Abstract
Purpose
Dyslipidemia, cardiovascular disease and hypertension are more frequently seen in patients with PCOS than in normal patients. We aimed at evaluating the distribution of Apo E alleles that can influence cardiovascular risk of the PCOS patients and control subjects.
Methods
In this study, 129 young women with PCOS and 91 healthy women were included. In all subjects we performed hormonal, biochemical and Apo E genetic analysis.
Results
The Apo E3 allele was found at a significantly higher frequency in the PCOS patient group compared with the control group. The Apo E2 allele was found at a significantly higher frequency in the control group compared with the patient group with PCOS.
Conclusions
Although there were genotype and allele differences between control and patient groups in this study, no statistically significant change was determined in lipid and other cardiovascular risk factors in connection with allele and genotype.
Keywords: Apolipoprotein E gene, Cardiovascular risk factors, Hyperlipidemia, Polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is one of the most frequent endocrine malfunctions, which typically occur with chronic anovulation and hyperandrogenism [1]. Almost 7% of women at reproductive age are diagnosed with PCOS [2].
Dyslipidemia, cardiovascular disease and hypertension are more frequently seen in patients with PCOS than in normal patients [3–5].
The disorder has also been reported to be associated with an increase in sub-clinical atherosclerotic disease [5]. Apolipoproteins that form the protein part of the lipoproteins are important in lipid metabolism. It regulates the transport and redistribution of lipoproteins in blood as a cofactor. Apolipoprotein E (ApoE) is a 34,200 kDa polymorphic glycoprotein consisting of 299 amino acids [6]. Three common alleles of ApoE are E2, E3, E4 code for three isoforms E2, E3 and E4 in exon 4 ApoE genes. These isoforms result in six common phenotypes, which are E2/2, E2/3, E2/4, E3/3, E3/4 and E4/4 [7]. Relation of ApoE2 to the threat of atherosclerosis is controversial. ApoE4 is connected with decreased longevity, increased plasma lipid and ApoB levels and higher prevalence of cardiovascular illness and also of Alzheimer’s [8, 9].
Addition of genetic risk factors (such as abnormality of Apo E, PPAR, and TPA-PAI) to increasing technology and inactivity increases the human susceptibility to cardiovascular disease in the future. To date, there is no data available for Turkish subjects in the genetic polymorphism of ApoE and its relation with increased cardiovascular risk factors in PCOS patients. In this study, we aimed at evaluating the distribution of ApoE alleles that can influence cardiovascular risk of the Turkish PCOS patients and control subjects.
Materials and methods
Patients
In this study, 129 young women with PCOS and 91 healthy women were included. PCOS was defined by Rotterdam PCOS consensus criteria [10].
Patients who had Diabetes Mellitus (diagnosed by 75 g Oral Glucose Tolerance Test), hyperprolactinemia, congenital adrenal hyperplasia (diagnosed with the adrenocorticotropic hormone stimulation test), thyroid disorders, Cushing’s syndrome, hypertension, hepatic or renal dysfunction were excluded from the study. At study entry, all subjects underwent venous blood drawing for complete hormonal assays, lipid profile, glucose, and insulin. All blood samples were obtained during early follicular phase of the spontaneous or progesterone-induced menstrual cycle in the morning between 8 AM and 9 AM respectively after an overnight fasting, and while resting in bed.
Biochemical assay
Serum concentrations of hs-CRP were determined by an immunonephelometric assay (N-hs-CRP; Dade Behring, Izmir, Turkey); intra- and inter-assay CVs were 1.72 and 2.80%, respectively. Serum total cholesterol, LDL and HDL cholesterol, triglyceride, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and -glutamyltransferase (GGT) were measured by Olympus AU 2,700 automated analyzer (Toshiba, Tokyo, Japan). Plasma insulin concentrations were determined by Immulite 2000 that uses two-site chemiluminescent immunometric assay. Insulin resistance was calculated by using homeostasis model assessment insulin resistance index (HOMA-IR) [11] according to the following formula:
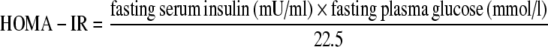 |
ApoE gene genotyping
Genomic DNA was extracted from peripheral leukocytes of the subjects using the High Pure PCR Template Preparation Kit (Roche Applied Science). For the detection of the presence of the three ApoE, E alleles E2, E3 and E4 (codon 112 and 158) were analyzed by the commercial LightCycler ApoE Mutation Detection Kit (Roche Diagnostics, Mannheim, Germany).
