Abstract
Purpose
To investigate the effects of male aging on sperm quality and sperm DNA fragmentation.
Methods
The ejaculates of 320 unselected men attending a fertility clinic and, as a control, 84 normozoospermic men without any history of ART were analyzed according to WHO guidelines. Sperm DNA fragmentation was measured by flow cytometry after staining with propidiumiodide.
Results
The patients were divided into four groups: <30 years, 30–35 years, 36–39 years and ≥40 years. Sperm motility decreased with increasing age whereas sperm concentration, morphology, and DNA fragmentation fluctuated throughout the four groups both among patients and among controls. However, we could not detect any significant correlation between male age and conventional semen parameters or sperm DNA fragmentation, respectively, neither in the patients’ group nor among the controls. This also applies to a classification of patients and controls into only two age groups with a cut-off point at 35 years.
Conclusions
Our findings suggest that neither the routinely assessed semen parameters nor the amount of spermatozoa with fragmented DNA are affected by male age.
Keywords: Aging male, Sperm DNA fragmentation, Flow cytometry, Nicoletti assay, Semen parameters
Introduction
The developed countries are currently witnessing an increase in maternal and paternal ages at the time of first parenthood. In Germany, the percentage of mothers older than 35 years nearly doubled from 9.9% in 1990 to 18.1% in the year 2000. The corresponding figures for fathers are also noticeable (23.1% in 1990 vs. 38.5% in 2001). Among couples undergoing treatment by assisted reproductive technologies (ART), fathers are significantly older compared with those not needing ART (36.6 vs. 33.5 years) [1]. Whereas it is well-established that women >35 years of age bear a higher risk of conceiving genetically abnormal offsprings [2–7], a correlation with paternal age is still at issue. For instance, Sloter et al. [8] have shown that advancing male age is associated with a gradual and significant increase in the risk of fathering children with various chromosomal defects. In contrast, Luetjens et al. [9] did not find a significantly higher risk of producing chromosomally abnormal offsprings for men of advanced age.
Sperm concentration, motility, and morphology are the most important parameters assessed by conventional semen analyses. It is evident that these variables describe only visible features of spermatozoa and do not allow any prediction on the genetic constitution of the male gamete, i.e., the integrity of its DNA. An intact DNA is necessary for the correct transmission of genetic material to the next generation [10]. DNA fragmentation could result in an early arrest in embryonic development or even prevent fertilization [11].
In view of the observed increase in paternal age, substantial interest exists in studying effects of aging on semen quality and sperm DNA damage and elucidating possible correlations between these parameters. Here, we present our results for a group of 320 patients and 84 control subjects undergoing standard analysis of sperm concentration, motility, and morphology. Sperm DNA fragmentation (expressed as DNA fragmentation index = DFI) has been measured by flow cytometry after propidium iodide staining. To our knowledge, this is the first report using a modification of the so-called Nicoletti assay [12] for evaluating sperm DNA damage.
Materials and methods
Patients and controls
A total of 320 unselected patients consulting our IVF and Urological Center were included in this study. These patients neither had any infections or antibiotic treatment during the past 3 months nor did they undergo x- ray or chemotherapy during the past 6 months. As controls we recruited 84 normozoospermic men without any history of ART, also without any infections or antibiotic treatment during the past 3 months or x- ray or chemotherapy during the past 6 months.
Semen analysis
The samples were collected by masturbation after a period of 3–5 days of sexual abstinence. An aliquot of 1 ml was taken from each sample after liquefaction to determine the DFI. The semen parameters were analyzed according to WHO guidelines [13]. Sperm concentration and motility were determined with a Makler chamber at 200× magnification. Sperm morphology was analyzed after staining with Neo-methylenblue and Kresylvioletacetat at 1,000× magnification. Seventeen patients displayed such a marginal concentration that it was not possible to determine motility or morphology (e.g. just 56 sperms within the whole Makler chamber). Therefore only 303 patients were taken into account for the calculations concerning the semen parameters.
DNA fragmentation
The DFI was measured by staining the sample with propidiumiodide (PI) as published before by Nicoletti [12] with some modifications in order to adjust the assay to spermatozoa. Briefly, the samples were washed and centrifuged twice with PBS and the supernatant was discarded. The samples were then stained with 1:1 solution of Na- Citrate and Triton × 100 containing 25 µl of a 10% PI solution. After staining, the samples were incubated at 4°C for 16–24 h and analyzed by flow cytometry. We used a FC 500 series flow cytometer system by Beckman Coulter.
