Abstract
Comparative molecular, developmental and morphogenetic analyses show that the three major segmented animal groups—Lophotrochozoa, Ecdysozoa and Vertebrata—use a wide range of ontogenetic pathways to establish metameric body organization. Even in the life history of a single specimen, different mechanisms may act on the level of gene expression, cell proliferation, tissue differentiation and organ system formation in individual segments. Accordingly, in some polychaete annelids the first three pairs of segmental peripheral neurons arise synchronously, while the metameric commissures of the ventral nervous system form in anterior-posterior progression. Contrary to traditional belief, loss of segmentation may have occurred more often than commonly assumed, as exemplified in the sipunculans, which show remnants of segmentation in larval stages but are unsegmented as adults. The developmental plasticity and potential evolutionary lability of segmentation nourishes the controversy of a segmented bilaterian ancestor versus multiple independent evolution of segmentation in respective metazoan lineages.
Key words: Sipuncula, evolution, segmentation, seriality, annelid, Echiura, nervous system, development, phylogeny, bodyplan
Ontogeny and Functional Implications of Segmentation
The evolution of a segmented bodyplan is often considered a crucial metazoan innovation because it allows the subdivision and specialization of individual body regions along the anterior-posterior axis of an animal.1,2 This partitioning typically involves both the ectodermal and the endodermal germ layers and often results in metameric ectodermal appendages (parapodia) and segmentally arranged, paired mesodermal body cavities (coelomic sacs). Traditionally, a condition where all segments have the same type of parapodia and house identical sets of internal organs such as ganglia, nephridia, gonads and muscles has been regarded as basal for annelids and arthropods (homonomic segmentation).1,2 From this basal (plesiomorphic) condition, concentration of individual organ systems into segments of specific body regions combined with reductions of organs in other segments are thought to have occurred multiple times within various lineages, and eventually led to morphologically distinct segments along the anterior-posterior body axis (heteronomic segmentation).1,2
While often considered an important hint towards ancestral segmentation of a species, serial repetition of organs along the anterior-posterior axis alone is not decisive for a segmental evolutionary history (cf., e.g., the multiple ring muscles in the non-segmented platyhelminths). On the cellular and organ system level, segmentation can only be proven with the aid of developmental studies, because segmented animals typically exhibit a posterior growth zone from which all segments are progressively budded off.3–7 Accordingly, ontogenetically older segments—and thus also the organs associated with them—are found anterior to the younger segments, a fact that is illustrated by the gradual decrease of the degree of organ system differentiation from anterior to posterior (Fig. 1).8 This makes the pattern of organogenesis an ideal marker to test for the segmental ancestry of worm-shaped lophotrochozoan taxa.8–12
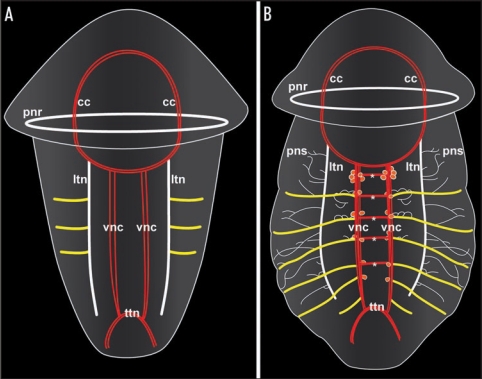
Figure 1.
Schematic representation of neurogenesis in the polychaete annelid Sabellaria alveolata based on serotonin immunoreactivity, revealing differences in the mode of establishment of metamery in the peripheral segmental neurons and the ventral commissures, respectively. Both aspects are ventral views with anterior facing upwards. Total length of the specimens is approximately 280 µm in (A) and 330 µm in (B). (A) Late larva with synchronously established peripheral segmental neurons (yellow). Ventral commissures and perikarya along the paired ventral nerve cord (vnc) are still lacking. The prototroch nerve ring (pnr) and the nerve ring underlying the telotroch (ttn) constitute subsets of the larval nervous system, while the circumoesophageal commissures (cc) and the longitudinal trunk neurons (ltn) are parts of the adult neural bodyplan. (B) Larva prior to metamorphosis. The ventral commissures (asterisks) of the first five segments have been established progressively, together with the paired, metameric sets of perikarya (red dots) along the ventral nerve cords (vnc). The six pairs of peripheral segmental neurons (yellow) correspond to the segments II–VII, because development of segment I is retarded in this species, resulting in development of the paired peripheral segmental neuron of this segment at a later stage. Note that ontogeny of the peripheral segmental neurons precedes development of the ventral commissures in segments VI and VII. pns - the nerves of the peripheral nervous system.
