Abstract
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family, which has been reported to regulate neurogenesis in the dentate gyrus, but the molecular control over this process remains unclear. We demonstrated that by activating TrkB receptor tyrosine kinase, BDNF controls the size of the surviving pool of newborn neurons at the time of connectivity. The TrkB-dependent decision regarding survival in these newborn neurons takes place at approximately four to six weeks of age. Before newborn neurons start to die they exhibit a drastic reduction in dendritic complexity and spine density, which may reflect a failure of these cells to integrate appropriately. Both the failure to become integrated, and subsequent dying, leads to impaired neurogenesisdependent plasticity and increased anxiety-like behavior in mice lacking a functional TrkB receptor in newborn neurons. Thus, our data demonstrate the importance of BDNF/TrkB signaling for the survival and integration of newborn neurons in the adult hippocampus and suggest a critical function of these neurons in regulating the anxiety state of the animal.
Key words: adult neurogenesis, synaptogenesis, neurotrophins, dentate gyrus, learning and memory, mood-related behavior
The lifelong genesis of new neurons is well documented in many animal species including humans,1,2 yet the fate of these new neurons and their functions are largely unknown.3–5 Adult neurogenesis in mammals has been observed mainly in two brain regions, the subependymal zone (SEZ) of the lateral ventricle6,7 and the subgranule zone (SGZ) in the dentate gyrus (DG) of the hippocampus.7 It has only recently been demonstrated that neurogenesis in these areas results from self replicating astroglial-like stem cells.8–10 Once generated, the vast majority of neurons in the DG remain located on the hilar side of the granule layer and integrate into the existing neuronal network by receiving afferent input from perforant path fibers,11,12 and providing efferent output to pyramidal CA3 cells.13,14 While new neurons have been regarded to enter the pre-existing circuitry via a stereotypical sequence of morphological transitions, a detailed description of this process has only recently been described in mice using retroviral mediated birth-dating analysis.15 Axons and dendrites initiate their growth around three days post retroviral injection. While axons enter the CA3 areas of the hippocampus after ten days, dendrites grow through the outer edge of the molecular layer where they attain a high level of complexity by approximately eight weeks of age, creating the basic organization of synaptic connections. Afferent inputs to adult-born neurons are regulated by prior development of dendritic spines and formation of functional synapses.12 Spine growth starts in neurons at day 16 after birth, whereas spine density is sharply increased only at day 28, slowly reaching the final plateau after six weeks.15 Although many factors are known to influence these processes indirectly, the molecular mechanisms regulating the functional integration and/or survival of new-born neurons are not yet fully understood. There is growing evidence emphasizing the effect of neuronal activity on newborn neurons differentiation and survival.16,17 Before being synaptically integrated, new neurons sense neuronal activity through tonic γ-aminobutyric acid (GABA). Defects in the GABA responsiveness of newborn neurons has been shown to lead to marked deficits in their morphology,16 suggesting that network activity controls key transitions in their maturation. In addition, glutamatergic inputs controlling newborn neuron survival at the initiation of connectivity have been described.17 Indeed, access to afferent inputs maybe the key to their ability to survive. An important consequence of this regulation is that adult-born neurons may contribute to the formation of new circuits in tune with the network's needs, which, in turn, require morphologically intact neurons to decide on their survival. Moreover, around connectivity time and later, adult-born neurons have shown enhanced synaptic plasticity16 and become preferentially recruited into functional networks, i.e., memory networks.18,19 As neurogenesis is an ongoing process, these features of newborn neurons' maturation may play a key role in the morphological and functional plasticity of hippocampal circuitry, which have been thought to underlie complex cognitive4,5 and emotional20 functions.
