Abstract
Health care information technology can be a means to improve quality and efficiency in the primary care setting. However, merely applying technology without addressing how it fits into provider workflow and existing systems is unlikely to achieve improvement goals. Improving quality of primary care, such as cancer screening rates, requires addressing barriers at system, provider, and patient levels. The authors report the development, implementation, and preliminary use of a new breast cancer screening outreach program in a large multicenter primary care network. This installation paired population-based surveillance with customized information delivery based on a validated model linking patients to providers and practices. In the first six months, 86% of physicians and all case managers voluntarily participated in the program. Providers intervened in 83% of the mammogram-overdue population by initiating mailed reminders or deferring contact. Overall, 63% of patients were successfully contacted. Systematic population-based efforts are promising tools to improve preventative care.
Introduction
Despite considerable resources devoted to cancer prevention in the United States, major deficiencies in the quality of care persist. Because primary care physicians (PCPs) are the major point of entry for most preventive and chronic illness care, efforts to measure and improve quality of care have often focused on these physicians. The application of health care information technology (IT) in the primary care setting has been offered as a potential solution. Yet, widespread adoption of information systems in the U.S. remains a challenge, and evidence to date supports only modest improvements in the quality of care and not the transformational change promised. However, health care IT tools alone are unlikely to result in the dramatic improvements in the efficiency and quality of care hypothesized. To be successful, efforts to improve care delivery require tailoring IT implementation to address barriers at all levels: system, individual provider, and patient. In their most basic form, primary care IT interventions should help correctly identify all eligible and at-risk patients in a health care system and direct pertinent clinical information to the responsible provider in an easily actionable way.
As an application of IT, we present an enterprise-wide breast cancer screening outreach and intervention program, called Mammography FastTrack, that pairs population-based surveillance with targeted information delivery to the appropriate care provider (physician or practice case manager) based on a validated model that accurately links patients to providers and practices. Here we report on the strategies we used to achieve our aim of increasing mammography screening reminders in a heterogeneous, multi-clinic primary care network. The main design principles used in our system are broadly applicable to others interested in developing population-based primary care screening and disease management initiatives.
Background
Breast cancer is the second leading cause of cancer death in women and is the most common cancer among women worldwide. 1 Mammography has been shown to be an effective breast cancer screening test 2,3 that provides an estimated 30% reduction in breast cancer mortality in women ages 50 to 69 years old. 4,5 Expert panels including the National Cancer Institute (2002), U.S. Preventive Services Task Force (2002), and the American Cancer Society (2003) have recommended routine annual or biennial screening mammography for all women age 40 and older. Despite these recommendations, studies show that 40% to 90% of eligible women report not having a mammogram in the preceding year. 6-8 Failure to diagnose breast cancer at a time when cure is possible is one of the most common reasons for malpractice claims against physicians. 9
For patients regularly seen within a health care system, suboptimal mammography screening rates represent a system-level failure in care. Although patient factors such as financial/insurance barriers and lack of education contribute to lower screening rates, a key factor may be lack of physician recommendation. 10 In one study, only 66% of eligible women received a screening recommendation from their physician. 11 Published evidence suggests patients were more likely to follow up with mammography screening when testing was ordered by their PCP. 12,13 This finding supports including PCPs, if possible, in any systematic approach to improving breast cancer screening. However, for patients cared for within a health care system but without close linkage to a specific PCP or otherwise lacking PCP contact, delegating the responsibility to a case manager (CM) may be a practical alternative.
Design Objectives
Our primary goal was to initiate a program to systematically identify and contact patients overdue for mammography screening within the Massachusetts General Primary Care Network (MGPCN). This heterogeneous network provides primary care services for over 150,000 patients and includes approximately 180 PCPs working in 14 clinically and demographically diverse practices (4 community health centers and 10 hospital-affiliated practices) with varied practice styles. All practices use electronic medical records (3 distinct systems), and have the same electronic billing and scheduling systems. Yet to date, there has been no network-level effort to identify or reach out to patients overdue for mammography screening. Prior to our initiative, practices relied solely on ad-hoc methods by PCPs at the point of care to identify patients as mammogram-overdue. Thus, no reminders were systematically sent to patients overdue for mammography screening prior to initiation of our outreach effort. Additionally because patient identification was usually at the point of care, despite these ad-hoc methods, patients without clinic appointments remained at risk. We present the following key design points to achieve our objectives.
-
1 Maintain Accurate and Up-to-Date Identification of the Population at Risk
The application of electronic registries derived from clinical systems has enabled a population management approach to quality improvement that facilitates identification of patients for specific interventions.14,15 Yet, to be trusted by care providers, these population-level registries must be both highly sensitive and specific with respect to identification of the at-risk population. Data must be kept up to date and be contextually complete. Because patients may be seen in different health care systems, the information from the primary institution's electronic medical record (EMR) may need to be augmented with other data, such as billing and insurance claims data.
To properly identify the at-risk population, a registry may need to be populated with aggregated data from multiple disparate sources from within and outside the clinical system. Additionally, processes must exist to ensure that data in this registry are regularly refreshed to maintain accuracy and usefulness.
Although there are different approaches to achieve this objective, we developed a service-oriented architecture that includes: (1) a population-at-risk registry with aggregated and contextually complete clinical and billing data, (2) a process to periodically update the registry to account for the dynamic nature of population management in the setting of day-to-day care, and (3) a service-oriented data access layer/wrapper. Wrapping a registry in a service-oriented abstraction layer facilitates data access and scaling to multiple clients. Additionally, a service-oriented architecture may result in lower implementation barriers for future applications, simplify code maintenance as quality metrics change, and provide more facile code reuse.