Coelomic compartmentalization of a cylindrical body has frequently been proposed to be of selective advantage due to the fact that these animals are able to regulate the hemolymphic pressure in each compartment (segment) individually. The interplay of coelomic pressure and the contractile ring and longitudinal muscles of the body wall enable direct and independent control over the diameter of the body in each individual segment, thus allowing for a diversity of complex movement patterns.2 However, while coelomic segmentation has often (but not always) been retained in large, burrowing annelids (e.g., earthworms), secondary loss is often observed in non-benthic free-living (e.g., leeches), interstitial (e.g., Protodrilus), or sessile forms (e.g., tube worms).
Loss of Segmentation
Despite the loss of segmentation in various annelid taxa, ontogenetic remnants of their segmented ancestry are present in a number of annelids that do not show obvious segmental features in the adult body (e.g., leeches).3,4 Recent developmental studies have shown that this holds also true for representatives of the Sipuncula (peanut worms), unsegmented lophotrochozoans that are regarded either as derived ingroup annelids or as a direct annelid sister clade.13–17 Interestingly, however, segmental traits in the sipunculans are restricted to the nervous system but have been completely lost on the level of coelom organisation.18 Accordingly, larvae of Phascolosoma agassizii develop four pairs of perikarya that are associated with the paired ventral nerve cord and express the common neurotransmitter serotonin (Fig. 2A). These paired perikarya form, together with commissures that interconnect the ventral nerve cords, in a typical annelid-like anterior-posterior progression, thus demonstrating the segmental ancestry of Sipuncula. During subsequent larval development, the ventral nerve cords fuse and the paired perikarya migrate towards each other, resulting in two cell clusters of five cells each, which are grouped around the now single ventral nerve cord (Fig. 2B). This results in loss of the metameric neural pattern and eventually in the establishment of the non-segmented ventral nervous system with only one single nerve cord.18
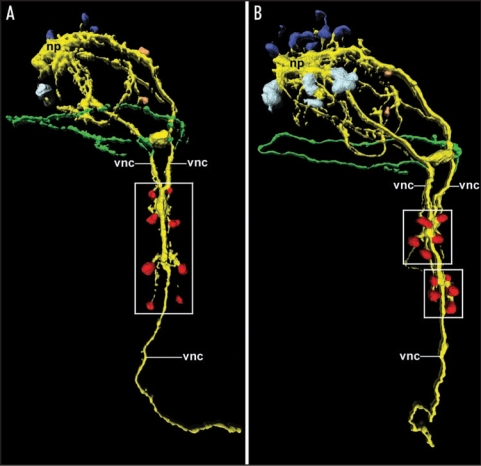
Figure 2.
Expression and loss of the segmental pattern of the ventral CNS in the sipunculan Phascolosoma agassizii, as depicted by 3D reconstruction of serotonin immunoreactivity. Both aspects are ventral views with anterior facing upwards. Total length of the specimens is approximately 150 µm. (A) Late larva with four pairs of metameric perikarya (red; boxed area) associated with the ventral nerve cord (vnc). The latter is already fused along the entire anterior-posterior axis except in the anterior-most region. Additional neural elements include the cells of the larval apical organ (dark blue) overlying the neuropil mass (np) of the adult brain, the first cell bodies of the developing adult brain (light blue), two cells of the peripheral nervous system (orange), and the larval prototroch nerve ring (green). (B) Larva prior to metamorphosis in which the metameric arrangement of the ventral perikarya (red) has been lost in favor of two cell clusters (boxed areas) comprising five cells each. Note the increased number of cells belonging to the adult brain (light blue).