Statistical analysis
The results were analyzed using the students T-test.
Results
A total of 320 semen samples from infertile patients were analyzed regarding the semen parameters and the DFI. The age of the patients ranged from 24 to 56 with a mean of 36.62 years. The results of our study were allocated to four age groups:
Younger than 30 years (27.13 years in average, n = 31)
30–35 years (32.81 years in average, n = 101)
36–39 years (37.4 years in average, n = 92)
40 years and older (42.95 years in average, n = 96)
The results of the analysis of the semen parameters and the DFI of these four groups are presented in Table 1. In a second step the patients were split into two age groups, the first up to 35 years with 132 patients and 31.48 years in average, and the second one 36 years and older with 188 patients and a mean of 40.23 years. The data for these two groups are also shown in Table 1.
Table 1.
Descriptive statistics and comparisons between the patients, divided into four age groups (<30, 30–35, 36–39, ≥40) and two summarized age groups (≤35, >35)
| Age (years) | DNA fragmentation (%) | Age (years) | Concentration (× 106/ml) | Motility (%) | Morphology (%) |
|---|---|---|---|---|---|
| <30 [Ø = 27.13, n = 31] | 23.12 ± 21.24 | <30 [Ø = 27.00, n = 28] | 39.73 ± 36.58 | 52.52 ± 17.84 | 11.79 ± 7.49 |
| 30–35 [Ø = 32.81, n = 101] | 18.42 ± 18.49 | 30–35 [Ø = 32.81, n = 97] | 57.85 ± 53.92 | 47.3 ± 19.36 | 14.95 ± 9.22 |
| 36–39 [Ø = 37.4, n = 92] | 18.66 ± 18.17 | 36–39 [Ø = 37.4, n = 86] | 56.53 ± 44.93 | 46.46 ± 19.09 | 14.3 ± 10.69 |
| ≥40 [Ø = 42.95, n = 96] | 24.1 ± 21.45 | ≥40 [Ø = 42.91, n = 92] | 44.96 ± 45.52 | 42.8 ± 19.67 | 13.06 ± 9.95 |
| ≤35 [Ø = 31.48, n = 132] | 19.52 ± 19.17 | ≤35 [Ø = 31.51, n = 125] | 54.61 ± 51.02 | 47.35 ± 19.14 | 15.14 ± 9.54 |
| >35 [Ø = 40.23, n = 188] | 21.44 ± 20.14 | >35 [Ø = 40.25, n = 178] | 50.59 ± 45.6 | 44.59 ± 19.47 | 11.86 ± 9.02 |
The results are presented as mean ± standard deviation. None of the collectives showed a statistical difference
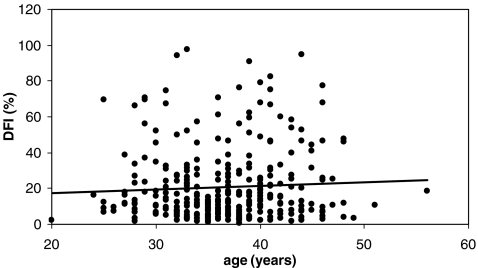
No statistical significance was demonstrable between any of the age groups of the patients. The correlations between the age of the patients and their semen parameters was very weak. The same applies to the correlation between the age of the patients and the DFI, as shown in Table 2 and graphically illustrated in Fig. 1.
Table 2.
Correlations of age with the conventional semen parameters and DNA fragmentation
| DNA fragmentation (%) | Concentration (× 106/ml) | Motility (%) | Morphology (%) | |
|---|---|---|---|---|
| Patients (total) | r = 0.06 p < 0.05 | r = −0.04 p < 0.05 | r = −0.12 p < 0.05 | r = −0.09 p < 0.05 |
| Patients (NORMO) | r = 0.08 p < 0.05 | r = −0.09 p < 0.05 | r = 0.22 p < 0.05 | r = 0.02 NS |
| Patients (SF) | r = 0.04 p < 0.05 | r = 0.05 p < 0.05 | r = −0.13 p < 0.05 | r = −0.04 p < 0.05 |
| Controls | r = −0.17 p < 0.05 | r = 0.09 p < 0.05 | r = 0.24 p < 0.05 | r = −0.06 p < 0.05 |
Fig. 1.
