Abstract
Wnt/β-catenin canonical pathway is critical for normal embryonic development; mutations and aberrant expression of specific components of this pathway can be oncogenic. Mitogen-activated protein kinase (MAPK) pathways, prominent in intracellular signaling, have been shown to have unique and provocative roles that impact the Wnt/β-catenin signaling. We discuss recent insights that implicate the three major pathways of the MAPK network, i.e., mediated by p38, c-Jun N-terminal (JNK) kinase and Extra-cellular-Regulated Kinases (ERK) and their downstream signaling elements in Wnt/β-catenin signaling. Novel “crosstalk” among MAPK and Wnt/β-catenin canonical signaling pathways is essential. A fuller understanding of how such signaling is integrated during development is a high-value target for future research.
Key words: Wnt, β-catenin, p38, ERK, JNK, frizzled, dishevelled, Lef/Tcf, G-protein
Introduction
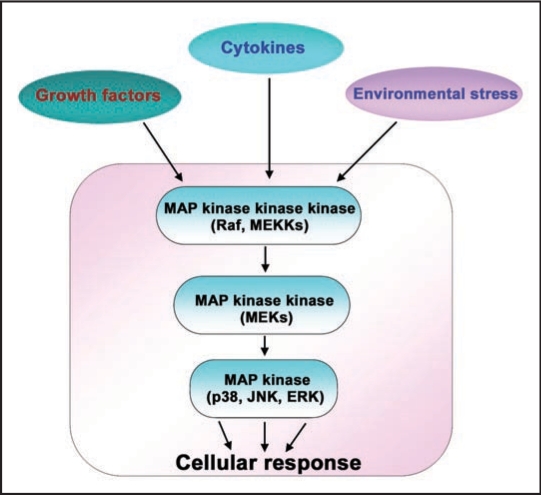
Mitogen-activated protein kinases are a family of signaling molecules whose activity is regulated in response to a variety of stimuli, including growth factors, cytokines, or even environmental stress. MAPKs are the primary end points for a cascade that included upstream activation of MAP kinase kinase kinases (e.g., MAPKKK, MAP3K) that phosphorylate MAP kinase kinases (e.g., MAPKK, MAP2K) that, in turn, phosphorylate the MAPKs themselves (i.e., p38 MAPK, JNK1/2 and ERK1/2) (Fig. 1).1,2 MAPK pathways of this level of complexity and reversible phosphorylation/dephosphorylation constitute a large set of targets for input from cell signaling molecules not routinely associated with their operation, especially the canonical Wnt/β-catenin signaling.
Figure 1.
Mitogen-activated protein kinase pathways. Mitogen-activated protein kinase (MAPK) pathways are stimulated by a variety of extra-cellular stimuli, including, growth factors, cytokines or environmental stress. The MAPK cascades constitute three sequentially activated kinase complexes. MAPKs, which include p38, c-Jun N-terminal kinase (JNK) and Extra-cellular regulated kinase (ERK) are substrates for phosphorylation by MAP kinase kinases (MKKs). The MAP kinase kinases are in turn phosphorylated by MAP kinase kinase kinases (MEKKs). The activated MAPK ultimately induce an appropriate cellular response. Different stimuli activate either p38 or JNK or ERK pathways via different combinations of MEKKs and MKKs. Note: for more information on MAP kinase proteins and their upstream kinases refer to Dong et al.2
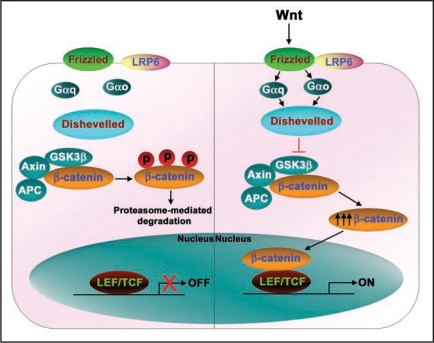
Wnt signaling is essential for normal embryonic development, patterning and cellular proliferation; dysregulation of the function/expression of components of this pathway is linked to many forms of human cancer.3–6 Wnt ligands bind to G-protein-coupled receptors, termed “Frizzleds” (Fzs) and initiate intracellular signaling cascades (Fig. 2).7,8 The Wnt-sensitive pathways include not only the “canonical” Wnt/β-catenin,7,9 but also the non-canonical planar cell polarity and Wnt/cyclic