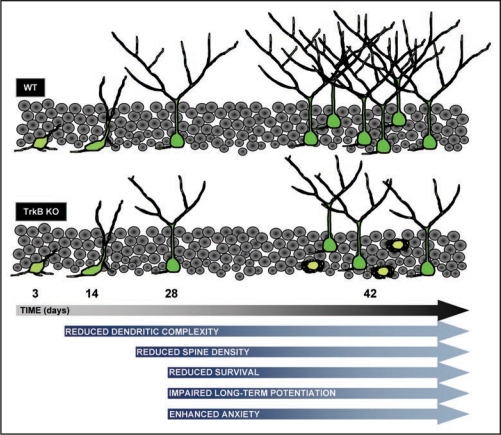
Our study assessed the role of the brain-derived neurotrophic factor (BDNF)/TrkB signaling on the lineage progression, differentiation and functional outputs of neurons generated in the adult hippocampus. We used a mouse line expressing inducible Cre in adult neural stem cells and retrovirus-mediated single-cell Cre expression to obtain a specific lack of BDNF receptor TrkB signaling in adult-born neurons (Fig. 1). We show that this receptor is required for the morphological maturation and survival of new neurons at the critical time period of connectivity. While the selective loss of NeuN positive newborn neurons is manifest only after four weeks post recombination, TrkB deficient newborn neurons start to exhibit the first signs of decreased dendritic arborization by two weeks. Noticeably, a similar reduction in dendritic complexity has been shown with knock down of the Na-K-2Cl co-transporter NKCC1.16 This manipulation affects the chloride gradient in newborn neurons, thereby converting GABA prematurely into an inhibitory transmitter on these neurons. The drastic loss of dendritic complexity in that study was also accompanied by a delayed synaptic innervation both of GABA and glutamatergic inputs. The fact that TrkB deficient newborn neurons exhibit a marked reduction in the density of dendritic spines suggests that loss of this neurotrophin receptor is also accompanied by a loss of glutamatergic synapses at four weeks of age. Both dendritic morphology and synapse density suggest that there may be a functional interdependence between GABA and TrkB signaling in immature neurons. Loss of functional synaptic input may indeed cause the subsequent cell death. Tashiro et al.,17 found that specific deletion of NMDAR1 in newborn neurons drastically shortens the survival of these cells beginning from about week three and continuing to week six. No apparent deficits in dendritic morphology were reported in that study, but one may speculate that the cause of cell death in TrkB deficient newborn neurons is not a direct consequence of the faulty morphological development. Instead it may be due to impaired functional synaptic input to these cells even though synapse formation per se does not require TrkB signaling. Currently, it is impossible to discern whether the deficit in dendritic arborization is a consequence of faulty synaptic input or, conversely, the deficit in synaptic input is due to faulty dendritic arborization. An interesting observation in the Tashiro study was that the shorter survival of immature neurons produced through loss of NMDAR1 could be rescued by pharmacological blockade of NMDA receptors in the dentate gyrus. This is taken as evidence for NMDA receptor activation mediating competitive selection among newborn neurons. Our observation that deletion of TrkB in the majority of newborn neurons did not prevent the effect on dendritic morphology and survival suggests that selective activation of TrkB is not involved in the competitive action through activation of the NMDA receptor.
Figure 1.
Scheme depicting the effects of TrkB deletion in newborn neurons.
While we cannot exclude that some aspects of axonal ramification are also affected in the absence of TrkB, we observed a normal development of the axonal projection to the CA3 region. This would suggest that the maturation of the dendritic and axonal compartments is differentially affected by the loss of TrkB and that the processes underlying their respective development are not tightly interwoven. Thus, one interesting finding arising from our study is that the survival of newborn neurons is primarily dependent upon the input received by these neurons rather than the output generated by them.
