Abstract
Purpose
To investigate immunostaining pattern of caspase-3, an apoptosis marker, and vascular endothelial growth factor (VEGF), an hypoxia marker in testis biopsy specimens collected either from smoking or non-smoking patients with azoospermia.
Methods
Testis biopsy specimens were obtained from thirty seven non-smoker and thirty eight smoker patients. Using immunochemistry technique, caspase-3 and VEGF were evaluated in all intratubular spermatogenic and interstitial Leydig cells.
Result(s)
Caspase-3 expression was significantly increased in germ cells in maturation arrest specimens in smoker azoospermic patients. No statistically significant difference was present between smokers and non-smokers for caspase-3 expression in Sertoli cell. However, the VEGF immunopositive Leydig cells were statistically higher in smokers. There were no differences between groups in terms of germ cell immunopositivity.
Conclusion
Our results support the hypothesis that increased apoptosis contributes significantly to impaired spermatogenesis. We conjecture that germ cell apoptosis may be augmented by hypoxic microenvironments and environmental toxicants in smoking azoospermic men.
Keywords: Azoospermia, Caspase-3, Apoptosis, VEGF, Hypoxia
Introduction
Spermatogenesis is a complex process among germ cells, in which spermatogonial stem cells undergo many cycles of mitotic and meiotic cell division and differentiation to generate mature haploid spermatozoa [1]. Azoospermia, defined as the complete absence of sperm in the ejaculate, is present in less than 1% of all men, but in 10–15% of all infertile men. There are many causes of azoospermia, but obstruction of the ductal system is responsible for approximately 40% of cases [2]. Non-obstructive azoospermia is caused by severe impairment of spermatogenesis, and is considered the most critical case of male infertility. Despite remarkable advances in the treatment of severe male factor infertility, many patients cannot be treated effectively, because they lack the ability to produce mature sperm.
Apoptosis is an active phenomenon resulting in cell death, and is crucial in maintaining the integrity of an organ [3]. Apoptosis is a common phenomenon during spermatogenesis, and its dysregulation has been associated with male infertility. Over-proliferation of early testicular germ cells is tempered by selective apoptosis normally and continuously throughout life [4, 5].
The roles of caspases in sperm differentiation and testicular maturity are of considerable interest. Two major pathways (intrinsic and extrinsic) are involved in the process of caspase activation and apoptosis in mammalian cells. The extrinsic pathway is characterized by the oligomerization of death receptors, such as Fas or tumor necrosis factor, followed by the activation of caspase-8 and caspase-3. The intrinsic pathway involves the activation of procaspase-9, which in turn activates caspase-3 [6]. Increased testicular apoptosis has been observed during maturation arrest and hypo-spermatogenesis states in rodent models, but little currently is known about its role in human testes with abnormal apoptosis [7].
Cigarette smoking is quite prevalent in the general population; approximately 35% of reproductive age men in the United States smoke cigarettes and 45% of men in Turkey [8]; but our knowledge of its effect on male reproductive function remains very limited. Smoking seems to impair sperm production and epididymal as well as accessory sex gland function, and could be one of the factors contributing to regional differences in sperm parameters [9, 10]. In recent studies, men whose mothers smoked during pregnancy have been noted to have lower sperm production, and to demonstrate increased apoptosis during abnormal spermatogenesis via several moleculer mechanisms [11]. Moreover, peripheral vasoconstriction, induced by the adrenergic effects of nicotine via smoking, may contribute to the observed decrease in subcutaneous tissue oxygen. Therefore, cigarette smoking decreases tissue oxygen [12]. Hypoxia, in turn, has been shown to stimulate the expression of vascular endothelial growth factor (VEGF), which is a major mediator of angiogenesis and vasculogenesis. During hypoxia, VEGF promotes angiogenesis in the testis. However, the effect of VEGF on cell apoptosis among seminiferous tubul germ cells remains unclear.