GMP/Ca2+ pathways.10–14 In the absence of Wnt, cellular β-catenin is phosphorylated by the Serine/Threonine protein kinase, glycogen synthase kinase-3β (GSK3β), which is a component of a large multiprotein complex that includes the scaffold Axin and the product of the adenomatous polyposis coli gene. This complex is referred to as a “destruction complex” since it catalyzes the ubiquitination and proteasome-mediated degradation of β-catenin when Wnts are not present. Wnt3a binding to Fz1 activates G-proteins, Gαo and Gαq and the downstream phosphoprotein Dishevelled (Dsh/Dvl), provoking reduced GSK3β kinase activity, decreased phosphorylation of β-catenin by the complex, and increased stability and accumulation of intracellular β-catenin. Nuclear accumulation of β-catenin provokes activation of lymphoid-enhancer factor/T-cell factor (Lef/Tcf)-sensitive transcription of developmentally-related genes (Fig. 2).15,16 The central role of β-catenin in this canonical pathway is highlighted in studies of human cancers. In colon cancer, cells display increased levels of β-catenin that appear to result from high-frequency mutations in the adenomatous polyposis coli (APC) protein.17 Mutations in β-catenin itself were also found to be responsible for several colon and other types of human cancers.18,19 The majority of the mutations of β-catenin reported are potential sites of GSK3β phosphorylation.20,21 Intracellular levels of β-catenin are tightly controlled, suggesting that multiple signaling pathways may be involved in regulating β-catenin levels and β-catenin-induced gene transcription. In this review, we highlight novel roles of the three MAPKs; p38 MAPK, c-Jun N-terminal kinase (JNK) and Extra-cellular regulated kinases (ERK) in the canonical Wnt/β-catenin signaling.
Figure 2.
Wnt/-catenin signaling pathway. Wnts are secreted glycoproteins that bind to their cognate receptors, Frizzleds. Frizzleds belong to a family of heptahelical, G-protein-coupled receptors that bind specific Wnts and transduce the signal to downstream signalling pathways. Under basal conditions, cellular -catenin is phosphorylated by a Serine/Threonine kinase, Glycogen Synthase Kinase-3 (GSK3) in a complex along with Axin, adenomatous polyposis coli (APC) and other elements. The phosphorylated -catenin is constantly subjected to a proteasome-mediated degradation. Wnt-activated Frizzled (with its co-receptor low-density lipoprotein-related receptor protein-6 (LRP6)) activate the downstream signalling components including G-proteins, Go and Gq, and a phosphoprotein, Dishevelled. Dishevelleds, by an unclear mechanism, lead to reduced GSK3 kinase activity. Suppression of GSK3 activity leads to elevation of cytosolic and nuclear -catenin levels. Elevated nuclear -catenin levels activates the lymphoid-enhancer factor/T-cell factor (Lef/Tcf)-sensitive transcription, turning on the genes that are necessary for development. Note: for complete list of proteins involved in Wnt signalling, refer to the Wnt home page (http://www.stanford.edu/~rnusse/wntwindow.html).