-
2 Ensure Reliable Patient–Provider Linkage for Accurate Information Delivery
Systematic screening and disease management (DM) initiatives such as efforts to increase breast cancer screening may be more successful if they can take advantage of an established patient–provider relationship. With regard to mammography screening, patients are more likely to respond positively to a recommendation directly from their physician than from a general letter or phone call.16,17 Because patients may see multiple providers in a health care system over time,18 it can be difficult to accurately link patients with the single provider who will consider her/him “my patient.”19,20 Simply using a patient's listed PCP from registration information or an insurer's roster may not be sufficient if the patient does not routinely see that physician. The PCPs receiving such lists as part of a quality-improvement initiative may be rightly frustrated if they do not recognize a sizable number of individuals for whom they are assigned responsibility and expected to intervene.
Our initiative to improve mammography screening was designed to use information about the linkage between a patient and her designated PCP (or lack thereof). Specifically, patients who could not be linked with a single PCP were linked to the primary care practice where they received most of their care, thereby maximizing the potential impact of the intervention while still being accepted for use by network PCPs.
-
3 Enable Visit-independent Population Surveillance
Primary care is in crisis due to an inadequate supply of physicians, increased demand for preventive and chronic care services, and insufficient reimbursement.21 This has resulted in tremendous time pressure during office visits that challenge quality improvement initiatives that compete with the issues patients bring to the visit. The resulting prioritization and tradeoff with other tasks make clinicians feel that they have inadequate visit time for activities such as addressing cancer prevention.22,23 Clinical reminders, traditionally real-time tools to support physicians at the point of care,24 have been used extensively to improve guideline compliance.25,26 Yet one reason they are underutilized is because of competing demands in the clinical setting.27,28 Evidence suggests that point-of-care reminders are associated with minimal improvement in outcomes such as mammography completion.29,30 Finally, point-of-care reminders are completely ineffective for patients without clinic appointments, especially to their PCP.
Increasingly, population management provides an alternative to the encounter-based care model by focusing on an entire patient cohort with a given condition. Population surveillance does not require an office visit with the patient's assigned provider, and may be a useful alternative for networks that provide care across large geographical catchments.31,32
Thus to support multiple practices with diverse organizational workflow and to target patients overdue for breast cancer screening regardless of visit frequency or continuity with a specific PCP, our system sought to implement a visit-independent, population-based solution.
-
4 Catalyze Change with Safe and Efficient “One-click” Mammography Ordering
A key shortcoming of many existing DM applications is that PCPs and practice CMs are provided potentially useful information without a simple mechanism to translate this information into action. Systems that provide information that is not directly actionable have been shown to have limited clinical impact.33 An ideal system should not only report clinical information to the “right” provider, as detailed above, but also catalyze change by providing all of the “raw materials” needed for clinical decision making with a simple means to take action.34 The user interface should be standalone (contextually complete) to minimize the amount of manual cross-referencing of data elements from disparate sources. Similarly, the interface should be actionable yet require the user to perform a minimum amount of work to initiate an entire chain of events to complete the DM or screening task.
-
5 Tailor the Application to Reduce Barriers to Care and Improve Workflow
Evidence suggests that the most effective DM interventions are population-based, multi-modal, and complement existing workflow by either circumventing system-level obstacles or augmenting existing processes of care.35-37 Focus groups and workflow analysis can help to identify barriers to care or other resource-consuming processes. In designing our system, we used both of these methods to identify a key requirement of providing a single web-based interface while preserving the heterogeneous workflow needs of each of our network's practices. We also leveraged our patient–provider linkage information to address barriers related to improper targeting of reminder information and provided centralized administrative support where deficiencies in resources were uniform across all practices (such as support for mailing materials to patients and medical record documentation).
-
6 Inform and Activate the Patient
Finally, our system needed to reach out to the at-risk (and overdue) patients with information designed to overcome knowledge barriers to successfully completing screening. Studies have shown that women with negative perceptions or inaccurate knowledge about mammography are less likely to participate,38,39 whereas women with positive perceptions of screening effectiveness are more likely to complete mammography testing.40,41 Ideally, patient correspondence should use the patient's primary language at an appropriate education level and address common barriers to breast cancer screening, such as not believing in the effectiveness or need for screening and the fear of adverse effects.42 Our system provided educational materials that included balanced, evidence-based information about mammography screening as well as simple, specific instructions to easily arrange for testing.
System Description
We developed the Mammography FastTrack (MFT) system using a series of workflow analyses, prototyping, and usability exercises in 2006 and 2007. We describe the system in terms of its clinical setting as well as the system architecture, including the population-at-risk registry, service-oriented data access and aggregation processes, and user interfaces.
The Physical Setting, Clinical Venue, and Patient–Provider Linkage
Mammography FastTrack was implemented for 6 of 14 primary care practices within the MGPCN. This network provided care to over 150,000 patients predominantly living in eastern Massachusetts, including nearly 1 million unique office visits between 2004 and 2006. While all practices use the same billing and scheduling systems and participate in network-level leadership and quality improvement activities, practices have a great deal of independence in terms of organization, workflow, and even the EMR used (three distinct EMRs). Some practices are organized in an independent provider model, whereas others use a team-based care delivery model in which patients may receive care from multiple providers without necessarily establishing a strong relationship with any single provider.
We designed our system to accommodate these varied practice styles by applying a previously validated algorithm to link patients with a specific PCP. 43,44 Patients who could not be linked with a specific PCP were linked to the primary care practice where they received most of their care. Variables used in this model included: PCP designee from hospital registration, PCP practice style (independent or team-based), months since last PCP visit, and in-state Massachusetts residence. Patients who were linked to a specific PCP were assigned to that PCPs population registry. Those not linked to a specific PCP but rather to a specific practice were assigned to that practice's population registry and managed by a practice CM.
