Abstract
CaMKII, calcium/calmodulin dependent protein kinase, is an active kinase in the cell that phosphorylates a number of substrates including several cytoskeletal and signaling proteins. In addition to kinase activity, the β isoform of CaMKII also contains an F-actin binding region. We recently identified a new F-actin rich structure in developing cortical neurons that endogenous CaMKIIβ bound. In nonneuronal cells and dendrite spines of hippocampal neurons where an interaction between CaMKIIβ and F-actin has been identified, CaMKIIβ was involved in regulating the differentiation of dendrite spines and formation of synapses. In this study, we took advantage of the temporal and spatial regulation of CaMKII isoforms to reveal a specific role for CaMKIIβ in binding and stability of a novel F-actin rich structure. We used FRAP and colocalization assays in this CaMKIIβ rich system to demonstrate a structural, rather than enzymatic, role of CaMKIIβ. In this addendum, we further discuss the significance of this study and the possible implication to the field.
Key words: CaMKII, calcium, calmodulin, F-actin, cytoskeleton, protein-protein interaction, subcellular localization, oligomerization
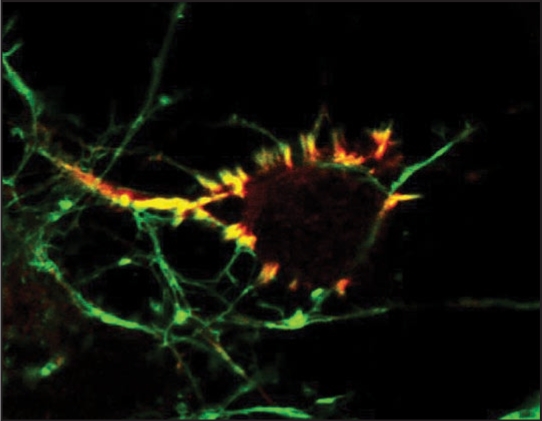
CaMKII is an evolutionarily conserved multimeric protein encoded by four isoforms in higher vertebrates: α, β, γ and δ.1 CaMKII is an abundant protein and constitutes up to 1∼2% of total protein in the adult brain.2,3 Although all isoforms of CaMKII can be found in the brain, CaMKIIα and β predominate and preferentially form α and β holoenzymes and αβ heteroenzymes.2–4 CaMKII isoform expression in the brain is developmentally and spatially regulated in vivo and in vitro. In the cerebral cortex CaMKIIβ expression is detected embryonically, while CaMKIIα expression is postnatal.5–7 CaMKII isoforms share 70∼90% sequence homology and consist of a catalytic, regulatory, variable and oligomerization domains.8 The variable domain of CaMKII is alternatively spliced which alters subcellular localization and enzymatic attributes of the enzyme.9,10 In the variable domain of CaMKIIβ lies a F-actin binding domain that targets expressed CaMKIIβ to actin filaments in stress fibers and dendrite spines.11–13 We recently found that endogenous CaMKIIβ was highly enriched in a unique F-actin rich structure in developing cortical neurons.14 This subcellular localization was pronounced and specific to CaMKIIβ (Fig. 1).
Figure 1.
CaMKIIβ(red) highly colocalizes with F-actin (green) in discrete F-actin rich structures in an embryonic cortical neuron. These F-actin rich protrusions are enriched around or underneath the cell body. CaMKIIβ binding to F-actin is regulated by calcium signals and is important for the F-actin filament stability. Image courtesy of Yu-Chih Lin and Lori Redmond.