Scatter graph illustrating the associations between age and DNA- fragmentation (r = 0.06, p < 0.05)
When splitting the patients into a group of normozoospermic men (NORMO) and a group of men with at least one impaired conventional semen parameter (SubFertile= SF), the two groups were significantly different from each other, concerning the semen parameters and the DFI (with p < 0.000001 in each case), whereas the age distribution showed no statistically significant difference. The results for the NORMO and the SF group are shown in Table 3. No statistically significant differences were observed within the NORMO group. Within the SF group the DFI of men aged 36–39 years and of men aged 40 years and older was significantly different (p < 0.05), as well as the motility of the youngest group compared to the 30–35 and the 36–39 group (p < 0.05) and to the 40 years and older group (p < 0.01). We also calculated the correlations between age and semen parameters and the DFI within these two groups, but as shown in Table 2, the relationships were very weak.
Table 3.
Descriptive statistics and comparisons of the patients group, divided into four age groups (<30, 30–35, 36–39, >39) and two summarized age groups (≤35, >35)
| Age (years) | DNA fragmentation (%) | Concentration (× 106/ml) | Motility (%) | Morphology (%) |
|---|---|---|---|---|
| NORMO | ||||
| <30 [Ø = 25.67, n = 6] | 7.92 ± 3.93 | 76 ± 35.94 | 62.17 ± 11.23 | 21.5 ± 5.85 |
| 30–35 [Ø = 32.89, n = 27] | 7.21 ± 5.62 | 105.7 ± 59.8 | 63.07 ± 8.93 | 25.74 ± 7.13 |
| 36–39 [Ø = 37.3, n = 23] | 12.08 ± 15.64 | 87.17 ± 46.91 | 62.17 ± 9.43 | 26.22 ± 9.57 |
| >39 [Ø = 42.54, n = 13] | 9.99 ± 11.86 | 87.92 ± 44.44 | 68.92 ± 11.29 | 26.23 ± 8.06 |
| ≤35 [Ø = 31.58, n = 33] | 7.34 ± 5.36 | 100.3 ± 57.37 | 62.91 ± 9.4 | 24.97 ± 7.1 |
| >35 [Ø = 39.19, n = 36] | 11.32 ± 14.42 | 87.44 ± 46.03 | 64.61 ± 10.65 | 26.22 ± 9.06 |
| Total [Ø = 35.55, n = 69] | 9.42 ± 11.24 | 93.59 ± 52.16 | 63.8 ± 10.11 | 25.62 ± 8.2 |
| SF | ||||
| <30 [Ø = 27.36, n = 22] | 22.72 ± 19.51 | 32.48 ± 31.2 | 52.45 ± 17.422 3 * | 9.68 ± 5.29 |
| 30–35 [Ø = 32.79, n = 70] | 21.12 ± 17.71 | 38.91 ± 36.52 | 42.73 ± 18.112 | 11.49 ± 6.78 |
| 36–39 [Ø = 37.43, n = 63] | 18.58 ± 151 | 48.65 ± 38.73 | 43.56 ± 16.053 | 10.86 ± 7.28 |
| >39 [Ø = 42.92, n = 79] | 25.00 ± 21.041 | 39.56 ± 41.96 | 39.39 ± 17.02* | 9.65 ± 6.67 |
| ≤35 [Ø = 31.49, n = 92] | 21.51 ± 18.17 | 37.37 ± 35.43 | 45.05 ± 18.42 | 11.05 ± 6.5 |
| >35 [Ø = 40.47, n = 142] | 22.15 ± 18.87 | 43.59 ± 40.81 | 41.24 ± 16.72 | 10.18 ± 6.97 |
| Total [Ø = 36.97, n = 234] | 21.9 ± 18.6 | 41.15 ± 38.9 | 42.74 ± 17.51 | 10.53 ± 6.8 |
The patients have also been divided into a normozoospermic group (NORMO) and a subfertile group (SF). The results are presented as mean ± standard deviation. Only 303 patients have been evaluated (see text). None of the collectives showed a statistical difference, except 1, 2, 3 ➔ p < 0.05 and * ➔ p < 0.01
In the control collective the distribution into the four groups was as follows:
Younger than 30 years (25.08 years in average, n = 24)
30–35 years (32.36 years in average, n = 28)
36–39 years (37.27 years in average, n = 22)
40 years and older (42.8 years in average, n = 10)
We only detected statistically significant differences between the two younger age groups regarding sperm motility and between the youngest and the eldest group concerning the DFI, as can be seen in Table 4. In the control group, the correlations between age and semen parameters or the DFI were also weak (Table 2).