Software and Architectural Design
Mammography FastTrack is a web-based application suite within an extensible, disease-agnostic framework designed to identify patients overdue for health care services based on specified criteria. This general architecture enables disease-specific cohorts to be presented as provider- or practice-linked populations with contextually specific “just-in-time” clinical decision support. 45 The MFT application is composed of: (1) a population registry, (2) data aggregation and update services, and (3) several user-specific front-end interfaces. These user interfaces, described later, include actionable screen elements to help catalyze the transformation of information to clinical activity.
Mammography FastTrack is hosted within one of MGH's EMR frameworks, known as Oncall. 46 Through web services, customized extensible markup language (XML), and practice-specific branding, Oncall supports the medical records of primary care and multiple specialty practices with several thousand users. Oncall also supports the MGPCN with a brand of Oncall for those providers that use an EMR other than Oncall.
Mammography FastTrack was primarily developed in Active Server Pages, a Microsoft's server-side script engine for dynamically generated web pages, using JavaScript 47 and using Microsoft Structured Query Language Server 48 as the system's database.
Population-at-Risk Registry, Nightly Data Processes, and Service-oriented Data Services
To identify the at-risk population within the MGPCN, we populated our registry with aggregated electronic data from a variety of sources including: (1) visit notes and health maintenance data from multiple EMRs used across the network, (2) prior mammogram results/testing dates, (3) radiology scheduling records via IDX, (4) patient demographic variables from the hospital registration system, and (5) payer billing and encounter claims. Granular data elements such as mammogram dates were used by our rules engine to populate the at-risk registry, and non-parsable (free text) data was used in point-of-care decision support.
Because much of the data in our disease registry are artifacts from usual practice and, by their very nature, are dynamic, we used a nightly process to maintain fresh data. For example, a patient who was initially up to date with screening when the system was activated only appeared in the at-risk registry if she became overdue. Patients who completed a mammogram were removed from the overdue registry for a 2-year screening interval (this time period was selected to work as part of a failsafe system). The nightly process also removed patients from the overdue registry who had pending (scheduled) mammograms. In these cases, once a scheduled mammogram appeared in the system, the patient was removed from the overdue roster until 1 week after the scheduled mammogram date, allowing time for the mammogram report to be finalized. At that point, the patient was again processed in the nightly batch job and either removed if there was a documented mammogram or added back to the overdue registry if screening was not completed or rescheduled.
To accommodate other computing tasks, the nightly processes to update the disease registry data, time-shared with other jobs by processing 10 patients at a time, running every minute, and running until completion. This nightly process also triggered once-weekly e-mails that alerted both central administrative and primary care office staff when new patients in need of contact appeared on their lists. Central administration used this e-mail to initiate mailings for all provider-approved, overdue patients, and designated office staff subsequently contacted patients by telephone for scheduling after a delay to permit for receipt of the mailed letter.
Although there was no immediate need to share our disease registry information with other enterprise applications, efforts were made to wrap our population-at-risk registry in a service-oriented data access layer potentially lowering implementation and maintenance barriers for future DM applications.
System Flow and User Interfaces
To support the mammography screening workflow, MFT used distinct user interfaces to accommodate each of 3 user classes: (1) providers (PCPs and CMs), (2) central administration, and (3) practice office staff (termed delegates for MFT). Based on the nightly updates, patients overdue for mammography screening initially appear on the Provider Mammography Review Interface, where providers can initiate screening outreach efforts. Patients who are selected for contact by providers transition through the system appearing on the central administrative interface where patient mailings are created, and later on the practice delegate interface where telephone contact is made. Each interface is described below.
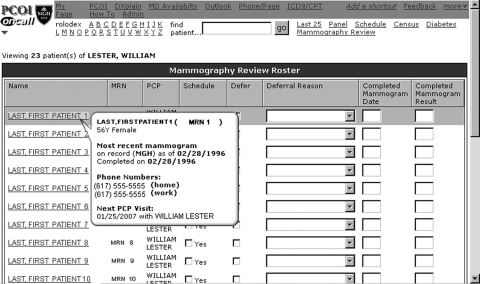
Provider Mammography Review Interface (▶)
Figure 1.
Provider mammography review interface.
The Provider Mammography Review Interface was used by providers to initiate mammography screening. Based on patient–provider linkage, PCPs used this page to screen patients linked to them, and CMs screened practice-linked patients. Patients appeared on this list if they: (1) had not completed a mammogram in the prior 2 years based on both billing and clinical data, and (2) were without any scheduled mammogram in the hospital's radiology scheduling system. Each provider's population management page included: (1) a list of eligible, overdue, and linked patients, (2) just-in-time clinical decision support, and (3) an actionable component to either initiate mammography screening or defer scheduling. Users could act on one or more patients in any given session depending on available time. Action of any type removed the patient from the provider queue for the remainder of the screening cycle.
Decision support was provided within a dynamic element via AJAX (asynchronous JavaScript and XML): by moving the cursor over a patient's name, a pop-up bubble appeared populated with: (1) patient age, gender, primary language, and contact information; (2) date of last completed screening mammogram on record; (3) date of next scheduled PCP visit; and (4) EMR health maintenance notes in particular non-parsable free text information, such as information on prior outside testing, prior refusals, or pending tests at outside facilities. If additional clinical data were desired, clicking on the patient's name hyperlink provided direct access to the patient's EMR in a separate window.