p38 MAPK
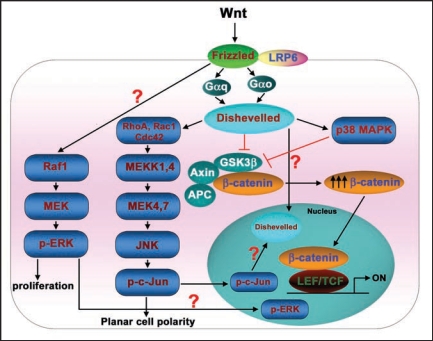
p38 is a family of MAPKs, highly conserved from yeast to mammals. p38 MAPKs also are activated in response to many extracellular stimuli including growth factors, cytokines and environmental stress.22 Interestingly, Wnts have been reported to be capable of activating p38 MAPKs.23–25 In mesenchymal stem cells (MSCs), Wnt4 mediated activation of p38 MAPK was reported to be critical for enhancing osteogenic differentiation of MSCs.24 Similarly, in C3H10T1/2 mesenchymal cells, Wnt3a induced transient activation of p38 and ERK MAPKs, which in turn regulate alkaline phosphatase activity and mineralization of nodules, suggesting a critical role for p38 in the progression of mesenchymal cells into osteoprogenitors.25 Wnt3a was shown recently to stimulate p38 MAPK activation in totipotent mouse F9 teratocarcinoma cells: this activation of p38 by Wnt was shown to be dependent both on heterotrimeric G-proteins and Dishevelleds (Fig. 3).23 More remarkably, Wnt-stimulated activation of p38 MAPK appeared to be regulating the canonical Wnt/β-catenin signaling.23 The mechanism by which Wnt stimulated the p38 MAPK activation and regulated canonical Wnt/β-catenin signaling was unclear. Reports of a novel role for p38 MAPK in regulating GSK3β inactivation may provide some insight.23,26 By utilizing specific chemical inhibitors, gene-targeting small interfering RNAs (siRNAs), or expression of kinase-dead mutants of p38 MAPK, Wnt3a stimulation of p38 MAPK was shown to inactivate GSK3β. The loss of GSK3β function provoked an increase in cytosolic β-catenin accumulation and Wnt-sensitive gene transcription.23 Thornton et al. have demonstrated that p38MAPK can phosphorylate GSK3β at Thr390 (corresponding to Ser389 in the mouse), inactivating GSK3β's kinase activity.26 Thus, Wnt3a activates p38 MAPK and this p38 pathway feeds into the canonical Wnt/β-catenin pathway minimally at the level of GSK3β (Fig. 3).
Figure 3.
Conversations between Wnt/β-catenin and MAPK signaling pathways. Wnt ligands apart from activating Wnt/β-catenin pathway also activate MAPK pathways. Wnts induce a strong activation of p38 MAPK and this activation is G-protein and Dishevelled dependent. Wnt-activated p38 MAPK regulates Wnt/β-catenin signalling by inactivating GSK3β kinase activity. Similarly, Wnt-induced JNK activation occurs through G-proteins, Dishevelleds, small molecular weight GTPases (RhoA, Rac1, Cdc42), MEKKs and MKKs. Activated JNK phosphorylates its prime substrate, c-Jun, that ultimately leads induction of planar cell polarity. The phosphorylated c-Jun may also translocate into the nucleus and along with nuclear Dishevelled regulates Lef/Tcf-sensitive gene transcription. ERKs are also activated by Wnt through Raf1/MEK/pERK pathway and induce cell proliferation. In addition, the activated ERKs may also translocate into the nucleus and regulate the Lef/Tcf-sensitive gene transcription.
c-Jun N-terminal Kinase (JNK) or Stress-Activated Protein Kinase (SAPK)
JNKs are activated in response to cytokines, UV irradiation or growth factor depletion, hence the name SAPKs. During Wnt signaling, activation of JNK is critical for the planar cell polarity pathway (PCP). Wnt ligands activate JNK in F9 teratocacrcinoma cells,10 HEK 293 cells11 and also in WEHI-231, BAJI and Raji B-cells.27 The Wnt3a-induced JNK activation operates via a pathway that is dependent on heterotrimeric G-proteins and Dishevelleds (Fig. 3).10 Interestingly, apart from this central role in the Wnt-induced PCP pathway, JNKs also regulate the canonical Wnt/β-catenin pathway.10,28 Treating cells with a highly-specific JNK inhibitor, SP600125, was found to attenuate Wnt-induced Lef/Tcf-sensitive gene transcription.10 Expression of kinase-dead mutants of the upstream protein kinases of the JNK MAPK cascade (RhoA, Rac1, Cdc42 and MEKKs), in contrast, had no significant effects on Wnt/β-catenin signaling.10 Interestingly, JNK was found to regulate only the Wnt-induced Lef/Tcf-sensitive transcription (as measured by the Topflash luciferase activities). While, β-catenin accumulation or primitive endoderm formation in response to Wnt stimulation were largely unaffected after SP600125 treatment.10,28 Earlier studies suggest that phospho c-Jun (i.e., the primary product of JNK activation) interacts with the HMG-box transcription factor Tcf4,29 while the AP-1 complex interacts with Lef1.30 Equally provocative, Gan et al. reported that nuclear c-Jun mediates Dvl association with the TCFβ-catenin complex,28 although the mechanism remains poorly described. Taken together, these observations suggest a novel insight, i.e., that the Wnt/Fz/JNK MAPK pathway and Wnt/Fz/β-catenin canonical pathway are in conversation with each other at the transcriptional level (Fig. 3).