In addition to variable regions among the CaMKII isoforms, oligomerization is important for proper CaMKII localization. For example, monomeric CaMKIIβ, which lacks an oligomerization domain, does not colocalize with F-actin even though the F-actin binding domain is intact.14 Although the F-actin binding domain in CaMKIIβ may be unique and required for CaMKIIβ targeting to F-actin, the domain itself is not sufficient to bind F-actin directly.13,14 CaMKIIδ binds to F-actin likely via a non-variable domain of the δ isoform that also requires oligomerization.15,16 This further emphasizes that proper assembly of the oligomer may be crucial for formation of a secondary structure which allows CaMKIIβ to anchor to actin filaments. In addition, all four isoforms of CaMKII can freely hetero-oligomerize via their oligomerization domains and the isoform composition of CaMKII influences enzyme localization.9,13,17 For example two CaMKIIβ subunits are sufficient for targeting the holoenzyme to cortical F-actin.13
CaMKII is readily activated in response to fluctuations of cellular calcium concentrations. Calcium induces calcium/calmodulin binding followed by two phosphorylation events.18 The first of these is phosphorylation of T286 (in α) or T287 (in β, γ and δ) by a neighboring calcium/calmodulin-bound subunit. T286/7 autophosphorylated CaMKII is enzymatically active, but a subsequent calcium/calmodulin-independent phosphorylation at T305/306 suppresses the total enzymatic activity. The calcium/calmodulin-binding site of CaMKIIβ is adjacent to the F-actin binding domain. The activation of CaMKIIβ via calcium/calmodulin binding likely masks the F-actin binding site and therefore, competes with F-actin binding for CaMKIIβ. We used KN93, which binds to the calcium/calmodulin binding site of CaMKII, and demonstrated that calcium/calmodulin binding was required to dissociate CaMKIIβ from F-actin.14 We further confirmed this by examining a mutant that cannot bind calcium/calmodulin, CaMKIIβ-A303R, and found that it bound F-actin similarly to wild-type CaMKIIβ. Likewise kinase and phosphorylation deficient mutants associated with F-actin to the same degree as wild-type CaMKIIβ whereas activated CaMKIIβ dissociated from F-actin.14 This data indicates that even though a kinase and capable of phosphorylating actin19 the kinase activity of CaMKIIβ was not required for F-actin binding. Our findings are consistent with biochemical studies indicating that dissociation of CaMKIIβ from F-actin requires calcium/calmodulin binding, but not kinase activity.11,12,14
We found that wild-type, calcium/calmodulin binding deficient, and kinase deficient CaMKIIβ increased the prominence of F-actin rich structures whereas active CaMKIIβ did not. This ability of CaMKIIβ to promote the F-actin rich structures was directly tied to CaMKIIβ binding to F-actin. When CaMKIIβ—F-actin binding was tight, F-actin rich structures were more prominent and when binding was weak, prominence decreased.14 This strong binding of CaMKIIβ to actin filaments and the loss of the F-actin rich structures after disruption of CaMKIIβ—F-actin binding are consistent with a role for CaMKIIβ in actin stabilization and bundling in vivo and in vitro.19,20
In summary, our work suggests that it is CaMKIIβ binding to F-actin that is the key to CaMKIIβ's ability to regulate actin filament stability. Although several kinases have been shown to bind F-actin and regulate actin dynamics, CaMKIIβ is unique in that it regulates the cytoskeleton independent of its kinase activity. CaMKIIβ's dual function, structural and enzymatic, expands it's role in cellular signaling, and suggests CaMKIIβ, and by inference other enzymes, lacks a purely inactive or nonfunctional state.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7426
References
- 1.Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca2+/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MK, Erondu NE, Kennedy MB. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983;258:12735–12744. [PubMed] [Google Scholar]
- 3.Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem. 1999;274:22713–22722. doi: 10.1074/jbc.274.32.22713. [DOI] [PubMed] [Google Scholar]
- 5.Bayer KU, Lohler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70:147–154. doi: 10.1016/s0169-328x(99)00131-x. [DOI] [PubMed] [Google Scholar]
- 6.Karls U, Muller U, Gilbert DJ, Copeland NG, Jenkins NA, Harbers K. Structure, expression and chromosome location of the gene for the beta subunit of brain-specific Ca2+/calmodulin-dependent protein kinase II identified by transgene integration in an embryonic lethal mouse mutant. Mol Cell Biol. 1992;12:3644–3652. doi: 10.1128/mcb.12.8.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahyoun N, LeVine H, 3rd, Burgess SK, Blanchard S, Chang KJ, Cuatrecasas P. Early postnatal development of calmodulin-dependent protein kinase II in rat brain. Biochem Biophys Res Commun. 1985;132:878–884. doi: 10.1016/0006-291x(85)91889-3. [DOI] [PubMed] [Google Scholar]
- 8.Lin CR, Kapiloff MS, Durgerian S, Tatemoto K, Russo AF, Hanson P, et al. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci USA. 1987;84:5962–5966. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith LC, Lu CS, Sun XX. CaMKII, an enzyme on the move: regulation of temporospatial localization. Mol Interv. 2003;3:386–403. doi: 10.1124/mi.3.7.386. [DOI] [PubMed] [Google Scholar]
- 10.Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:73–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 11.Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 12.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 13.Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- 14.Lin YC, Redmond L. CaMKII{beta} binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0804399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Easley CA, Faison MO, Kirsch TL, Lee JA, Seward ME, Tombes RM. Laminin activates CaMK-II to stabilize nascent embryonic axons. Brain Res. 2006;1092:59–68. doi: 10.1016/j.brainres.2006.03.099. [DOI] [PubMed] [Google Scholar]
- 16.Caran N, Johnson LD, Jenkins KJ, Tombes RM. Cytosolic targeting domains of gamma and delta calmodulin-dependent protein kinase II. J Biol Chem. 2001;276:42514–42519. doi: 10.1074/jbc.M103013200. [DOI] [PubMed] [Google Scholar]
- 17.Lantsman K, Tombes RM. CaMK-II oligomerization potential determined using CFP/YFP FRET. Biochim Biophys Acta. 2005;1746:45–54. doi: 10.1016/j.bbamcr.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 19.O'Leary H, Lasda E, Bayer KU. CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol Biol Cell. 2006;17:4656–4665. doi: 10.1091/mbc.E06-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci USA. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]