All experiments were carried out on LightCycler™ Instrument (Roche Applied Science, Germany) according to the protocols provided by manufacturer. Polymorphic alleles were identified by specific melting temperature (Tm) of the resulting amplicons. In the case of ApoE, codon 112 and 158 were analyzed simultaneously and therefore two Tm values were obtained for each allele: for E2, these were 56°C and 57.5°C; for E3, 56°C and 66°C; and for E4, 62.5°C and 66°C respectively.
Statistical analysis
SPSS 14.0 for Windows (SPSS Inc. Chicago, USA) was used for statistical analysis of the results. P < 0.05 values were accepted as statistically significant. The characteristics of the patients with PCOS and the mean plasma homocystein, glucose, and insulin, dehydroepiandrosterone sulphate, 17ß-estradiol, homocystein, 17-hydroxyprogesterone, prolactin, testosterone levels between the two clinical groups were compared by Student’s t test for unpaired data and between and within the different groups (E2/E3, E3/E3, E3/E4) of ApoE genotypes with the ANOVA. To determine which of the parameters, for which Student’s t test found a statistically significant difference between patient and control groups, was more strongly associated with PCOS, we performed univariate logistic regression analysis. Differences in the genotype distribution between different groups were assessed by logistic regression analysis of heterogeneity. All results were expressed as means ± SD.
Results
Biochemical and hormonal parameters of the patients with PCOS and the control group are seen in Table 1. The levels of total cholesterol (p = 0.004), LDL-cholesterol (p = 0.023), triglyceride (p = 0.004), fasting glucose (p < 0.001), fasting insulin (p < 0.001), homocystein (p < 0.001), fibrinogen (p < 0.001), FSH, LH, 17-OHP and free-testosterone (p < 0.001) in patients with PCOS were determined to be statistically significantly high. Statistically significant difference was not determined for age and BMI between both groups. Again, no statistically significant difference was determined between control and patient groups for DHEAS, estradiol, total testosterone, prolactin, HDL-cholesterol, and hs-CRP levels.
Table 1.
Clinical characteristics of patients and controls
| Characteristic | Patients * n = 129 | Control* n = 91 | P& |
|---|---|---|---|
| Age (years) | 24.58 ± 4.61 | 25.48 ± 3.38 | 0.115 |
| BMI (k/m2) | 24.47 ± 4.64 | 24.2 ± 3.31 | 0.65 |
| Total-cholesterol (mg/dl) | 199.86 ± 38.82 | 187.33 ± 13.77 | 0.004 |
| LDL-C (mg/dl) | 117.75 ± 29,35 | 110.31 ± 11.40 | 0.023 |
| HDL-C (mg/dl) | 56.76 ± 13.89 | 57.33 ± 4.49 | 0.705 |
| Triglycerides (mg/dl) | 122.34 ± 55.27 | 115.09 ± 24.07 | 0.004 |
| Fasting Glucose (mg/dl) | 91.43 ± 7.55 | 88.08 ± 3.96 | < 0.001 |
| Fasting insulin (mIU/ml) | 12.16 ± 9.07 | 4.71 ± 1.17 | < 0.001 |