If appropriate, the user initiated the screening process by simply clicking on the Contact Patient check box, thereby queuing the patient for central administration to create and mail materials. To indicate that screening mammography was not needed or inappropriate for a particular patient, users clicked the Defer check box, thereby activating and highlighting a Deferral Reason drop down menu. Selecting a deferral reason was required, and choices included: (1) mammogram completed, (2) mammogram scheduled, (3) bilateral mastectomy, (4) patient deceased, (5) not eligible, (6) not my patient, (7) informed refusal, and (8) other, with a free-text response required. Providers could also enter data for completed mammograms via this interface. Action of any type removed the patient from the overdue registry for the remainder of the screening cycle.
Central Administration Interface
The project coordinator acted centrally to track all eligible patients and generate patient letters and educational materials. Patients identified for screening appeared on the central administration interface. Clicking the Export link created a Microsoft Excel spreadsheet used in conjunction with practice-specific Microsoft Word mail merge templates to generate customized mailed materials. Personalized letters from the patient's PCP (for PCP-linked women) or from the practice's medical director (for practice-linked women) informed patients of the value of mammography screening and included detailed instructions for patients to directly schedule mammograms with the hospital's radiology department.
Mailed materials also included a central mammography reporting telephone number where patients could confidentially leave information about outside mammogram results or other pertinent information. Information about outside mammograms left on the Mammography Line was entered into the patient's EMR by a central administrative nurse as “patient reported data,” thereby removing these patients from the overdue registry. This centralized administration function facilitated mammography tracking and documentation with minimal practice effort and personnel while still allowing practices to use customized versions of the patient letter to facilitate provider/practice participation.
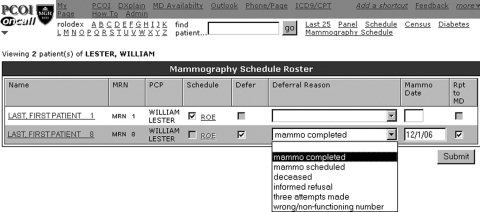
Practice Delegate Interface (▶)
Figure 2.
Practice delegate interface.
Each PCP and CM was linked with a member of the practice's office staff who was responsible for contacting the selected overdue patients. These delegates used a different version of the population management page designed to be used while speaking directly with patients by telephone. Overdue patients without pending mammograms appeared on their designated delegate's population management page 1 week after patient letters were mailed to accommodate for transit time of postal materials. If patients scheduled a mammogram independently as instructed by mailed patient materials, they were automatically removed from the overdue registry and therefore did not appear on the delegate's interface.
The interface supported screening workflow in that delegates used this interface to: (1) schedule mammogram appointments directly via the hospital's web-based radiology order entry system, (2) provide documentation for completed tests or informed patient refusals, and (3) documented when multiple telephone calls were made without patient contact. Activity of any kind removed patient from the registry for the remainder of the DM cycle.
Delegates were provided Health Insurance Portability and Accountability Act (HIPAA) compliant scripts and a list of frequently asked questions (with answers) when speaking with patients by telephone or leaving messages.
Status Report
We created the MFT system after performing detailed workflow analyses of our network's mammography screening processes followed by a series of design, prototyping, and usability exercises over the course of a 1-year period starting in 2006. Prior to implementation, there was no network-level effort to contact patients overdue for mammography screening. Practices relied solely on ad-hoc methods by PCPs to identify patients as mammogram-overdue. Our group regularly met with PCP representatives from each of the installation sites at network-level leadership meetings and interviewed potential users from each of our user groups (PCPs, CMs, and delegates). We also provided marketing and training sessions for each of our installation practices. We established as a measure of implementation success an a priori goal of at least 75% participation by physicians and case managers.
Mammography FastTrack was implemented for continuing use in six practices within our primary care network, with a total of 64 PCP users and 6 CMs acting on behalf of 3,054 overdue patients. The mean age of PCPs was 47.4 years (median 46.3), 48.4% were women, and on average they had graduated from medical school 19 years ago (median 18 years) and had been at MGH for 14 years (median 11 years). Patients eligible for mammography screening had a mean age of 54, were predominantly non-Hispanic white, and had health insurance (▶). Roughly half of the at-risk population was linked to a particular PCP (n = 1,689), whereas the remaining patients were practice-linked (n = 1,365).
Table 1.
Table 1 Characteristics of Patients in the Installation Practices
| Patient Characteristics, N (%) | Eligible N = 3,054 |
|---|---|
| Age, mean (SD) | 53.7 (7.9) |
| Median | 52.7 |
| Ethnicity | |
| Non-Hispanic white | 2,395 (78%) |
| Hispanic | 143 (5%) |
| African American | 211 (7%) |
| Asian | 170 (6%) |
| Other /unknown | 135 (4%) |
| Primary language spoken, English | 2,820 (92%) |
| Insurance status | |
| Commercial health insurance | 2,057 (67%) |
| Government insurance | 748 (24%) |
| Medicare | 393 (13%) |
| With secondary Medicaid | 155 (5%) |
| Medicaid | 355 (12%) |
| No insurance, self-pay | 249 (8%) |
| Months since last practice visit, mean (SD) | 9.4 (8.1) |
| Number of practice visits in past 3 years, mean (SD) | 6.2 (6.1) |
| Patient-physician linkage status | |
| MD-linked | 1,689 (55%) |
| Practice-linked | 1,365 (45%) |
| Practice type, community health center | 982 (32%) |
SD = standard deviation.
Utilization of Mammography FastTrack over First 6 Months
In the 6 months after deployment, 55 of 64 PCPs (86%) in participating practices used MFT to screen at least a portion of their patient population (▶). Usage by PCPs varied among practices and ranged from 75% to 100%. The CMs at all of the practices used MFT to screen patients.
