therapy at the peak of activity of the added drug, but no significant improvements at the trough of activity.21 A recent positive trial of combination therapy involved the metabolic agent ranolazine given on top of atenolol (50 mg/day), amlodipine (5 mg/day), or diltiazem (180 mg/day) in patients with severe angina pectoris. The combination showed an improvement in total exercise duration, although changes in time to 1 mm ST-segment depression were not significant.22 The present study, in terms of the size of the trial, the compliance with regulatory recommendations, and the consistency of significant improvements across all ETT criteria and time points, represents perhaps the most compelling single demonstration of the benefit of any combination of anti-anginal drugs published to date.
In the present study, treadmill ETTs were performed using the standard Bruce protocol, in which higher workloads are reached more rapidly than in the modified Bruce protocol.23 The more demanding standard Bruce protocol was chosen in view of the fact that patients were receiving background therapy known to improve exercise capacity. At baseline, resting heart rate was ∼67 b.p.m. and mean angina attack frequency was only 1.6–1.8 attacks per week, compared with approximately 3.3 attacks per week in the published INITIATIVE study of ivabradine vs. atenolol in 939 patients with stable angina pectoris.12 In the current study, 264 patients (30%) recorded no angina attacks during the run-in period on background therapy alone. The low mean number of angina attacks at baseline may explain that we did not reach statistical significance for the reduction of angina attacks. The improvements in total exercise duration in the present study (on top of background beta-blockade) were numerically smaller than in the previous INITIATIVE study of ivabradine monotherapy12 that used the modified Bruce ETT protocol. Nevertheless, the additional 16.3 s in total exercise duration with ivabradine treatment in the present study, relative to placebo, was, on average, achieved at a walking speed of 5.5 km/h up a gradient of 14%. This represents a substantial additional workload in this context.
Ivabradine in combination with beta-blocker therapy was well tolerated in this study. Adverse events related to low heart rate are predictable with any heart rate-lowering therapy, and the low rate of withdrawal for this cause (1.1% in the ivabradine group) is notable, especially in the light of the low heart rate criterion (≥50 b.p.m.) for up-titration of the study drug. Visual symptoms, typically phosphenes, are thought to be related to the pharmacological action of ivabradine on ion channels in the retina that are similar to those responsible for the If current in the sino-atrial node. Visual symptoms with ivabradine are typically mild and transient, without impact on daily activity of the patient. The incidence of adverse events related to visual symptoms with ivabradine (2.0% compared with 0.9% in the placebo group) was markedly lower than in previous studies.11,12 In these previous studies, patients were specifically asked about visual symptoms at study visits. In the present study, patients were informed of the possibility of visual symptoms before study commencement, but were not specifically asked about them during visits.
Ivabradine directly and selectively inhibits the ionic channel in the sino-atrial node responsible for If, a current that is important in pacemaker activity and the physiological regulation of heart rate.10,24,25 At therapeutic concentrations, ivabradine has no action at other cardiac ion channels or receptors and does not act via altering intracellular cyclic adenosine monophosphate levels. Consequently, ivabradine does not depress myocardial contractility26,27 or intracardiac conduction28 and has only minor effects on blood pressure as seen in this and previous studies.12 Thus, the unique haemodynamic profile of ivabradine can provide anti-ischaemic efficacy in addition to heart rate lowering with beta-blocker therapy, and this combination therefore represents a potential therapeutic strategy to treat patients with stable angina.
In conclusion, ivabradine treatment resulted in significant dose-dependent improvements in all ETT criteria relative to placebo in patients with stable angina pectoris receiving background therapy with atenolol. The starting dose of ivabradine was 5 mg b.i.d., which was increased at 2 months to 7.5 mg b.i.d. for a further 2 months. Improvements in all ETT criteria were significant at 2 months as well as at 4 months, indicating the efficacy of both ivabradine doses. The combination of ivabradine and atenolol was well tolerated, and the incidence of adverse events related to visual symptoms was markedly lower than reported in previous ivabradine studies. Thus, the combination of ivabradine 7.5 mg b.i.d. and atenolol at the commonly used dosage in clinical practice in patients with chronic stable angina pectoris produced additional efficacy with no untoward effect on safety or tolerability.