| Homocysteine (µmol/L) | 2.63 ± 1,60 | 1.33 ± 0.22 | < 0.001 |
| FSH (mIU/ml) | 5.43 ± 1,77 | 4.17 ± 1.24 | < 0,001 |
| LH (mIU/ml) | 6.53 ± 3.68 | 4.18 ± 0.99 | < 0.001 |
| Estradiol (pg/ml) | 34.90 ± 20.83 | 39.02 ± 32.35 | 0.254 |
| DHEA-S (µg/dl) | 225.92 ± 89.32 | 222.29 ± 42.84 | 0.721 |
| 17-OHP (ng/ml) | 1.72 ± 0.87 | 0.90 ± 0.31 | < 0.001 |
| Total-Testosterone (ng/ml) | 0.99 ± 1.36 | 1.07 ± 0.69 | 0.632 |
| Free-Testosterone (pg/ml) | 3.39 ± 1,80 | 1,52 ± 0.40 | < 0.001 |
| Prolactine (pmol) | 17.08 ± 7.27 | 15.51 ± 4.49 | 0.07 |
| Fibrinogen (mg/dl) | 367.00 ± 75.07 | 277.12 ± 44.07 | < 0.001 |
| hs-CRP(mg/dl) | 0.41 ± 0.63 | 0.29 ± 0.22 | 0.730 |
*mean ± SD
&2-tailed t test
P < 0.05 values were accepted as statistically significant
ApoE genotype distribution in patient and control groups is seen in Table 2. There is no patient or healthy subject with E2/E2 and E4/E4 genotypes in our study. E2/E3 and E3/E3 genotypes were determined to be higher in control group (p < 0.001) and E3/E3 was determined to be higher in the group of patients with PCOS (p < 0.001, OR: 6.34, CI: 2.93–13.74). However, E3/E4 genotype was determined to be at similar levels in patient and control groups (p = 0.07, OR: 1.94, CI: 0.67–5.66). The ApoE3 allele showed a significantly higher frequency in PCOS patient group (91.8%) when compared with control group (75.6%) (p < 0.001). The ApoE2 allele showed a significantly higher frequency in the control group (16.7%) when compared with patient group with PCOS (4.3%) (p < 0.001). In this study, ApoE4 allele frequency was not statistically significantly different in patient and control groups (p > 0.513).
Table 2.
Distrubution of ApoE haplotypes and genotypes
| Haplotypes/Genotypes | Patients* | Control* | p& | OR& | 95% CI& |
|---|---|---|---|---|---|
| E2 | 11 | 30 | < 0.001 | ||
| 4.3% | 16.7% | R | |||
| E3 | 235 | 136 | < 0.001 | ||
| 91.8% | 75.6% | 0.212 | 0.103–0.437 | ||
| E4 | 10 | 14 | 0.513 | ||
| 3.9% | 7.8% | 0.513 | 0.177–1.490 | ||
| E2/E3 | 11 | 31 | < 0.001 | ||
| 8.6% | 33.3% | R | |||
| E3/E3 | 108 | 46 | < 0.001 | ||
| 83.6% | 51.1% | 6.34 | 2.93–13.74 | ||
| E3/E4 | 10 | 14 | 0.072 | ||
| 7.8% | 15.6% | 1.94 | 0.67–5.66 |
P < 0.05 values were accepted as statistically significant
*% frequency
&Logistic regression analysis
Metabolic and hormonal levels were analyzed in patients with PCOS according to different allele situations (E2, E3, E4) of Apo E. (Table 3). As a result of this analysis, a relation was found only between Apo E alleles and prolactin and LH levels. LH levels were found to be low in patients with PCOS in the existence of E3 and E4 allele (p = 0.018). However, prolactin levels was statistically lower in the ApoE4 allele-carrying PCOS patients (p = 0.046).
Table 3.