Table 4.
Descriptive statistics and comparisons of the control group, divided into four age groups (<30, 30–35, 36–39, >39) and the two summarized age groups (≤35, >35)
| Age (years) | DNA fragmentation (%) | Concentration (x 106/ml) | Motility (%) | Morphology (%) |
|---|---|---|---|---|
| <30 [Ø = 25.08, n = 24] | 13.10 ± 14.31* | 88.96 ± 38.26 | 55.67 ± 11.85** | 16.67 ± 9.56 |
| 30–35 [Ø = 32.36, n = 28] | 7.19 ± 6.31 | 106.04 ± 44.41 | 63.75 ± 11.54** | 16.86 ± 5.24 |
| 36–39 [Ø = 37.27, n = 22] | 11.54 ± 16.87 | 115.82 ± 109.68 | 60.73 ± 10.45 | 17.55 ± 6.65 |
| >39 [Ø = 42.8, n = 10] | 4.11 ± 2.83* | 103.5 ± 51.17 | 60.4 ± 8.79 | 14.6 ± 5.83 |
| ≤35 [Ø = 29, n = 52] | 9.92 ± 11.17 | 98.15 ± 42.54 | 60.02 ± 12.36 | 16.77 ± 7.55 |
| >35 [Ø = 39, n = 32] | 9.22 ± 14.49 | 111.97 ± 95.5 | 60.63 ± 9.96 | 16.63 ± 6.55 |
The results are presented as mean ± standard deviation
*p < 0.01, **p < 0.02, the other parameters were not significantly different concerning the age of the patients
Discussion
Sperm DNA damage has been attributed to a variety of intra- and extratesticular factors [14]. Probably the most important is the production of reactive oxygen species (ROS) which is excited, for example, by excessive stress, competitive sports, alcohol and drug abuse or nicotine. If produced in abundance, ROS can enter the cell nucleus, bind to the DNA and cause its fragmentation [15–20]. However, DNA fragmentation is also a feature of physiological processes like apoptosis and necrosis. For instance, during spermatogenesis apoptosis controls the amount of spermatogonia one Sertoli cell has to feed [11, 21, 22].
Several techniques are currently available that assess sperm DNA damage directly or indirectly by evaluating sperm chromatin compaction [14]. The most common tests are the sperm chromatin structure assay (SCSA), the Comet assay and the TUNEL assay. The SCSA measures the susceptibility of sperm DNA to denaturation, whereas the other two assess DNA strand breaks [14]. These methods have already been used to assess whether male age influences the semen parameters or the DFI but the studies revealed partially different results. Singh et al. [23] found by means of the Comet assay that male age may predispose for DNA double strand breaks. In contrast Schmid et al. [24], using the same method, reported that male age only influences single strand breaks. However, single strand breaks do not have any influence on fertilization, because they can be repaired by the oocyte. Two further studies based on the TUNEL assay provided more coherent results. Both groups reported that male aging affects the chromatin integrity of spermatozoa, but only in infertile populations [25, 26]. The modified Nicoletti assay, used in our study, is based on the long incubation time of 16–24 h, during which the DNA fragments diffuse to the extracellular medium, since the plasma membrane has been perforated by Triton- X and Na- citrate. Subsequently the amount of DNA inside the cells can be detected by flow cytometry. All cells with minor fluorescence intensity (which means minor content of DNA) are counted as DNA fragmented.
A multitude of studies have focused on possible relations between aging and male reproduction in the past few years [8, 27–33]. However, most of them either examined congenital malformations or chromosomal aberrations [8, 29] and sometimes the results were not clear [28, 29]. Levitas et al. [31] detected a statistically significant and inverse relationship between semen volume, sperm quality, and patient age, but the patients had a longer period of sexual abstinence before the testing. Some of these studies analyzed a fewer amount of patients [26, 30] and used other methods, for example the TUNEL assay, to assess the DFI [26] which targets apoptotic cells rather than DNA fragmented cells. In the latter study [26], sperm concentration even improved with increasing age. On the other hand, Luetjens et al. [9] reported that men of advanced age still wanting to become fathers do not have a significantly higher risk of producing offsprings with chromosomal abnormalities compared with younger men. Thiemann- Boge et al. [33] found that the increased risk with paternal age to father a child with achondroplasia or Apert syndrome cannot be explained from the number of spermatozoa carrying the corresponding mutations.