The primary purpose of our study was to investigate smoking-related immunochemical expression of caspase-3 related apoptotic changes and VEGF in testis tissue from male patients with obstructive and non-obstuctive azoospermia.
Materials and methods
Patients and tissue preparation
Biopsy materials were taken from patients with a normal karyotype who attended the ART (Assisted Reproduction Techniques) Clinic at Zekai Tahir Burak Women’s Health, Education and Research Hospital. Prior to any data collection, the experimental protocol was reviewed and approved by the local ethics committee.
A total of 75 men with azoospermia aged between 23 and 45 years were included in the study and assigned to two groups: non-smokers (Group 1; n═37) and smokers (Group2; n═38). In Group 2, current smokers, who had been smoking for at least 5 years and had a habit of smoking at least ten cigarettes per day, were included. Patients included in both groups had no health problems other than azospermia. Using the open micro-testiculer biopsy technique, testicular specimens were obtained from the patients with obstructive or nonobstructive azoospermia.. As a routine procedure of our clinic, informed consents were obtained from all patients prior to participation in the study.
Immunohistochemistry
Immediately after surgical removal, tissue samples were fixed in Bouine solution for about 72 h. The samples were dehydrated through a graded ethanol series and embedded in paraffin wax for conventional histological diagnosis. 4 µm thick sections were mounted onto glass slides, treated with poly-L-lysine, and then incubated overnight at 37°C and subsequently for 1h at 60°C. Following two changes of xylol applications (15 min each), slides were immersed in 96% absolute alcohol and 80% ethanol for 10 min, followed by immersion in distilled water, twice for 5 min. Slides were heated in a high-temperature microwave oven for antigen retrieval. Upon cooling at room temperature for 20 min, the tissues on the glass slides were circled with a pap-pen (hydrophobic pen). Upon washing the slides with distilled water and phosphate-buffered saline (PBS), hydrogen peroxide (Fisher Scientific, Melrose Park. IL) was added dropwise. After washing with PBS, an ultra V block was applied. The tissues were then incubated for 1 h with either primary antibody (Caspase-3 Catalog #RB-1197, Vascular Endothelial Growth Factor-VEGF Ab-1 Catalog #RB-222 Thermo Scientific, 47777Warm Spring Blvd, Fremont CA,94539, USA) diluted with antibody diluent (Ultra Ab Atibody Diluent catalog # TA-125-UD, Thermo Scientific, 47777 Warm Spring Blvd, Fremont CA,94539, USA). After rewashing with PBS, the specimens were placed in AEC (3-Amino-9-Ethyl Carbazole, Substrate system, Cat number TA-060-HA, Lab Vision, USA) chromogen for 10 min. Finally, the slides were counterstained with Mayer’s hematoxilyn for 2 min and coveslipped with Ultra mount (Lab Vision, Fremont, CA, USA). Slides were evaluated using a Leica DM 4000 B light microscope (Wetzlar, GERMANY).
Microscopic analysis
All intratubular spermatogenic and Sertoli cells in ten tubules were evaluated randomly visualized at X400 magnification, using a Leica DM 4000 B (Wetzlar, GERMANY) image analysing microscope. The number of apoptotic bodies was determined as [1] the total number of apoptic cells and [2] the number of apoptic Sertoli cells within the seminiferous tubule, so as to provide apoptotic indices [13]. For quantitative evaluation, the percentage of caspase-3 immunopositive cells for every 300 cells of each cell type (Sertoli cells, germ cells, and interstitial Leydig cells) was calculated for each patient.
All tissues were evaluated by the same histologist, who was blinded to the clinical characteristics of the patients to prevent ascertainment bias. The relative intensity of VEGF immunoreactivity staining was assessed quantitatively, as previously described by McCarty et al. [14], taking into account both the intensity and the distribution of specific staining. A histological score (HSCORE) was calculated as the sum of the percentages of positively-stained epithelial cells multiplied by the weighted intensity of staining: HSCORE = ∑Pi(I + 1), where ‘I’ represents staining intensity (0=no expression, 1=mild, 2=moderate, and 3=intense) and ‘Pi’ is the percentage of stained cells for each intensity [15].