Biochemical and Hormonal parameters between ApoE haplotypes in patient group
| Haplotypes and parameters (Mean ± SD) | ||||
|---|---|---|---|---|
| E2 | E3 | E4 | P value | |
| Total-cholesterol (mg/dl) | 188.20 ± 40.13 | 200.36 ± 39.43 | 207.40 ± 30.45 | 0.503 |
| LDL- cholesterol (mg/dl) | 111.20 ± 29.32 | 118.12 ± 29.82 | 121.00 ± 25.76 | 0.712 |
| HDL- cholesterol (mg/dl) | 59.00 ± 16.18 | 55.66 ± 12.37 | 66.00 ± 22.68 | 0.067 |
| Triglycerides (mg/dl) | 110.50 ± 45.34 | 125.00 ± 54.90 | 106.90 ± 69.22 | 0.468 |
| Fasting Glucose (mg/dl) | 92.70 ± 6.91 | 91.03 ± 7.32 | 94.30 ± 10.37 | 0.360 |
| Basal Insulin (mIU/ml) | 11,20 ± 7..61 | 12.18 ± 9.56 | 13.10 ± 5.32 | 0.893 |
| Homocysteine(µmol/L) | 2.73 ± 1.62 | 2.57 ± 1.61 | 3.13 ± 1.49 | 0.569 |
| FSH (mIU/ml) | 5.48 ± 1.80 | 5.54 ± 1.74 | 4.19 ± 1.80 | 0.069 |
| LH (mIU/ml) | 8.50 ± 5.16 | 6.29 ± 3.33 | 6.98 ± 5.00 | *0.018 |
| Estradiol (pg/ml) | 38.20 ± 20.70 | 33.53 ± 19.79 | 46.00 ± 29.43 | 0.167 |
| DHEA-S (µg/dl) | 247.11 ± 127.18 | 222.39 ± 86.19 | 240.30 ± 78.69 | 0.597 |
| 17-OHP (ng/ml) | 1.98 ± 1.25 | 1.74 ± 0.85 | 1.29 ± 0.51 | 0.177 |
| Total-Testosterone (ng/ml) | 0.86 ± 0.29 | 1.04 ± 1.48 | 0.63 ± 0.20 | 0.503 |
| Free-Testosterone (pg/ml) | 3.30 ± 1.53 | 3.48 ± 1.84 | 2.61 ± 1.52 | 0.343 |
| Prolactine(pmol) | 18.36 ± 10.55 | 17.33 ± 7.09 | 13.00 ± 3.09 | *0.046 |
| Fibrinogen (mg/dl) | 343.81 ± 72.34 | 369.50 ± 74.70 | 365.70 ± 85.13 | 0.560 |
*P < 0.05 values were accepted as statistically significant
Discussion
In the present study, we compared the frequency of the ApoE gene polymorphism in PCOS patients and healthy controls living in Western Turkey. Women with PCOS are more prone to have metabolic syndrome than healthy women [12]. Additionally, different markers of atherosclerosis, such as fibrinogen, high sensitive C-reactive protein, homocysteine, oxidative stress markers, PAI-1, TPA, carotid intimae-media thickness, and echocardiographic findings have also been found to be changed [13–16]. Dyslipidemia is very common and includes elevated triglyceride levels and low high-density lipoprotein (HDL) cholesterol concentrations in PCOS patients [17].
Dyslipidemia may be the most common metabolic abnormality in PCOS, although the type and extent of the findings have been variable. PCOS patients have an atherogenic lipid profile characterized by lower high-density lipoprotein (HDL) cholesterol and/or HDL2 cholesterol levels, and higher triglyceride and low-density lipoprotein (LDL) cholesterol levels than the age- and weight-matched control women [18]. Thus, the presence of abdominal obesity, insulin resistance and dyslipidemia predispose women with PCOS to cardiovascular diseases (CVDs) [19].
Another study, found that E4 carriers carry a higher risk of hyperuricaemia and postprandial hypertriglyceridaemia after a fat excess in patients with metabolic syndrome [35]. Some researchers described a connection between E4 variant and increased fasting plasma glucose levels and plasma insulin levels [20, 21]. In our study, E4 allele frequency was determined to be similar in patient and control groups. Our results demonstrate a statistically significant increase in triglyceride, LDL-cholesterol and total cholesterol levels of the PCOS patients when compared to those of controls. However, no statistically significant difference was found in LDL-cholesterol, total cholesterol, triglyceride, and HDL-cholesterol levels in E3, E4, and E2 alleles of ApoE gene. Some researchers presented similar study and they demonstrated similar results in patients with PCOS from Finland [22]. On the other hand, in Western Anatolia ApoE4 allele frequency (3.9%) was lower than that of Finland (17.2%) in women with polycystic ovary syndrome.