Table 2.
Table 2 Mammography FastTrack Physician Usage by Practice
| Practice | Total PCPs in Practice | PCPs with Use | % PCPs with Use |
|---|---|---|---|
| A | 5 | 5 | 100 |
| B | 15 | 14 | 93 |
| C | 8 | 7 | 88 |
| D | 7 | 6 | 86 |
| E | 13 | 11 | 85 |
| F | 16 | 12 | 75 |
| Total | 64 | 55 | 86 |
PCP = primary care physician.
Provider Usage
Among the 3,054 patients in the at-risk population, PCPs and practice CMs screened 2,534 patients (83%). Overall, they chose to send letters to 2,167 patients, or 71% of the at-risk population using MFT, and deferred 367 patients (12%). Practice-linked patients screened by CMs were more likely to be sent a letter, whereas provider-linked patients screened by PCPs were more likely to be deferred. The PCPs screened 1,228 of 1,689 (72.7%) patients, including choosing to send letters to 957 (56.7%) patients and deferring 271 (16%) patients. The CMs screened 1,306 of 1,365 (95.7%) patients, including choosing to send letters to 1,210 (89%) patients and deferring 96 (7%) patients (▶).
Table 3.
Table 3 Provider Utilization for Installation Practices
| Provider Utilization, N (%) | All Patients N = 3,054 | PCP Managed Patients N = 1,689 | Case Manager Managed Patients N = 1,365 |
|---|---|---|---|
| Any action taken | 2,534 (83.0) | 1,228 (72.7) | 1,306 (95.7) |
| Contact patient by letter | 2,167 (71.0) | 957 (56.7) | 1,210 (88.6) |
| Returned/unmailed letters | 167 (7.7) | 22 (2.3) | 145 (12.0) |
| Defer patient contact | 367 (12.0) | 271 (16.0) | 96 (7.0) |
| Mammogram complete | 180 (49.0) | 111 (41.0) | 69 (71.9) |
| Mammogram scheduled | 17 (4.6) | 15 (5.5) | 2 (2.1) |
| Patient deceased | 6 (1.6) | 6 (2.2) | 0 (0.0) |
| Informed refusal | 77 (21.0) | 57 (21.0) | 20 (20.8) |
| Not eligible | 9 (2.4) | 8 (3.0) | 1 (1.0) |
| Not my patient | 28 (7.6) | 27 (10.0) | 1 (1.0) |
| Prior bilateral mastectomy | 30 (8.2) | 27 (10.0) | 3 (3.1) |
| Other | 20 (5.4) | 20 (7.4) | 0 (0.0) |
| No action taken | 520 (17.0) | 461 (27.3) | 59 (4.3) |
PCP = primary care physician.
Among patients deferred by a PCP or CM (n = 367, 12.0%), most (n = 271, 73.8%) were deferred by PCPs rather than CMs, with completed mammograms (n = 180, 49%) and prior informed refusals (n = 77, 21%) being the most common deferral reasons. Patient deferrals due to exclusion criteria (not eligible, not my patient, and prior bilateral mastectomy) were uncommon (n = 67) and were almost all identified by PCPs (n = 62). Criteria linking patients to the correct PCP were highly accurate, with only 27 patients (2.2%) among those reviewed by the PCP deferred because she was “not my patient.”
Central Administration Usage
Central administrative staff facilitated mammography tracking and EMR documentation for all patients in all practices. During the first month of system deployment, central administrative usage was high with a large numbers of reminder letters sent to patients, consuming roughly 8 hours per week. After this initial bolus was accommodated for, administrative staff spent roughly 2 hours per week performing all central functions including sending patient letters, taking phone messages, and updating EMR health maintenance data.
Patient Mammography Reporting
Among 2,000 patients mailed a letter (167 letters returned/unmailed due to incorrect address), 118 patients (5.9%) contacted our central office by telephone, fax, or mail. Among these patients, 90 reported a previously completed mammogram at a facility outside our network (later entered into the patient's medical record as “patient reported data” by administrative nursing staff). An additional 9 patients reported completed mammograms at MGH after our study start date, 7 patients reported having an outside mammogram but did not supply enough information to update the medical record, and 2 patients reported the date for a future scheduled mammogram. Ten patients called to decline screening or provided a reason to be excluded.
Delegate Usage
Eight hundred sixty-six patients (40%) of our initial contacted population were either scheduled or deferred by delegate staff using the MFT interface. When broken down by linkage status, delegate action occurred in 315 of 957 (33%) provider-linked patients screened by PCPs and 551 of 1,210 (46%) practice-linked patients screened by CMs (▶). Delegates scheduled mammograms for 180 of the contacted patients (8%), whereas another 686 (32%) were identified as not needing or wanting mammography screening. An additional 278 patients (13%) scheduled a mammogram independently with the hospital's radiology department as instructed in the patient letter.
Table 4.