Noticeably, within Sipuncula the degree of preservation of neural segmentation appears to be dependent on the duration of the larval phase and is thus directly correlated with the basal versus the derived mode of sipunculan development. The segmented nervous system in Phascolosoma is expressed in the so-called pelagosphera larva. This larval stage is a defining (apomorphic) character for Sipuncula and is thus considered part of the ancestral sipunculan life cycle.19 Neurogenesis in another sipunculan species that lacks the pelagosphera stage, Phascolion strombi, shows that the remnants of the metameric nervous system have been reduced even further. As such, while also having a primarily paired ventral nerve cord, this species lacks the associated perikarya and only has retained three transitional ventral commissures as the sole remnants of the ancestral segmented neural bodyplan.20
Cryptic segmentation in taxa with close annelid affinities seems to be more common than previously assumed. The echiurans (spoon worms), now considered as clustering within Annelida,13,16,17 do not show any segmental traits in their adult gross morphology. However, neurogenesis revealed the same ontogenetic mechanisms as found in annelids and sipunculans, namely that paired sets of perikarya are formed in a discrete anterior-posterior progression.9–11 In contrast to the sipunculans and similar to the condition found in “typical” annelids, this metameric organization of the nervous system persists in the adult echiurans. Accordingly, the annelid-echiuran-sipunculan lineage shows a gradual decrease of preservation of nervous system segmentation, a notion that is further supported by patterns of myogenesis. Hereby, annelids exhibit the typical anterior-posterior progression of ring and dorsoventral muscle formation, while sipunculan myogenesis starts with synchronous formation of early ring muscle rudiments, followed by the emergence of additional ring muscles along the entire anterior-posterior axis by fission from already existing myocytes.8,20 Accordingly, Sipuncula represents a developmental mosaic of segmental and non-segmental bodyplan patterning mechanisms, whereby the ectoderm-derived nervous system has to some degree maintained its segmental ancestry while the mesodermal musculature is formed entirely non-metamerically.
Plasticity of Segmentation
Despite the long standing definitions concerning the characteristics of a segmented bodyplan (see above), recent data have shown that the ontogenetic establishment of annelid segmentation may follow quite different developmental pathways. Traditionally, it had been proposed that the first three (larval) segments form more or less synchronously by schizocoely from the paired lateral mesodermal band, while the following (adult) segments develop from a pre-anal growth zone.21 Accordingly, one would assume that the organ systems associated with the first three segments also arise synchronously, while only the subsequent segmental organs follow the anterior-posterior differentiation gradient. However, this is only partly true for the polychaete Sabellaria alveolata. While the three larval segments indeed arise synchronously in this species, only the corresponding pairs of peripheral segmental neurons form synchronously, while the ventral commissures develop subsequently one after another (Fig. 1).22 While this may be interpreted as (secondary) chronological dissociation of larval segment formation and the development of the ventral commissures, it could alternatively be explained as the result of a heterochronic shift of the first three segments, which were originally derived from a posterior growth zone, into the larval stage of the animal. This notion is supported by reports that describe an—albeit rapid—progressive formation of these first three pairs of coelomic sacs in several polychaete taxa.23 Whatever alternative holds true, this example demonstrates that the developmental mechanisms that underlie annelid segmentation are much more complex than previously assumed. This is confirmed by recent cell proliferation pattern analyses, which suggest that the location of the growth zone might have shifted from a posterior-median position to both lateral sides in some species, indicating positional variability of the annelid growth zone.24 However, despite the high morphogenetic plasticity of segmentation, some molecular mechanisms appear similar even between distant phylogenetic entities such as arthropods and vertebrates.25,26
Ancestry of Segmentation
Comparative analyses of the developmental mechanisms that form metameric organs in segmented lophotrochozoan worms demonstrate a high plasticity of ontogenetic patterns that lead to a segmented bodyplan and show that segmentation may be lost during evolution. This raises the question as to what extent such an evolutionary loss has yet remained unrecognized in other phyla, thus reviving the discussion about a possible segmented ancestor of Lophotrochozoa and Bilateria as a whole. Such a scenario has been repeatedly proposed by the advocates of a conserved molecular pathway that is thought to underlie the ontogeny of metazoan segmentation.7,26 However, despite some similarities on the molecular level, there are also significant differences in the way typical “segmentation genes” are expressed, and cellular and tissue differentiation pathways that eventually give rise to individual segments vary between lophotrochozoans, vertebrates and ecdysozoans.25 To complicate matters further, segment formation is highly variable even between phyla within the respective “superclades” Ecdysozoa and Annelida.25 Moreover, morphologically similar segments may follow different ontogenetic pathways even within the same individual, and distinct metamerically arranged subunits of the nervous system may form differently in individual segments of the same animal (e.g., the ventral commissures versus the peripheral segmental neurons in polychaetes; see above). Lastly, a mosaic of segmental and non-segmental modes of organogenesis may occur within an individual, indicating the occurrence of dissociation of organogenesis from the segmentation process in some species (e.g., muscle formation in sipunculans; see above).