Increasing evidence suggests that atherosclerosis is a chronic inflammatory process and due to this fact the markers of the inflammatory response such as CRP and fibrinogen might be useful in assessing the risk of cardiovascular disease [23]. Women’s Health Study has shown that CRP is a strong independent risk factor for cardiovascular diseases in females [24]. Endothelial dysfunction and high concentrations of high-sensitivity CRP were detected even in normal-weight women with PCOS [25].
No statistical correlation was found between different alleles of Apo gene and homocysteine, fibrinogen, and hs-CRP in our current study. The Asian people usually have lesser apo ε4 frequency than European people. The APOE4 allele has also been connected to increased plasma LDL-C concentrations in healthy subjects [26, 27]. The negative effects of lifestyle factors can interact with genetic factors (e.g. ApoE4), therefore increasing cardiovascular risk [28, 29]. These values are similar to those in other populations, such as Italians (ApoE4: 9.4%) and the French (ApoE4: 12.0%) [30, 31]. In our study, the ApoE2 allele frequency in western Turkish healthy population (16.7%) was found to be higher than in Caucasian (8.0%) and Greek populations (8.1%) [32, 33].
The occurrence of the ApoE4 allele may therefore contribute to the variation in the risk of these diseases such as coronary artery disease, hyperlipidemia, type 2 diabetes, metabolic syndrome, and polycystic ovary syndrome across populations. ApoE4 allele frequency was not determined to be higher in patients with PCOS than the controls in our study.
The ApoE3 allele is the most common allele in each population [34]. The ApoE3 allele showed a significantly higher frequency in the PCOS patient group when compared with the control group (p < 0.001). E3/E3 genotype of ApoE gene in patients with polycystic ovary syndrome was found to be statistically significantly higher than that of healthy control group in this study. But no relation was found between ApoE gene E3/E3 and lipid parameters and cardiovascular risk factors such as hs-CRP, fibrinogen, and homocysteine. However, there is a need for long-term prospective studies for explaining the relation between ApoE3 allele elevation and reduction of cardiovascular disease risk in patients with PCOS.
Although there was a genotype and allele difference between control and patient group in this study, no statistically significant change was determined in lipid and other cardiovascular risk factors in connection with allele and genotype. ApoE gene polymorphism has no effect on cardiovascular risk factors such as lipid and hs-CRP, fibrinogen, and homocysteine in Turkish patients with PCOS.
Acknowledgements
We would like to thank Hatice Uluer for her assistance in statistical evaluation of this study. We are thankful to Technicians of Nail Tartaroglu Endocrinology Laboratory for their assistance in coordinating this study.
Abbreviations
- ApoE
Apolipoprotein E
- PCOS
polycystic ovary syndrome
- BMI
body mass index
- CI
confidence interval
- CV
coefficient(s) of variation
- MLRA
Multiple Logistic Regression Analysis
- CVD
cardiovascular disease
- DHEAS
dehydroepiandrosterone sulphate
- E2
17ß-estradiol
- Hcy
homocysteine
- 17-OHP
17-hydroxyprogesterone
- P
progesterone
- PRL
prolactin
- T
testosterone
- FSH
follicle stimulating hormone
- LH
luteinizing hormone
- HOMA-IR
homeostasis model of assessment insulin resistance
References
- 1.Chang RJ. A practical approach to the diagnosis of polycystic ovary syndrome. Am J Obstet Gynecol. 2004;191(3):713–7. doi:10.1016/j.ajog.2004.04.045. [DOI] [PubMed]