Table 4 Delegate Utilization for Installation Practices
| Delegate Usage, N (%) | All Patients N = 2,167 | PCP Managed Patients N = 957 (44.2) | Case Manager Managed Patients N = 1,210 (55.8) |
|---|---|---|---|
| Delegate action | 866 (40.0) | 315 (33.0) | 551 (45.5) |
| Mammogram scheduled | 180 (8.3) | 68 (7.1) | 112 (9.3) |
| Defer scheduling mammogram | 686 (31.7) | 247 (25.8) | 439 (36.3) |
| Mammogram complete | 138 (20.1) | 73 (29.6) | 65 (14.8) |
| Mammogram scheduled | 86 (12.5) | 58 (23.5) | 28 (6.4) |
| Deceased | 5 (0.7) | 0 (0.0) | 5 (1.1) |
| Informed refusal | 59 (8.6) | 21 (8.5) | 38 (8.7) |
| Not eligible | 1 (0.1) | 0 (0.0) | 1 (0.2) |
| Not a practice patient | 94 (13.7) | 10 (4.1) | 84 (19.1) |
| Prior bilateral mastectomy | 6 (0.9) | 2 (0.8) | 4 (0.9) |
| Three attempts made | 63 (9.2) | 35 (14.2) | 28 (6.4) |
| Wrong/nonfunctioning number | 218 (31.8) | 37 (15.0) | 181 (41.2) |
| Other | 16 (2.3) | 11 (4.5) | 5 (1.1) |
PCP = primary care physician.
Discussion
Mammography FastTrack is a population-based, multi-modal system initiated to identify and contact patients with screening reminders for mammogram-overdue patients across a heterogeneous primary care network. Prior to implementation, there were no systematic efforts to identify or send reminders to patients overdue for mammography screening. By addressing barriers to care at the clinical system, individual provider, and patient levels, 49 MFT, through voluntary use, resulted in over 85% of network physicians and case managers across all practices to take action on 83% of our mammogram-overdue population. Over 63% of the mammogram-overdue population was successfully contacted by letter within the first six months of use.
We applied the results of a previously validated algorithm to classify patients as PCP- or practice-linked, and successfully targeted information to the appropriate provider. When patient–provider linkage was strong, we directed our reminders to PCPs and sent customized educational mailings from the PCP's office to reinforce the need for mammography screening. In cases in which patient–provider linkage was weaker, practice CMs were key physician-extenders of the clinical effector arm. In these cases, customized educational mailings were addressed from the affiliated practice's medical director.
Approximately 2% of patients were identified by PCPs as inaccurately linked. This high degree of accuracy served to overcome PCP reluctance to review lists and likely resulted in our high utilization rates. Overall, preliminary usage data indicates that only 28 (1.1%) patients among those reviewed by a PCP or practice CM were identified as “not my patient,” indicating that our validated provider-to-patient linkage model worked reliably in a heterogeneous primary care clinical network. Other institutions interested in network-level quality improvement initiatives may benefit from such a unique linkage model.
The user interfaces of MFT were designed for ease of use. Our intent was to create contextually complete and standalone interfaces to minimize the amount of manual cross-referencing of clinical data elements. Voluntary PCP use of MFT was high, with over 85% of physicians screening at least some proportion of the population. Because physician use of this tool was entirely voluntary, ongoing system usage may be considered a key measure of success. Interestingly, a larger percentage of practice-linked patients were screened by CMs, likely indicating that a small percentage of PCPs may be reluctant to embrace new clinical systems. However, physicians were more than twice as likely to defer patient contact compared to practice CMs, likely reflecting better knowledge of the individual patient. For both PCP and practice-linked patients, the majority who had not had a screening mammogram in 2 years received a reminder letter. However, the differential usage data between PCPs and CMs also informs our knowledge about user acceptance of systematic efforts to improve health care quality.
It is highly unlikely that software and the application of our patient linkage model were solely responsible for the high degree of system use and overall success of the project. The development of central administrative support tightly integrated with our system's mammography workflow removed certain tasks from providers and practices and became a potent “clinical effector arm.” Central administrative staff facilitated mammography tracking and documentation for all patients (practice- and provider-linked) while still accommodating individual practice needs with customized patient educational materials. Additionally, we created a single result-reporting phone line, fax number, and mail address where patients from any practice could confidentially provide information about outside mammograms. Such results were subsequently incorporated into the patient's EMR by a nurse. In this way, our system used central human resources to augment the system's information technology.
One limitation of our system was that even with efforts to permit as much local workflow flexibility as possible, we could not meet every practice's preferences. Practices supplied varied levels of support for the CM and delegate roles, and personnel changes left some practices without these key individuals for varying periods of time. This required some use of our project administration to perform functions that the practices were intended to fulfill. Although this approach was feasible as part of a demonstration project, budgeting additional central administrator resources may be needed for continuing use.
Another limitation of our current system relates to its lack of “turn-key” sustainability. In this version, at the beginning of each DM cycle the population registry must be manually “loaded” with: (1) the mammogram-at-risk cohort, (2) mammography due dates, and (3) up-to-date patient–provider linkage. Because patient inclusion in the registry is static throughout the cycle, influx and efflux of patients from the network are not automatically updated, resulting in diminishing accuracy over time. Future implementations will incorporate a dynamic patient–provider linkage representation. Similarly, future versions should provide a reliable method for tracking of report results and provide an interface for PCPs to manually review and edit their list of linked patients. Future versions of the tool could also allow for more customized population rules and generate patient outreach materials written in the patient's native language.
Despite these limitations, MFT was successfully implemented and used in a heterogeneous primary care network. Although many similar initiatives have shown only modest uptake by providers, MFT was voluntarily used by nearly all providers. Because process metrics of care do not always translate into clinical outcomes, longer-term follow-up will be needed to determine whether use of this system results in higher mammogram completion rates over time compared to a control population. Full results reporting 1-year mammogram completion rates are unavailable at this time and will be reported in a future publication.
As system-level quality initiatives such as pay-for-performance, 50,51 at-risk financial withholds, and physician report cards 52,53 become more commonplace, health care organizations will need to allocate adequate resources to support population-level registry management initiatives such as MFT. To be successful, population-based health care information technology solutions must accommodate provider and practice workflow in a flexible system that uses highly accurate patient lists, simple and convenient provider screening, and automated surveillance and patient outreach methods.