Statistical analysis
Data analyses were performed using SPSS for Windows, version 11.5. Whether the distributions of continuous and discreet variables were normal or not was determined by means of the Kolmogorov Smirnov test. Data were presented as mean ± standard deviation (stdev) for numerical data as well as percentages for nominal data. Differences between groups (e.g., smokers vs. non-smokers) were evaluated by Pearson’s chi-square or Mann Whitney U tests, where appropriate. The degree of association between continuous variables was calculated using Pearson's correlation coefficients. A p value of less than 0.05 was considered statistically significant.
Results
Data were obtained from 75 biopsy specimens, collected from 37 non-smokers (Group 1) and 38 smokers (Group 2) with obstructive or non-obstructive azoospermia. The mean ages of smokers and non-smokers were 32.3 ± 6 years and 32.4 ± 6, respectively. Among the 37 patients in Group 1, 11 had obstructive and 26 had non-obstructive azoospermia. In Group 2, 10 patients had obstructive and 28 had non-obstructive azoospermia. Histopathological evaluation was defined as normal spermatogenesis, maturation arrest, and Sertoli cell only syndrome.
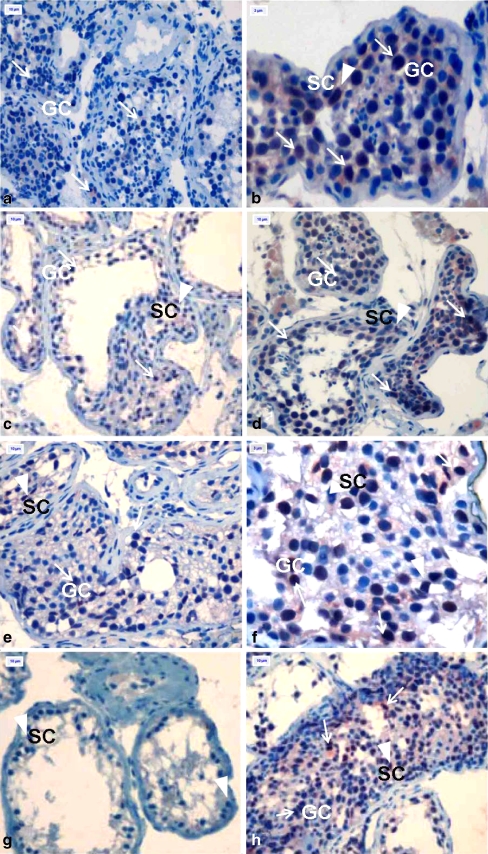
Immunostaining in all specimens was accomplished by use of caspase-3 and VEGF immunohistochemistry kits. The average percentages of apoptotic cells for every 300 are shown in Table 1. In samples of patients with normal spermatogenesis; germ cells, spermatids, and Sertoli cells were observed in seminiferous tubules. There were no statistically significant differences in terms of Sertoli cell apoptosis between Groups 1 and 2 with respect to normal spermatogenesis, maturation arrest, or Sertoli cell only syndrome (p = 0.827, 0.593, 0.389, respectively) (Fig. 1a-h). The degree of germ cell apoptosis was not statistically different between non-smokers and smokers with normal spermatogenetic histology, the frequency of apoptotic cells in smokers was greater (p = 0.05) (Fig. 1a and b). In cases of maturation arrest, increased germ cell apoptosis was observed in smokers versus non-smokers (p = 0.037) (Fig. 1c, d, e, f, h).
Table 1.