Besides a critical time period for survival and connectivity, newborn neurons also exhibit a critical time period for synaptic plasticity. For instance, Ge et al.,19 reported that newborn neurons are particularly susceptible to synaptic modification during the postnatal 4- to 6-week period. Synaptic potentiation during this time period depends on the activation of an NMDA receptor containing the NR2B subunit that is selectively expressed in these cells, but absent in mature neurons. Interestingly, LTP mediated by this NMDA receptor can be elicited in the absence of GABA-A receptor antagonists,21,22 suggesting that this LTP is exclusively localized to synapses on newborn neurons. Consistent with this interpretation is the fact that LTP induced in the absence of GABA-A antagonists is abrogated upon ablation of neurogenesis, for instance by irradiation.21–23 Our study shows that while an ifenprodil-sensitive LTP can be induced in wild-type mice, LTP is drastically impaired in mice in which the vast majority of new born neurons lack TrkB (Fig. 1). While an early, albeit reduced, LTP could be observed the late phase was totally abolished. The slight reduction in the early phase of LTP may already reflect the reduced number of surviving neurons, while the lack of a late phase of LTP is strikingly similar to the effect of the loss of TrkB or its ligand BDNF at the Schaffer collateral synapses. The lack of a long-term LTP may ultimately cause the reduced number of synapses since these may not undergo activity-dependent stabilization. However, we currently cannot rule out that the deficit in LTP is a secondary consequence of the morphological maturation deficit. In any case the apparent deficit in synaptic long-term plasticity is likely to have a major bearing on the participation of newborn neurons in the functional network activity. It has recently been proposed that newborn neurons between four to six weeks are preferentially recruited in the context of exposure of mice to novel environments,18 an effect attributed to new neurons' enhanced synaptic plasticity and excitability.19,23,24
Lastly, we show for the first time that mice lacking functional full-length TrkB specifically in the newborn neuron population of four to six weeks of age exhibited a markedly enhanced anxiety- like behavior as evidenced by their decreased explorative activity in the open field and elevated plus maze tests (Fig. 1). The fact that deletion of TrkB in newborn neurons is sufficient to cause an enhanced anxiety-like behavior suggests that this mood state may be particularly affected by the functional recruitment of newborn neurons. Intriguingly, a recent publication reported that ablation of hippocampal neurogenesis does not result in anxiety-like behavior,22 suggesting that the phenotype we observed here may be due to an inappropriate integration of the surviving neurons rather than the death of the others. This interpretation supports the current idea that neurogenesis per se20,25 and BDNF26 are not etiological factors for anxiety, but rather, contribute indirectly to the activity of hippocampal network involved in this emotional state. Hence, deficits in hippocampal circuitry have been implicated to influence the activity of structures receiving input from the hippocampus (i.e., prefrontal cortex, amigdala and nucleus accumbens), and are associated with mood behavior. In addition, BDNF regulation of newborn neurons' integration into the existing hippocampal circuitry could represent a molecular mechanism for plastic refinement of hippocampal network activity adapting to external inputs, which, in turn, may influence anxiety-like behavior. These inputs include physical exercise,27 environmental enrichment28 and common antidepressant drugs,29 that have been shown to influence mood-related behavior by regulating the rate of neurogenesis.
Our identification that BDNF is an extrinsic factor regulating anxiety-related functions by active neurogenesis will aid future efforts to understand the mutual interaction of network activity and the physiological properties of young neurons for the control of distinct emotional outputs.
Acknowledgements
We thank Bendetto Amarone for suggestions and insightful comments on the manuscript. The research in the authors' laboratories was partially supported by PRIN.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7349
References
- 1.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 2.Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savati M. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 4.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 5.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 9.Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori T, Tanaka K, Buffo A, Wurst W, Kühn R, Götz M. Inducible gene deletion in astroglia and radial glia—a valuable tool for functional and lineage analysis. Glia. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- 11.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 13.Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 15.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 18.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 19.Ge S, Yang CH, Hsu KS, Ming GL, Song HA. Critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 21.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 22.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol. 2000;42:248–257. [PubMed] [Google Scholar]
- 24.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 25.Castrén E, Voikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 27.Ernst C, Olson AK, Pinel JP, Lam RW, Christie BR. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci. 2006;31:84–92. [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]