References
- 1.World Health Organization Screening for Breast Cancerhttp://www.who.int/cancer/detection/breastcancer/en/Accessed December 4, 2007.
- 2.Tabar L, Fagerberg G, Duffy SW, Day NE. The Swedish two county trial of mammographic screening for breast cancer: recent results and calculation of benefit J Epidemiol Community Health 1989;43:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabar L, Fagerberg G, Chen HH, et al. Efficacy of breast cancer screening by age. New results from the Swedish Two-County Trial. Cancer 1995;75:2507-2517. [DOI] [PubMed] [Google Scholar]
- 4.Kerlikowske K. Efficacy of screening mammography. A meta-analysis [see comment]. JAMA 1995;273:149-154. [PubMed] [Google Scholar]
- 5.Elwood M, Cox B, Richardson A. The effectiveness of breast cancer screening by mammography in younger women: correction[Erratum for Online J Curr Clin Trials 1993;Feb 25:Doc No 32; PMID: 8305999] Online J Curr Clin Trials 1994. Doc No 121. [PubMed]
- 6.Martin LM, Calle EE, Wingo PA, Heath Jr CW. Comparison of mammography and Pap test use from the 1987 and 1992 National Health Interview Surveys: are we closing the gaps? Am J Prev Med 1996;12:82-90. [PubMed] [Google Scholar]
- 7.Burns RB, McCarthy EP, Freund KM, et al. Variability in mammography use among older women J Am Geriatr Soc 1996;44:922-926. [DOI] [PubMed] [Google Scholar]
- 8.Caplan LS, Wells BL, Haynes S. Breast cancer screening among older racial/ethnic minorities and whites: barriers to early detection J Gerontol 1992;47:101-110. [PubMed] [Google Scholar]
- 9.Paquette D. Performance of screening mammography in organized programs in Canada in 1996. The Database Management Subcommittee to the National Committee for the Canadian Breast Cancer Screening Initiative. [see comment] CMAJ 2000;163:1133-1138. [PMC free article] [PubMed] [Google Scholar]
- 10.Physician Insurers Association of America and American College of Radiology Practice Standards Claims SurveyRockville, MD: Physician Insurers Association of America; 1997.
- 11.Preston JA, Scinto JD, Ni W. Mammography underutilization among older women in Connecticut J Am Geriatr Soc 1997;45:1310-1314. [DOI] [PubMed] [Google Scholar]
- 12.Tinley ST, Houfek J, Watson P, et al. Screening adherence in BRCA1/2 families is associated with primary physicians' behavior Am J Med Genetics 2004;125A:5-11. [DOI] [PubMed] [Google Scholar]
- 13.Otero-Sabogal R, Owens D, Canchola J, Golding JM, Tabnak F, Fox P. Mammography rescreening among women of diverse ethnicities: patient, provider, and health care system factors J Health Care Poor Underserved 2004;15:390-412. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim MA, Savitz LA, Carey TS, Wagner EH. Population-based health principles in medical and public health practice J Public Health Manag Pract 2001;7:75-81. [DOI] [PubMed] [Google Scholar]
- 15.Grant RW, Hamrick HE, Sullivan CM, et al. Impact of population management with direct physician feedback on care of patients with type 2 diabetes Diabetes Care 2003;26:2275-2280. [DOI] [PubMed] [Google Scholar]
- 16.Fox SA, Stein JA. The effect of physician-patient communication on mammography utilization by different ethnic groups Med Care 1992;29:1065-1082. [DOI] [PubMed] [Google Scholar]
- 17.Grady KE, Lemkau JP, McVay JM, Reisine ST. The importance of physician encouragement in breast cancer screening of older women Prev Med 1992;21:766-780. [DOI] [PubMed] [Google Scholar]
- 18.Gray DP, Evans P, Sweeney K, et al. Towards a theory of continuity of care J R Soc Med 2003;96:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kingston PO. Patient ties to ambulatory care providers: the concept of provider loyalty J Health Care Marketing 1983;3:27-34. [PubMed] [Google Scholar]
- 20.Roberge D, Lebeau R, Haddad S, Beaulieu, MD, Pineault R. Loyalty to primary physician: consumers and clinicians' views and measurement issuesCentre de recherche du CHUM, Montreal, Quebec H2L 4M1, Canada Abstr Book Assoc Health Serv Res Meet. 1998;15:293. [Google Scholar]
- 21. The impending collapse of primary care medicine and its implications for the state of the nation's health careAmerican College of Physicians, January 30, 2006www.acponline.org 1998. Accessed August 1, 2008.