Quantitative analysis of immunopositivity from non-smoking (group1) and smoking (group2) azoospermic men with different histologic diagnoses
| Groups | Apoptotic index (%) | VEGF imm.pos. (HSCORE Mean ±SD) | |||
|---|---|---|---|---|---|
| Histologic Diagnosis | Sertoli C | Germ C. | Leydig C. | Germ C. | |
| Group 1 | Normal spermatogenesis (n:11) | 8.00 ± 1.26 | 8.00 ± 2.09 | 163.63 ± 40.31 | 218.18 ± 53.0 |
| Maturation arrest (n:14) | 34.64 ± 8.12 | 37.28 ± 6.28 | 141.42 ± 35.26 | 231.4 ± 66.1 | |
| Sertoli cells only (n:12) | 37.4 ± 6.92 | - | 170.0 ± 58.6 | - | |
| Group 2 | Normal spermatogenesis (n: 10) | 7.5 ± 1.58 | 33.4 ± 10.2 | 260 ± 31.62 | 224 ± 70.11 |
| Maturation arrest (n:16 ) | 34.81 ± 7.63 | 89.8 ± 14.81 | 268.7 ± 45.29 | 225 ± 51.51 | |
| Sertoli cells only (n:12) | 33.5 ± 8.90 | - | 268.3 ± 45.69 | - | |
Data are means ± SD. Three hundered cells were evaluated for each cell type in each patient. VEGF imm.pos.:Vasculer endothelial Growth factor Immunopositivity. C:Cell
Fig. 1.
In subjects with normal spermatogenesis, numbers of apoptotic spermatocytes and Sertoli cells were determined in seminiferous tubules in non-smokers. (Fig. 1). In smokers, increased apoptosis was observed in primary spermatocytes (Fig. 2). Caspase-3 imunoreaktivity in human testes from non-smokers and smokers. a Testis from a non-smoker with normal spermatogenesis (Group 1); b Testis from a smoker with normal spermatogenesis (Group 2); c, e Testis at different stages of maturation arrest (Group 1); d, f, h Testis at different stages of maturation arrest (Group 2); g Testis with Sertoli cell only syndrome (Group 1). NOTE: Arrows indicate immunopositive germ cells(GC) in seminiferous tubules, and arrow heads indicate immunopositive Sertoli cells(SC) in seminiferous tubules. Magnification was determined for ( A, C, D, E, G X 400) and (B, F X1000)
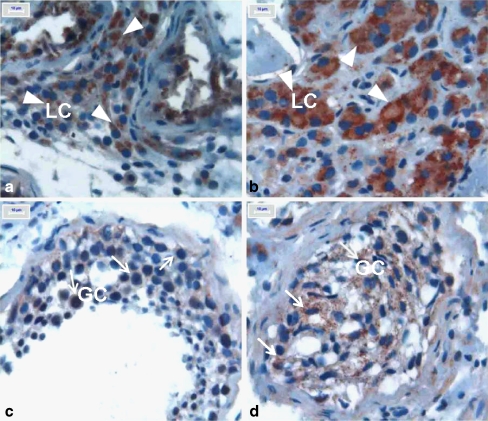
A widespread and variable cytoplasmic VEGF immunopositivity was observed in all seminiferous tubule cells, germ cells, Sertoli cells and peritubular Leydig cells, in both groups. In terms of VEGF immunopositivity, there were no statistically significant differences between the two groups for germ cells, either in maturation arrest or normal spermatogenetic patients (p = 0.972, 0.612, respectively) (Fig. 2c and d); however, there was a significant difference between the two groups for the Leydig cells.. The VEGF immunopositivity was siginificantly greater in Leydig cells of smokers with normal spermatogenesis and maturation arrest histology (p = 0.01 for both) (Fig. 2a and b).
Fig. 2.