We analyzed the semen parameters and the DFI in 320 unselected patients and 84 normozoospermic controls. Our current findings suggest that neither the semen parameters measured by WHO guidelines [13] (concentration, motility, and morphology) nor the DFI degrades in aging men. This is also supported by the fact that the only parameter that was not significant after splitting the patients into a NORMO and an SF group was the age of the patients, whereas all the other parameters were statistically significant (p < 0.000001). Furthermore there were only weak correlations between age and the analyzed parameters that did not reach statistical significance.
All in all, this topic needs additional investigation. For instance the different techniques to measure the DFI should be compared, to be able to make a clear statement on the advantages and disadvantages of these methods. We are currently planning these studies in our laboratory.
Acknowledgements
This work was gratefully supported by a grant from Merck Serono.
Footnotes
Capsule Neither conventional parameters of semen quality nor sperm DNA fragmentation were significantly correlated with paternal age in 320 infertility patients and 84 control subjects.
References
- 1.Engel W, Sancken U, Laccone F. Paternal age from a genetic point of view. J Reproduktionsmed Endokrinol. 2004;1:263–7. German.
- 2.Hook EB, Schreinemachers DM, Willey AM, Cross PK. Rates of mutant structural chromosome rearrangements in human foetuses: data from prenatal cytogenetic studies and associations with maternal age and parental mutagen exposure. Am J Hum Genet. 1983;35:96–109. [PMC free article] [PubMed]
- 3.Hecht F, Hecht BK. Aneuploidy in humans: Dimensions, demography, and dangers of abnormal numbers of chromosomes. In: Vig BK, Sandberg AA, editors. Aneuploidy part II: Incidence and etiology. New York: Alan R. Liss; 1987. p. 9–49.
- 4.Hassold T, Hunt PA, Sherman S. Trisomy in humans: incidence, origin and etiology. Curr Opin Genet Dev. 1993;3:398–403. doi:10.1016/0959-437X(93)90111-2. [DOI] [PubMed]
- 5.Wyrobek AJ, Aardema M, Eichenlaub-Ritter U, Ferguson L, Marchetti F. Mechanisms and targets involved in maternal and paternal age effects on numerical aneuploidy. Environ Mol Mutagen. 1996;28:254–64. doi:10.1002/(SICI)1098-2280(1996)28:3<254::AID-EM9>3.0.CO;2-D. [DOI] [PubMed]
- 6.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91. doi:10.1038/35066065. [DOI] [PubMed]
- 7.Nicolaidis P, Petersen MB. Origin and mechanisms of non-disjunction in human autosomal trisomies. Hum Reprod. 1998;13:313–9. doi:10.1093/humrep/13.2.313. [DOI] [PubMed]
- 8.Sloter ED, Marchetti F, Eskenazi B, Weldon RH, Nath J, Cabreros D, et al. Frequency of human sperm carrying structural aberrations of chromosome 1 increases with advancing age. Fertil Steril. 2007;87:1077–86. doi:10.1016/j.fertnstert.2006.08.112. [DOI] [PubMed]
- 9.Luetjens CM, Rolf C, Gassner P, Werny JE, Nieschlag E. Sperm aneuploidy rates in younger and older men. Hum Reprod. 2002;17:1826–32. doi:10.1093/humrep/17.7.1826. [DOI] [PubMed]
- 10.Donnelly ET, O’Connell M, McClure N, Lewis SE. Differences in nuclear DNA fragmentation and mitochondrial integrity of semen and prepared human spermatozoa. Hum Reprod. 2000;15:1552–61. doi:10.1093/humrep/15.7.1552. [DOI] [PubMed]
- 11.Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–7. doi:10.1530/ror.0.0040031. [DOI] [PubMed]
- 12.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139(2):271–9. doi:10.1016/0022-1759(91)90198-O. [DOI] [PubMed]
- 13.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction, 4th ed. Cambridge, UK: Published on behalf of the World Health Organization by Cambridge University Press; 1999.