- 22.Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services J Fam Pract 1994;38:166-171. [PubMed] [Google Scholar]
- 23.Kottke TE, Brekke ML, Solberg LI. Making “time” for preventive services Mayo Clin Proc 1993;68:785-791. [DOI] [PubMed] [Google Scholar]
- 24.McDonald CJ. Computer reminders, the quality of care and the nonperfectability of man N Engl J Med 1976;295:1351-1355. [DOI] [PubMed] [Google Scholar]
- 25.McDonald C. Use of a computer to detect and respond to clinical events: its effect on clinician behavior Ann Intern Med 1976;84:162-167. [DOI] [PubMed] [Google Scholar]
- 26.Khoury AT, Wan GJ, Niedermaier ON, et al. Improved cholesterol management in coronary heart disease patients enrolled in an HMO J Health Care Quality 2001;23:29-33. [DOI] [PubMed] [Google Scholar]
- 27.Sittig DF, Krall MA, Dykstra RH, Russell A, Chin HL. A survey of factors affecting clinician acceptance of clinical decision support BMC Med Inform Decis Mak 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schellhase KG, Koepsell TD, Norris TE. Providers' reactions to an automated health maintenance reminder system incorporated into the patient's electronic medical record J Am Board Fam Pract 2003;16:312-317. [DOI] [PubMed] [Google Scholar]
- 29.McPhee SJ, Bird JA, Fordham D, Rodnick JE, Osborn EH. Promoting cancer prevention activities by primary care physicians. Results of a randomized, controlled trial. JAMA 1991;266:538-544. [PubMed] [Google Scholar]
- 30.Burack RC, Gimotty PA. Promoting screening mammography in inner-city settings. The sustained effectiveness of computerized reminders in a randomized controlled trial. Med Care 1997;35:921-931. [DOI] [PubMed] [Google Scholar]
- 31.Baker DW, Einstadter D, Husak SS, Cebul RD. Trends in postdischarge mortality and readmissions: has length of stay declined too far? Arch Intern Med 2004;164:538-544. [DOI] [PubMed] [Google Scholar]
- 32.Berger CS, Cayner J, Jensen G, Mizrahi T, Scesny A, Trachtenberg J. The changing scene of social work in hospitals: a report of a national study by The Society for Social Work Administrators in Health Care and NASW Health Social Work 1996;21:167-177. [DOI] [PubMed] [Google Scholar]
- 33.Maviglia SM, Zielstorff RD, Paterno M, Teich JM, Bates DW, Kuperman GJ. Automating complex guidelines for chronic disease: lessons learned J Am Med Inform Assoc 2003;10:154-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success BMJ 2005;330:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis Med Care 1998;36:1138-1161. [DOI] [PubMed] [Google Scholar]
- 36.Haynes RB, McKibbon K, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications Lancet 1996;348:383-386. [DOI] [PubMed] [Google Scholar]
- 37.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success BMJ 2005;330:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clemow L, Costanza ME, Haddad WP, et al. Underutilizers of mammography screening today: characteristics of women planning, undecided about, and not planning a mammogram Ann Behav Med 2000;22:80-88. [DOI] [PubMed] [Google Scholar]
- 39.Pearlman DN, Clark MA, Rakowski W, Ehrich B. Screening for breast and cervical cancers: the importance of knowledge and perceived cancer survivability Women Health 1999;28:93-112. [DOI] [PubMed] [Google Scholar]
- 40.Fajardo LL, Saint-Germain M, Meakem Jr. T, Rose C, Hillman BJ. Factors influencing women to undergo screening mammography Radiology 1992;184:1. [DOI] [PubMed] [Google Scholar]
- 41.Eisner EJ, Zook EG, Goodman N, Macario E. Knowledge, attitudes, and behavior of women ages 65 and older on mammography screening and Medicare: results of a national survey Women Health 2002;36:1-18. [DOI] [PubMed] [Google Scholar]
- 42.Leupker RV. Patient adherence: a “risk factor” for cardiovascular disease Heart Dis Stroke 1993;2:418-421. [PubMed] [Google Scholar]
- 43.Lasko TA, Atlas SJ, Barry MJ, Chueh HC. Automated identification of a physician's primary patients J Am Med Inform Assoc 2006;13:74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atlas SJ, Chang Y, Lasko TA, Chueh HC, Grant RW, Barry MJ. Is this “my” patient?. Development and validation of a predictive model to link patients to primary care providers. J Gen Intern Med 2006;21:973-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chueh H, Barnett GO. “Just-in-time” clinical information Acad Med 1997;72:512-517. [DOI] [PubMed] [Google Scholar]
- 46.Rabbani U, Morgan M, Barnett GO. A COSTAR interface using WWW technology. Submitted for presentation at the Proceedings of the 1998 AMIA Annual Fall Symposium, 1998 November 7–11, Orlando, FL. [PMC free article] [PubMed]
- 47.MicrosoftActive Server Pageshttp://msdn2.microsoft.com/enus/library/aa286483.aspx 1997. Accessed July 1, 2008.
- 48.MicrosoftMicrosoft SQL Server 2000http://msdn2.microsoft.com/enus/library/ms950404.aspx 1997. Accessed July 1, 2008.
- 49.Phillips KA, Kerlikowske K, Baker LC, Chang SW, Brown ML. Factors associated with women's adherence to mammography screening guidelines Health Serv Res 1998;33:29-53. [PMC free article] [PubMed] [Google Scholar]
- 50.Bufalino V, Peterson ED, Burke GL, et al. American Heart Association's Reimbursement, Coverage, and Access Policy Development Workgroup. Payment for quality: guiding principles and recommendations: principles and recommendations from the American Heart Association's Reimbursement, Coverage, and Access Policy Development Workgroup. Circulation 2006;113:1151-1154. [DOI] [PubMed] [Google Scholar]
- 51.Petersen LA, Woodard LD, Urech T, Daw C, Sookanan S. Does pay-for-performance improve the quality of health care? Ann Intern Med 2006;145:265-272. [DOI] [PubMed] [Google Scholar]
- 52.Hofer TP, Hayward RA, Greenfield S, Wagner EH, Kaplan SH, Manning WG. The unreliability of individual physician “report cards” for assessing the costs and quality of care of a chronic disease JAMA 1999;281:2098-2105. [DOI] [PubMed] [Google Scholar]
- 53.Balas EA, Boren SA, Brown GD, Ewigman BG, Mitchell JA, Perkoff GT. Effect of physician profiling on utilization. Meta-analysis of randomized clinical trials. J Gen Intern Med 1996;11:584-590. [DOI] [PubMed] [Google Scholar]