Funding
The study was supported by Servier, France. Funding to pay the Open Access publication charges for this article was provided by Les Laboratoires SERVIER, France.
Conflict of interest: J.-C.T., P.P., and T.K. have received honoraria from Servier.
Appendix
Study coordinators: J.-C. Tardif, Canada (principal coordinator); J.E. Tronje, R.A. Ahuad Guerrero, Argentina; M. Machado Cesar, Brazil; N.N. Gotcheva, Bulgaria; M. Vejar, Chile; V. Chaloupka, Czech Republic; W. Delius, Germany; I. Edes, Hungary; F. Crea, Italy; P.-K. Ronnevik, Norway; M. Banasiak, A. Swed, Poland; C. Macarie, Romania; Y. Karpov, Russia; D. Pella, Slovakia; P.J. Commerford, South Africa; T. Kahan, Sweden; J.J. Alonso Martin, Spain; A. Parshomenko, Ukraine; A. Timmis, UK.
Investigators: Argentina: R.A. Ahuad Guerrero, F.J. Sokn, O.A. Allall, L.R. Cartasegna, L.L. del V. Lobo Marquez, A. Hirschson Prado, A.D. Hrabar, J.O. Ibañez, J.J. Fuselli, H.L. Luciardi, S.M. Macin, M. Rusculleda, A.C. Piombo, M.S. Sanjurjo, F.L. Gadaleta, J.C. Pomposiello, R.M. Colgue, M. Hominal, E. Kuschnir, H.A. Luquez, R. Ronderos, A.C. Amico; Brazil: L.A. Machado Cesar, M. Maranhao, O.R. Coelho, E. Manenti, A.H. Herdy, J.F. Kerr Saraiva, D.B. Precoma, J.P. Ribeiro, I. Castro, J.R.C. de B Silveira; Bulgaria: V. Baycheva, S. Denchev, D. Raev, A. Tschirkov, A. Dzhurdzhev, A. Penev, M. Tzekova; Canada: P. Ma, G. Bailey, R. Saint-Hilaire, D. Desai, M.F.J. O’Mahony; Chile: M. Vejar, F. Lanas, R. Lamich, J. Abadal, L. Lucio; Czech Republic: M. Rubacek, O. Jerabek, E. Zidkova, K. Gorican, J. Danczik; Germany: A. Al Zoebi, A. Förster, W. Rein; Hungary: A. Nagy, A. Katona, J. Takacs, F. Magel, J. Lippai, B. Nyuzo, A. Kovacs, G. Polak, J. Nagy, T. Sydo, A. Papp, K. Toth, M. Sereg, S. Timar, J. Tenczer, F. Lakatos; Italy: A. Gaspardone, C. Vitale; Norway: P. Ronnevik, H. Istad, P.A. Sirnes; Poland: W. Banasiak, A. Budaj, M. Dluzniewski, H. Szwed, K. Kawecka-Jaszcz, W. Piwowarska, P. Achremczyk, J. Kwiecien, M. Szpajer, J. Kopaczewski, K. Szymczak, A. Kowalisko, W. Pluta, R. Trojnar, A. Drzewiecki; Romania: C. Macarie, M.M. Vintila, A.G. Dan, R. Capalneanu, D.D. Ionescu, I. Manitiu, M. Popescu, G. Aaron; Russian Federation: Y.A. Karpov, D. Aronov, S.A. Boytsov, N.A. Gratsiansky, A.Y. Konyakhin, A.K. Starodubtsev, S.N. Tereschenko, O.P. Shevchenko, Y.N. Grishkin, A.S. Svistov, N. Khasanov, V. Naumov, E.A. Zharova, S. Martsevitch, E. Kokourina, M.G. Glezer, A.Y. Ivleva, Y. Lukjanov, S. Tchurina, L. Ermoshkina, T. Treshkur, G. Usova, E. Volkova, I. Shaposhnik, A. Timofeev, G.A. Chumakova, A. Masin, O.L. Barbarash, P. Yakhontova, A. Kolomiets; Slovakia: D. Pella, A. Dzupina, S. Filipova, P. Loviska, J. Mazur; South Africa: J. Bayat, M.M de V. Basson, M. Mpe, D.P. Naidoo; Spain: J.J. Alonso Martin, V. Manuel, I. Plaza; Thailand: R. Krittayaphong; Ukraine: A. Parkhomenko, A. Bazilevich, B. Goloborodko, I. Krayz, V. Lyzogub, V. Tseluyko, M. Vlasenko, V. Volkov: UK: A. Pell.