In subjects with normal spermatogenesis, numbers of apoptotic spermatocytes and Sertoli cells were determined in seminiferous tubules in non-smokers. (Fig. 1). In smokers, increased apoptosis was observed in primary spermatocytes (Fig. 2). VEGF imunopositivity in testes from non-smokers and smokers. a Immunopositive interstitial Leydig cells (non-smokers, Group 1); b Immunopositive interstitial Leydig cells (smokers, Group 2); c Immunopositive germ cells in seminiferous tubule epithelium (Group 1); d Immunopositive germ cells(GC) in seminiferous tubule epithelium (Group 2). NOTE: Arrows indicate immunopositive germ cells and arrowheads indicate immunopositive Leidig cells(LC). Magnification was determined for A, B, C and D X1000
Discussion
In this study, we examined the incidence of apoptosis and the expressions of apoptosis regulator, caspase-3, in human testis with obstructive and non-obstructive azoospermia. Using an immunohistochemical method, this study demonstrated that the degree of apoptosis is significantly increased in histologic states of maturation arrest especially in smokers. Numerous studied have been conducted to evaluate the role of cigarette smoking on impaired spermatogenesis. Apart from those studies, this study evaluated the effect of smoking on apoptosis in a group of patients with azoospermia which, to our knowledge, was not undertaken before.
Three hypotheses have been postulated to explain the source of DNA damage in sperm. First, it is believed that sperm DNA damage is caused by improper packaging and ligation during sperm maturation [16]. Second, it is felt that oxidative stress causes DNA damage, and the increased levels of specific forms of oxidative DNA damage, like 8-hydroxydeoxyguanosine in sperm DNA, supports such a theory [17, 18]. Third, observed sperm DNA fragmentation is believed to be caused by apoptosis [19].
Cigarette smoke extract is abundant with oxidants and free radicals. Oxidative stress caused by cigarette smoking results in the destruction of cells. Oxidative stress caused by cigarette smoke extract may be the radical factor leading to apoptosis, as well as cell growth inhibition in cells [20]. Chronic exposure to cigarette smoke has been shown to induce apoptosis in rat testis. Apoptosis may be one of the pathogenic mechanisms responsible for defective spermatogenesis in the rats, following such chronic exposure to cigarette smoke [21]. In men, cigarette smoking reduces sperm production, increases oxidative stress, and promotes DNA damage. Spermatozoa from smokers have reduced fertilizing capacity, and embryos display lower implantation rates [22].
Recently, apoptosis also has been considered to play an important role in spermatogenesis in the human testis [23], and increased apoptosis has been found to occur during maturation arrest (MA), hypo-spermatogenesis, and Sertoli cell only syndrome [24, 25]. The results of our study support the conjecture that increased apoptosis contributes significantly to impaired spermatogenesis. Using immunohistochemical methods, we identified a significantly increased incidence of apoptosis evidenced by caspase-3 immunopositivity in testes of azoospermic smokers in maturation arrest, but we did not observe a significant change in normal spermatogenesis and Sertoli cell only tissue samples, relative to the same tissues in non-smokers.
Rajpurkar et al. have demonstrated the harmful effects of chronic inhalation of cigarette smoke on the testes of Sprague-Dawley rats [21, 26, 27]. They also demonstrated an association between chronic cigarette smoke inhalation and increases in the levels of oxidants, as well as simultaneous decreases in the levels of antioxidants in rat testis. This abnormality in the normal oxidant-antioxidant balance may be one of the mechanisms leading to testicular tissue damage and abnormal spermatogenesis in rat testis following chronic cigarette smoke exposure. A more recent study by the same group of investigators revealed that chronic cigarette smoke induces apoptosis in rat testis [21]. Apoptosis may be one of the pathogenic mechanisms responsible for defective spermatogenesis in the rat, following chronic cigarette smoke exposure.
In our study, similar to the study by Rajpurkar et al. [21, 26], the apopotic index was higher and caspase-3 immunopositivity was more intense in the germ cells of smokers with normal spermatogenesis compared to their non-smoking counterparts. The differences in these parameters were even more remarkable in maturation arrest. Spermatogenesis in the seminiferous tubules of the testis occurs at a high proliferation rate, suggesting considerable oxygen consumption [28]. Thus, cigarette smoking and chronic hypoxia can contribute to the impaired spermatogenesis in patients with azoospermia.