- 2.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi:10.1210/jc.83.9.3078. [DOI] [PubMed]
- 3.Holte JL, Gennarelli G, Wide L, Lithell H, Berne C. High prevalence of polycystic ovaries and associated clinical, endocrine, and metabolic features in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 1998;83:1143–50. doi:10.1210/jc.83.4.1143. [DOI] [PubMed]
- 4.Wild RA. Obesity, lipids, cardiovascular risk, and androgen excess. Am J Med. 1995;98:27S–32S. doi:10.1016/S0002-9343(99)80056-4. [DOI] [PubMed]
- 5.Chambers JC, Kooner JS. Homocysteine: a novel risk factor for coronary heart disease in UK Indian Asians. Heart. 2001;86(2):121–2. doi:10.1136/heart.86.2.121. [DOI] [PMC free article] [PubMed]
- 6.Utermann G. Apolipoprotein E polymorphism in health and disease. Am Heart J. 1987;113:433–40. doi:10.1016/0002-8703(87)90610-7. [DOI] [PubMed]
- 7.Reilly SL, Ferrell RE, Sing CF. The gender-specific apolipoprotein E genotype influence on the distribution of plasma lipids and apolipoproteins in the population of Rochester, MN. III. Correlations and covariances. Am J Hum Genet. 1994;55(5):1001–18. [PMC free article] [PubMed]
- 8.Smith JD. Apolipoprotein E4: an allele associated with many diseases. Ann Med. 2000;32:118–27. doi:10.3109/07853890009011761. [DOI] [PubMed]
- 9.Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–54. doi:10.1016/j.tibs.2006.06.008. [DOI] [PubMed]
- 10.The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7. doi:10.1093/humrep/deh098. [DOI] [PubMed]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi:10.1007/BF00280883. [DOI] [PubMed]
- 12.Bhattacharya SM. Metabolic syndrome in females with polycystic ovary syndrome and International Diabetes Federation criteria. J Obstet Gynaecol Res. 2008;34(1):62−6. [DOI] [PubMed]
- 13.Tarkun I, Arslan BC, Cantürk Z, Türemen E, Sahin T, Duman C. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89(11):5592–6. doi:10.1210/jc.2004-0751. [DOI] [PubMed]
- 14.Tarkun I, Cetinarslan B, Türemen E, Sahin T, Cantürk Z, Komsuoglu B. Effect of rosiglitazone on insulin resistance, C-reactive protein and endothelial function in non-obese young women with polycystic ovary syndrome. Eur J Endocrinol. 2005;153(1):115–21. doi:10.1530/eje.1.01948. [DOI] [PubMed]
- 15.Karadeniz M, Erdogan M, Berdeli A, Tamsel S, Saygili F, Yilmaz C. The progesterone receptor PROGINS polymorphism is not related to oxidative stress factors in women with polycystic ovary syndrome. Cardiovasc Diabetol. 2007;5(6):29. doi:10.1186/1475-2840-6-29. [DOI] [PMC free article] [PubMed]
- 16.Karadeniz M, Erdogan M, Berdeli A, Saygili F, Yilmaz C. 4G/5G polymorphism of PAI-1 gene and Alu-repeat I/D polymorphism of TPA gene in Turkish patients with polycystic ovary syndrome. J Assist Reprod Genet. 2007;24(9):412–8. doi:10.1007/s10815-007-9160-7. [DOI] [PMC free article] [PubMed]
- 17.Pirwany IR, Fleming R, Greer IA, Packard CJ, Sattar N. Lipids and lipoprotein subfractions in women with PCOS: relationship to metabolic and endocrine parameters. Clin Endocrinol (Oxf). 2001;54(4):447–53. doi:10.1046/j.1365-2265.2001.01228.x. [DOI] [PubMed]
- 18.Wild RA, Painter PC, Coulson PB, Carruth KB, Ranney GB. Lipoprotein lipid concentrations and cardiovascular risk in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1985;61:946–51. [DOI] [PubMed]
- 19.Ehrmann DA, Schneider DJ, Sobel BE, Cavaghan MK, Imperial J, Rosenfield RL, Polonsky KS. Troglitazone improves defects in insulin action, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2108–16. doi:10.1210/jc.82.7.2108. [DOI] [PubMed]