References
- 1.Hjemdahl P, Eriksson SV, Held C, Forslund L, Näsman P, Rehnqvist N. Favourable long term prognosis in stable angina pectoris: an extended follow up of the angina prognosis study in Stockholm (APSIS) Heart. 2006;92:177–182. doi: 10.1136/hrt.2004.057703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katritsis DG, Ioannides JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation. 2005;111:2906–2912. doi: 10.1161/CIRCULATIONAHA.104.521864. [DOI] [PubMed] [Google Scholar]
- 3.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Fihn SD, Fraker TD, Jr, Gardin JM, O’Rourke RA, Pasternak RC, Williams SV American College of Cardiology; American Heart Association Task Force on practice guidelines (Committee on the Management of Patients with Chronic Stable Angina) ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients with Chronic Stable Angina) J Am Coll Cardiol. 2003;41:159–168. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 5.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, Jacobs AK, Kern MJ, King SB, III, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B American College of Cardiology/American Heart Association Task Force on Practice Guidelines; American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography Interventions Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. Circulation. 2006;113:156–175. doi: 10.1161/CIRCULATIONAHA.105.170815. [DOI] [PubMed] [Google Scholar]
- 6.Fox K, Garcia MA, Ardessino D, Buszman P, Camici PG, Crea F, Daly C, De Backer G, Hjemdahl P, Lopez-Sendon J, Marco J, Morais J, Pepper J, Sechtem U, Simoons M, Thygesen K. Guidelines on the management of stable angina pectoris: executive summary: the Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–1381. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 7.Guth BD, Heusch G, Seitelberger R, Ross J. Mechanism of benefit of β-adrenergic blockade on exercise-induced myocardial ischaemia in conscious dogs. Circ Res. 1987;60:738–746. doi: 10.1161/01.res.60.5.738. [DOI] [PubMed] [Google Scholar]
- 8.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif J-C, Tavazzi L, Tendera M. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 9.Daly CA, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F, Fox KM. The initial management of stable angina in Europe, from the Euro Heart Survey: a description of pharmacological management and revascularization strategies initiated within the first month of presentation to a cardiologist in the Euro Heart Survey of Stable Angina. Eur Heart J. 2005;26:1011–1022. doi: 10.1093/eurheartj/ehi109. [DOI] [PubMed] [Google Scholar]
- 10.DiFrancesco D. Funny channels in the control of cardiac rhythm and mode of action of selective blockers. Pharmacol Res. 2006;53:399–406. doi: 10.1016/j.phrs.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Borer JS, Fox K, Jaillon P, Lerebours G. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107:817–823. doi: 10.1161/01.cir.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- 12.Tardif J-C, Ford I, Bourassa MG, Fox K INITIATIVE Investigators. Efficacy of ivabradine, a new selective If inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J. 2005;26:2529–2536. doi: 10.1093/eurheartj/ehi586. [DOI] [PubMed] [Google Scholar]
- 13.Ruzyllo W, Tendera M, Ford I, Fox KM. Antianginal efficacy and safety of ivabradine compared with amlodipine in patients with stable effort angina pectoris: a 3-month randomised, double-blind, multicentre, noninferiority trial. Drugs. 2007;67:393–405. doi: 10.2165/00003495-200767030-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hettmansperger TP, Mckean JW. Robust, Nonparametric Statistical Methods. London: Arnold; 1998. [Google Scholar]