Given the incongruencies and the plasticity of the processes involved in the ontogeny of segmentation in the various major bilaterian subgroups, no final statement as to whether or not Urbilateria was segmented can yet be made. In any case, assuming a segmented urbilaterian would imply a wide range of evolutionary modifications of the ancestral segmentation pathway on the molecular, cellular and morphogenetic level, as well as secondary loss of a segmented body in a number of lineages. Both, experimental developmental genetics employing RNAi experiments and comparative morphogenetic analyses provide exciting tools that enable us to directly test for evolutionary hypotheses concerning shared molecular segmentation pathways on the one hand and for cryptic remnants of a segmented bodyplan in seemingly non-segmented recent phyla on the other. This should eventually lead to a sound reconstruction of the ancestry of one of the key innovations of metazoan evolution: the origin of segmented bodies.
Acknowledgements
Research in the lab of Andreas Wanninger is funded by the EU Early Stage Research Training Network MOLMORPH under the 6th Framework Programme (contract number MEST-CT-2005—020542). Both Alen Kristof and Nora Brinkmann are recipients of a fellowship within the MOLMORPH programme.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7505
References
- 1.Brusca RC, Brusca GJ. Invertebrates. Sunderland: Sinauer Associates; 2003. [Google Scholar]
- 2.Ruppert EE, Fox RS, Barnes RD. Invertebrate Zoology. Belmont: Thomson, Brooks/Cole; 2004. [Google Scholar]
- 3.Weisblat DA, Price DJ, Wedeen CJ. Segmentation in leech development. Development. 1988;104:161–168. doi: 10.1242/dev.104.Supplement.161. [DOI] [PubMed] [Google Scholar]
- 4.Shankland M. Leech segmentation: cell lineage and the formation of complex body patterns. Dev Biol. 1991;144:221–231. doi: 10.1016/0012-1606(91)90416-z. [DOI] [PubMed] [Google Scholar]
- 5.Davis GK, Patel NH. The origin and evolution of segmentation. Trends Biochem Sci. 1999;12:68–72. [PubMed] [Google Scholar]
- 6.Nielsen C. Animal Evolution. Interrelationships of the Living Phyla. Oxford: Oxford University Press; 2001. [Google Scholar]
- 7.De Rosa R, Prud'homme B, Balavoine G. Caudal and even-skipped in the annelid Platynereis dumerilii and the ancestry of posterior growth. Evol Dev. 2005;7:574–587. doi: 10.1111/j.1525-142X.2005.05061.x. [DOI] [PubMed] [Google Scholar]
- 8.Wanninger A. Shaping the things to come: Ontogeny of lophotrochozoan neuromuscular systems and the Tetraneuralia concept. Biol Bull. 2009 doi: 10.1086/BBLv216n3p293. In press. [DOI] [PubMed] [Google Scholar]
- 9.Hessling R. Metameric organization of the nervous system in developmental stages of Urechis caupo (Echiura) and its phylogenetic implications. Zoomorphology. 2002;121:221–234. [Google Scholar]
- 10.Hessling R. Novel aspects of the nervous system of Bonellia viridis (Echiura) revealed by the combination of immunohistochemistry, confocal laser-scanning microscopy and three-dimensional reconstruction. Hydrobiologia. 2003;496:225–239. [Google Scholar]