- 14.Zini A, Libman J. Sperm DNA damage: clinical significance in the era of assisted reproduction. Curr Opin Urol. 2006;16:428–34. doi:10.1097/01.mou.0000250283.75484.dd. [DOI] [PubMed]
- 15.Lopes S, Jurisicova A, Casper RF. Gamete-specific DNA fragmentation in unfertilized human oocytes after intracytoplasmic sperm injection. Hum Reprod. 1998;13(3):703–8. doi:10.1093/humrep/13.3.703. [DOI] [PubMed]
- 16.Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod. 1998;13:896–900. doi:10.1093/humrep/13.4.896. [DOI] [PubMed]
- 17.Potts RJ, Norarianni LJ, Jefferies TM. Seminal plasma reduces exogenous damage to human sperm, determined by the measurement of DNA strand breaks and lipid peroxidation. Mutat Res. 2000;2:249–56. doi:10.1016/S0027-5107(99)00215-8. [DOI] [PubMed]
- 18.Agarwal A, Ramadan AS, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–43. doi:10.1016/S0015-0282(02)04948-8. [DOI] [PubMed]
- 19.Baumber J, Ball BA, Linfor JJ, Meyers SA. Reactive oxygen species and cryopreservation promote DNA fragmentation in equine spermatozoa. J Androl. 2003;4:621–8. [DOI] [PubMed]
- 20.Agarwal A, Prabakaran SA. Oxidative stress and antioxidants in male infertility: a difficult balance. Iranian J Reprod Med. 2005;3:1–8.
- 21.Gandini L, Lombardo F, Paoli D, Caponecchia L, Familiari G, Verlengia C, et al. Study of apoptotic DNA- fragmentation in human spermatozoa. Hum Reprod. 2000;15:830–9. doi:10.1093/humrep/15.4.830. [DOI] [PubMed]
- 22.Sina Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in testis. Rev Reprod. 1999;4:38–47. doi:10.1530/ror.0.0040038. [DOI] [PubMed]
- 23.Singh NP, Muller CH, Berger RE. Effects of age on DNA double strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80:1420–30. doi:10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed]
- 24.Schmid TE, Eskenazi B, Baumgartner A, Marchetti F, Young S, Weldon R, et al. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod. 2007;22:180–7. doi:10.1093/humrep/del338. [DOI] [PubMed]
- 25.Vagnini L, Baruffi RL, Mauri AL, Petersen CG, Massaro FC, Pontes A, et al. The effects of male age on sperm DNA damage in an infertile population. Reprod Biomed Online. 2007;15:514–9. [DOI] [PubMed]
- 26.Plastira K, Msaouel P, Angelopoulou R, Zanioti K, Plastiras A, Pothos A, et al. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J Assist Reprod Genet. 2007;24:437–43. doi:10.1007/s10815-007-9162-5. [DOI] [PMC free article] [PubMed]
- 27.Auger J, Jouannet P. Age and male fertility: biological factors. Rev Epidemiol Sante Publique. 2005;53:2S25–35. Spec No 2. [PubMed]
- 28.Sun Y, Vestergaard M, Zhu JL, Madsen KM, Olsen J. Paternal age and Apgar scores of newborn infants. Epidemiology. 2006;17:473–4. doi:10.1097/01.ede.0000220690.43455.22. [DOI] [PubMed]
- 29.Zhu JL, Madsen KM, Vestergaard M, Olesen AV, Basso O, Olsen J. Paternal age and congenital malformations. Hum Reprod. 2005;20:3173–7. doi:10.1093/humrep/dei186. [DOI] [PubMed]
- 30.Sloter E, Schmid TE, Marchetti F, Eskenazi B, Nath J, Wyrobek AJ. Quantitative effects of male age on sperm motion. Hum Reprod. 2006;21:2868–75. doi:10.1093/humrep/del250. [DOI] [PubMed]
- 31.Levitas E, Lunenfeld E, Weisz N, Friger M, Potashnik G. Relationship between age and semen parameters in men with normal sperm concentration: analysis of 6,022 semen samples. Andrologia. 2007;39:45–50. doi:10.1111/j.1439-0272.2007.00761.x. [DOI] [PubMed]
- 32.Hellstrom WJ, Overstreet JW, Sikka SC, Denne J, Ahuja S, Hoover AM, et al. Semen and sperm reference ranges for men 45 years of age and older. J Androl. 2006;27:421–8. doi:10.2164/jandrol.05156. [DOI] [PubMed]
- 33.Tiemann-Boege I, Navisi W, Greewal R, Cohn D, Eskenaz B, Wyrobek AJ, et al. The observed human sperm mutation frequency cannot explain the achondroplasia paternal age effect. Proc Natl Acad Sci USA. 2002;99:14952–7. doi:10.1073/pnas.232568699. [DOI] [PMC free article] [PubMed]