Extra-Cellular Regulated Kinase (ERK1/2)
The ERK signaling cascade transmits signals from a variety of extracellular stimuli to multiple cellular processes, including cell proliferation, differentiation and development.31 Wnt3a has been shown to activate ERK, stimulating proliferation of NIH3T3 cells via the Raf-1/MEK/ERK pathway (Fig. 3).32,33 Chemical inhibition of ERK activation (using MEK-specific inhibitors such as PD98059 or U0126) in either totipotent F9 teratocarcinoma cells or C3H10T1/2 mesenchymal cells has been shown to attenuate (although not knock-out) Wnt3a-sensitive Lef/Tcf gene transcription; whereas Wnt3a-induced β-catenin accumulation or primitive endoderm formation remain largely unaffected after treatment with such inhibitors.10,25 How Wnt3a-induced ERK activation can influence the read-out of Lef/Tcf-sensitive gene transcription, but yet not block the overall pathway to primitive endoderm formation suggests that the relationships between these pathways are neither linear nor fully appreciated in the functional sense. Wnt3a has been shown to provoke a strong nuclear accumulation of phospho-ERK in NIH3T3 fibroblasts.33 Perhaps, Wnts can likewise provoke differential nuclear accumulation of phospho-c-Jun, phospho-ERK, and even phospho-p38. Such differential nuclear retention of phospho-MAPKs may in fact play some key role/s in the Wnt-induced Lef/Tcf-dependent transcription that can differentially effect the downstream signaling to the ultimate readouts (Fig. 3).
Conclusions and Perspectives
MAPK have long been appreciated to play key roles in cell proliferation, homeostasis and development. It is now evident that MAPK pathways also play crucial roles that can be characterized as a crosstalk with the canonical Wnt/β-catenin pathway central to development. Wnts are capable of stimulating both the Wnt/β-catenin pathway, MAPKs themselves, as well as signaling molecule intermediates, that constitute an intricate downstream network (Fig. 3). This organization provides for a conversation between these major signaling pathways, which are essential to not only signaling per se, but also to the broader landscape of spatial and temporal control of signaling events.1,2 This spatiotemporal quality of the cell signaling that undergirds development remains a vital and attracting target for novel biosensors and new technologies. Even with the current understanding on how these pathways cross-talk, probing key molecular interactions in such complex pathways will be required to eavesdrop on these subtle conversations. Molecules such as the Dishevelleds, which can clearly regulate three different Wnt-sensitive pathways as well as elements of the MAPK network, might well be the ideal nodes at which to tap into these molecular conversations. Recent studies in F9 teratocarcinoma cells and genetically modified mice clearly demonstrate Dishevelled 3, one of the three homologues of Dishevelleds, as a prime candidate for surveillance.34,35
Acknowledgements
We thank Dr. H.-Y. Wang, Department of Physiology and Biophysics, State University of New York at Stony Brook, for the critical reading of the manuscript. We also thank members of the Malbon and Wang laboratories for their constant support and encouragement. This work has been generously supported by USPHS research awards from the National Institutes of Diabetes, Digestive, and Kidney Disease, National Institutes of Health to Craig C. Malbon.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7503
References
- 1.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 2.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 3.Logan CY, Nusse R. The Wnt signalling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 4.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signalling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 5.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 6.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 8.Liu T, DeCostanzo AJ, Liu X, Wang Hy, Hallagan S, Moon RT, Malbon CC. G protein signalling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- 9.Liu T, Liu X, Wang H, Moon RT, Malbon CC. Activation of rat frizzled-1 promotes Wnt signalling and differentiation of mouse F9 teratocarcinoma cells via pathways that require Galpha(q) and Galpha(o) function. J Biol Chem. 1999;274:33539–33544. doi: 10.1074/jbc.274.47.33539. [DOI] [PubMed] [Google Scholar]