- 11.Hessling R, Westheide W. Are Echiura derived from a segmented ancestor? Immunohistochemical analysis of the nervous system in developmental stages of Bonellia viridis. J Morphol. 2002;252:100–113. doi: 10.1002/jmor.1093. [DOI] [PubMed] [Google Scholar]
- 12.Wanninger A, Haszprunar G. Chiton myogenesis: Perspectives for the development and evolution of larval and adult muscle systems in molluscs. J Morphol. 2002;251:103–113. doi: 10.1002/jmor.1077. [DOI] [PubMed] [Google Scholar]
- 13.McHugh D. Molecular evidence that echiurans and pogonophorans are derived annelids. Proc Natl Acad Sci USA. 1997;94:8006–8009. doi: 10.1073/pnas.94.15.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boore JL, Staton JL. The mitochondrial genome of the sipunculid Phascolopsis gouldii supports its association with Annelida rather than Mollusca. Mol Biol Evol. 2002;19:127–137. doi: 10.1093/oxfordjournals.molbev.a004065. [DOI] [PubMed] [Google Scholar]
- 15.Halanych KM, Dahlgren TG, McHugh D. Unsegmented annelids? Possible origins of four lophotrochozoan worm taxa. Int Comp Biol. 2002;42:678–684. doi: 10.1093/icb/42.3.678. [DOI] [PubMed] [Google Scholar]
- 16.Struck TH, Schult N, Kusen T, Hickman E, Bleidorn C, McHugh D, Halanych KM. Annelid phylogeny and the status of Sipuncula and Echiura. BMC Evol Biol. 2007;7:57. doi: 10.1186/1471-2148-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–749. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- 18.Kristof A, Wollesen T, Wanninger A. Segmental mode of neural patterning in Sipuncula. Curr Biol. 2008;18:1129–1132. doi: 10.1016/j.cub.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 19.Jaeckle WB, Rice ME. In: Atlas of Marine Invertebrate Larvae. Young CM, editor. San Diego: Academic Press; 2002. pp. 375–396. [Google Scholar]
- 20.Wanninger A, Koop D, Bromham L, Noonan E, Degnan BM. Nervous and muscle system development in Phascolion strombus (Sipuncula) Dev Genes Evol. 2005;215:509–518. doi: 10.1007/s00427-005-0012-0. [DOI] [PubMed] [Google Scholar]
- 21.Iwanoff PP. Die Entwicklung der Larvalsegmente bei den Anneliden. Z Morph Ökol Tiere. 1928;10:161–162. (Ger). [Google Scholar]
- 22.Brinkmann N, Wanninger A. Larval neurogenesis in Sabellaria alveolata reveals plasticity in polychaete neural patterning. Evol Dev. 2008;10:606–618. doi: 10.1111/j.1525-142X.2008.00275.x. [DOI] [PubMed] [Google Scholar]
- 23.Anderson DT. Embryology and phylogeny in annelids and arthropods. Oxford: Pergamon Press; 1973. [Google Scholar]
- 24.Seaver EC, Thamm K, Hill SD. Growth patterns during segmentation in the two polychaete annelids, Capitella sp. I and Hydroides elegans: comparisons at distinct life history stages. Evol Dev. 2005;7:312–326. doi: 10.1111/j.1525-142X.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- 25.Blair SS. Segmentation in animals. Curr Biol. 2008;18:991–995. doi: 10.1016/j.cub.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 26.De Robertis EM. The molecular ancestry of segmentation mechanisms. Proc Natl Acad Sci USA. 2008;105:16411–16412. doi: 10.1073/pnas.0808774105. [DOI] [PMC free article] [PubMed] [Google Scholar]