- 15.Hollander M, Wolfe AD. Nonparametric Statistical Methods. New York: John Wiley & Sons, Inc.; 1973. [Google Scholar]
- 16.Committee for Medicinal Products For Human Use (CHMP) Guideline on the clinical investigation of anti-anginal medicinal products in stable angina pectoris. 2006 CPMP/EWP/234/95/rev.1. [Google Scholar]
- 17.Gislason GH, Rasmussen JN, Abildstrøm SZ, Gadsbøll N, Buch P, Friberg J, Rasmussen S, Køber L, Stender S, Madsen M, Torp-Pedersen C. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27:1153–1158. doi: 10.1093/eurheartj/ehi705. [DOI] [PubMed] [Google Scholar]
- 18.Fox KM, Mulcahy D, Findlay I, Ford I, Dargie HJ TIBET Study Group. The Total Ischaemic Burden European Trial (TIBET): effects of atenolol, nifedipine SR and their combination on the exercise test and the total ischaemic burden in 608 patients with stable angina. Eur Heart J. 1996;17:96–103. doi: 10.1093/oxfordjournals.eurheartj.a014699. [DOI] [PubMed] [Google Scholar]
- 19.Knight CJ, Fox KM. Amlodipine versus diltiazem as additional antianginal treatment to atenolol. Centralised European Studies in Angina Research (CESAR) Investigators. Am J Cardiol. 1998;81:133–136. doi: 10.1016/s0002-9149(97)00893-x. [DOI] [PubMed] [Google Scholar]
- 20.Pehrsson SK, Ringqvist I, Ekdahl S, Karlson BW, Ulvenstam G, Persson S. Monotherapy with amlodipine or atenolol versus their combination in stable angina pectoris. Clin Cardiol. 2000;23:763–770. doi: 10.1002/clc.4960231014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein WW, Jackson G, Tavazzi L. Efficacy of monotherapy compared with combined antianginal drugs in the treatment of chronic stable angina pectoris. Coron Artery Dis. 2002;13:427–436. doi: 10.1097/00019501-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, Wang W, Skettino SL, Wolff AA Combination Assessment of Ranolazine in Stable Angina (CARISA) Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309–316. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- 23.McInnis RJ, Balady GJ, Weiner DA, Ryan TJ. Comparison of ischemic and physiologic responses during exercise tests in men using the standard and modified Bruce protocols. Am J Cardiol. 1992;69:84–89. doi: 10.1016/0002-9149(92)90680-w. [DOI] [PubMed] [Google Scholar]
- 24.Bucchi A, Tognati R, Milanesi M, Baruscotti M, DiFrancesco D. Properties of ivabradine-induced block of HCN1 and HCN4 pacemaker channels. J Physiol. 2006;572:335–346. doi: 10.1113/jphysiol.2005.100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bucchi A, Baruscotti M, Robinson RB, DiFrancesco D. Modulation of rate by autonomic agonists in SAN cells involves changes in diastolic depolarization and the pacemaker current. J Mol Cell Cardiol. 2007;43:39–48. doi: 10.1016/j.yjmcc.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Manz M, Reuter M, Lauck G, Omran H, Jung W. A single dose of ivabradine, a novel I(f) inhibitor, lowers heart rate but does not depress left ventricular function in patients with left ventricular dysfunction. Cardiology. 2003;100:149–155. doi: 10.1159/000073933. [DOI] [PubMed] [Google Scholar]
- 27.Joannides R, Moore N, Iacob M, Compagnon P, Lerebours G, Menard JF, Thuillez C. Comparative effects of ivabradine, a selective heart rate-lowering agent, and propranolol on systemic and cardiac haemodynamics at rest and during exercise. Br J Clin Pharmacol. 2006;61:127–137. doi: 10.1111/j.1365-2125.2005.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camm AJ, Lau CP. Electrophysiological effects of a single intravenous administration of ivabradine (S 16257) in adult patients with normal baseline electrophysiology. Drugs R &D. 2003;4:83–89. doi: 10.2165/00126839-200304020-00001. [DOI] [PubMed] [Google Scholar]