- 10.Bikkavilli RK, Feigin ME, Malbon CC. Galphao mediates WNT-JNK signalling through dishevelled 1 and 3, RhoA family members, and MEKK 1 and 4 in mammalian cells. J Cell Sci. 2008;121:234–245. doi: 10.1242/jcs.021964. [DOI] [PubMed] [Google Scholar]
- 11.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signalling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–571. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Wang HY. Mitogen-activated protein kinase p38 regulates the Wnt/cyclic GMP/Ca2+ non-canonical pathway. J Biol Chem. 2007;282:28980–28990. doi: 10.1074/jbc.M702840200. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Wang HY. Suppression of cyclic GMP-dependent protein kinase is essential to the Wnt/cGMP/Ca2+ pathway. J Biol Chem. 2006;281:30990–31001. doi: 10.1074/jbc.M603603200. [DOI] [PubMed] [Google Scholar]
- 15.Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 16.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 17.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 18.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 19.Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 20.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signalling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 21.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/betacatenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 22.Martin-Blanco E. p38 MAPK signalling cascades: ancient roles and new functions. BioEssays. 2000;22:637–645. doi: 10.1002/1521-1878(200007)22:7<637::AID-BIES6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Bikkavilli RK, Feigin ME, Malbon CC. p38 mitogen-activated protein kinase regulates canonical Wnt-{beta}-catenin signalling by inactivation of GSK3{beta} J Cell Sci. 2008;121:3598–3607. doi: 10.1242/jcs.032854. [DOI] [PubMed] [Google Scholar]
- 24.Chang J, Sonoyama W, Wang Z, Jin Q, Zhang C, Krebsbach PH, Giannobile W, Shi S, Wang CY. Noncanonical Wnt-4 signalling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–30948. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- 25.Caverzasio J, Manen D. Essential role of Wnt3a-mediated activation of mitogen-activated protein kinase p38 for the stimulation of alkaline phosphatase activity and matrix mineralization in C3H10T1/2 mesenchymal cells. Endocrinology. 2007;148:5323–5330. doi: 10.1210/en.2007-0520. [DOI] [PubMed] [Google Scholar]
- 26.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi CS, Huang NN, Harrison K, Han SB, Kehrl JH. The mitogen-activated protein kinase kinase kinase kinase GCKR positively regulates canonical and noncanonical Wnt signalling in B lymphocytes. Mol Cell Biol. 2006;26:6511–6521. doi: 10.1128/MCB.00209-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, beta-catenin and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J Cell Biol. 2008;180:1087–1100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- 30.Rivat C, Le Floch N, Sabbah M, Teyrol I, Redeuilh G, Bruyneel E, Mareel M, Matrisian LM, Crawford HC, Gespach C, Attoub S. Synergistic cooperation between the AP-1 and LEF-1 transcription factors in activation of the matrilysin promoter by the src oncogene: implications in cellular invasion. FASEB J. 2003;17:1721–1723. doi: 10.1096/fj.03-0132fje. [DOI] [PubMed] [Google Scholar]
- 31.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 32.Kim SE, Choi KY. EGF receptor is involved in WNT3a-mediated proliferation and motility of NIH3T3 cells via ERK pathway activation. Cell Signal. 2007;19:1554–1564. doi: 10.1016/j.cellsig.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY. Both ERK and Wnt/beta-catenin pathways are involved in Wnt3a-induced proliferation. J Cell Sci. 2005;118:313–322. doi: 10.1242/jcs.01601. [DOI] [PubMed] [Google Scholar]
- 34.Lee YN, Gao Y, Wang HY. Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, -2 and -3. Cell Signal. 2008;20:443–452. doi: 10.1016/j.cellsig.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, Chen P, Wynshaw-Boris A. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea and neural tube development. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]