- 20.Scuteri A, Najjar SS, Muller D, Andres R, Morrell CH, Zonderman AB, Lakatta EG. ApoE4 allele and the natural history of cardiovascular risk factors. Am J Physiol Endocrinol Metab. 2005;289:E322–7. doi:10.1152/ajpendo.00408.2004. [DOI] [PubMed]
- 21.Stiefel P, Montilla C, Muniz-Grijalvo O, Garcia-Lozano R, Alonso A, Miranda ML, Pamies E, Villar J. Apolipoprotein E gene polymorphism is related to metabolic abnormalities, but does not influence erythrocyte membrane lipid composition or sodium-lithium countertransport activity in essential hypertension. Metabolism. 2001;50:157–60. doi:10.1053/meta.2001.19429. [DOI] [PubMed]
- 22.Heinonen S, Korhonen S, Hippeläinen M, Hiltunen M, Mannermaa A, Saarikoski S. Apolipoprotein E alleles in women with polycystic ovary syndrome. Fertil Steril. 2001;75(5):878–80. doi:10.1016/S0015-0282(01)01691-0. [DOI] [PubMed]
- 23.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi:10.1056/NEJM199901143400207. [DOI] [PubMed]
- 24.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. [DOI] [PubMed]
- 25.Atiomo WU, Bates SA, Condon JE, Shaw S, West JH, Prentice AG. The plasminogen activator system in women with polycystic ovary syndrome. Fertil Steril. 1998;69:236–41. doi:10.1016/S0015-0282(97)00486-X. [DOI] [PubMed]
- 26.Wilson PWF, Schaefer EJ, Larson MG, Ordovas JM. Apolipoprotein E alleles and risk of coronary disease: a meta-analysis. Arterioscler Thromb Vasc Biol. 1996;16:1250–5. [DOI] [PubMed]
- 27.Srinivasan SR, Ehnholm C, Elkasabany A, Berenson G. Influence of apolipoprotein E polymorphism on serum lipids and lipoprotein changes from childhood to adulthood the Bogalusa Heart study. Atherosclerosis. 1999;143:435–43. doi:10.1016/S0021-9150(98)00304-9. [DOI] [PubMed]
- 28.Komatsu F, Hasegawa K, Watanabe S, Kawabata T, Yanagisawa Y, Kaneko Y, Miyagi S, Sakuma M, Kagawa Y, Ulziiburen C, Narantuya L. Comparison of electrocardiogram findings and lifestyles between urbanized people and ger-living people in Ulaanbaatar, Mongolia. Atherosclerosis. 2004;175:101–8. doi:10.1016/j.atherosclerosis.2004.03.005. [DOI] [PubMed]
- 29.Ordovas JM. Nutrigenetics, plasma lipids, and cardiovascular risk. J Am Diet Assoc. 2006;106:1074–81. doi:10.1016/j.jada.2006.04.016. [DOI] [PubMed]
- 30.James RW, Boemi MG, Giansanti R, Fumelli P, Pometta D. Underexpression of the apolipoprotein E4 isoform in an Italian population. Arterioscler Thromb. 1993;13:1456–9. [DOI] [PubMed]
- 31.Bailleul S, Couderc R, Landais V, Lefevre G, Raichvarg D, Etienne JV. Direct phenotyping of human apolipoprotein E in plasma: application to population frequency distribution in Paris (France). Hum Hered. 1993;43:159–65. doi:10.1159/000154172. [DOI] [PubMed]
- 32.Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8(1):1–21. [DOI] [PubMed]
- 33.Kolovou GD, Daskalova DC, Hatzivassiliou M, Yiannakouris N, Pilatis ND, Elisaf M, Mikhailidis DP, Cariolou MA, Cokkinos DV. The epsilon 2 and 4 alleles of apolipoprotein E and ischemic vascular events in the Greek population-implications for the interpretation of similar studies. Angiology. 2003;54(1):51–8. doi:10.1177/000331970305400107. [DOI] [PubMed]
- 34.Sklavounou E, Economou-Petersen E, Karadima G, Panas M, Avramopoulos D, Varsou A, Vassilopoulos D, Petersen MB. Apolipoprotein E polymorphism in the Greek population. Clin Genet. 1997;52(4):216–8. [DOI] [PubMed]
- 35.Olivieri O, Martinelli N, Bassi A, Trabetti E, Girelli D, Pizzolo F, Friso S, Pignatti PF, Corrocher R. ApoE epsilon2/epsilon3/epsilon4 polymorphism, ApoC-III/ApoE ratio and metabolic syndrome. Clin Exp Med. 2007;7(4):164–72. doi:10.1007/s10238-007-0142-y. [DOI] [PubMed]