VEGF is a paracrine mitogenic and angiogenic factor, responsible for modulating the capillarization of human testicular tissue and maintaining the functions of testicular microvasculature. Endothelial cells in certain segments of human testicular microvasculature also stain positive for VEGF. In addition, VEGF may influence the permeability of capillaries passing through groups of Leydig cells, and of capillaries localized within the lamina propria of human seminiferous tubules. The differences in the expression patterns of VEGF receptors in human testicular tissue probably reflect different VEGF effects in different compartments of human testis. Moreover Leydig cells secrete angiogenic factors and are the source of inflammatory mediator(s) produced in the testis [29, 30].
Hypoxia has been shown to stimulate the expression of vascular endothelial growth factor (VEGF), which is a major mediator of angiogenesis and vasculogenesis. During hypoxia, VEGF promotes angiogenesis in the testis. Hypoxia, in turn, stimulates cell proliferation and testosterone release in Leydig cells via an increase of VEGF production [31]. In our study, we also found an increased VEGF immunoactivity in Leydig cells in the specimens of smokers. Such an increased expression may be due to hypoxic microenvironment related to smoking. We could not find a significant difference for VEGF immunoactivity in germ cells between groups. These findings were not in agreement with those of Marti et al., who assesed the effect of experimental systemic hypoxia on different organs [32]. However, our study evaluated chronic hypoxia in human testicular tissue caused by smoking. The increased apoptotic index in the smoking group suggests that smoking might destroy the microenvironment of testis tissue, as it does in other tissues.
We think that evaluating VEGF subgroup receptors in both intrinsic and extrinsic apoptosis pathways together, and the relationship between smoking, apoptosis, hypoxia and spermato-genesis across a broad spectrum of tissues, might tell us even more in the near future.
Footnotes
Capsule
The evaluation of caspase-3 and VEGF immunopositivity in smoking patients with azoospermia
Contributor Information
Sevtap Kilic, Phone: +90-312431-6162, FAX: +90312430-0718, Email: sevtapkilic@gmail.com.
Nese Lortlar, Email: doctornese@yahoo.com.
Yesim Bardakci, Email: yesimbardakci@yahoo.com.
Erkan Ozdemir, Email: dreroz@hotmail.com.
Beril Yuksel, Email: berilyuksel@gmail.com.
Ufuk Ozturk, Email: ufukozturk71@gmail.com.
Gurer Budak, Email: drgurerbudak@yahoo.com.
Muammer Dogan, Email: muadogan@gmail.com.
References
- 1.Rodriguez I, Ody CK, Araki C, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997;16:2262–70. doi:10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed]
- 2.Jarow JP, Espeland MA, & Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142–62–5. [DOI] [PubMed]
- 3.Lin WW, Lamb DJ, Wheeler TM, Lipshultz LI, Kim ED. In situ end-labeling of human testicular tissue demonstrates increased apoptosis in conditions of abnormal spermatogenesis. Fertil Steril. 1997;68:1065–89. doi:10.1016/S0015-0282(97)00372-5. [DOI] [PubMed]
- 4.Bartke A. Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon. Endocrinology. 1995;136:3–4. doi:10.1210/en.136.1.3. [DOI] [PubMed]
- 5.Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hseuh AJ. Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology. 1995;136:5–12. doi:10.1210/en.136.1.5. [DOI] [PubMed]
- 6.Moreno RD, Lizama C, Urzúa N, Vergara SP, Reyes JG. Caspase activation throughout the first wave of spermatogenesis in the rat. Cell Tissue Res. 2006;325(3):533–40. doi:10.1007/s00441-006-0186-4. [DOI] [PubMed]
- 7.Lin WW, Lamb DJ, Wheeler TM, Abrams J, Lipshultz LI, Kim ED. Apoptotic frequency is increased in spermatogenic maturation arrest and hypo-spermatogenic states. J Urol. 1997;158(5):1791–93. doi:10.1016/S0022-5347(01)64130-2. [DOI] [PubMed]
- 8.Kıter G, Başer S, Akdağ B, Ekinci A, Ünal N, Öztürk E. The characteristics of smoking habit among patients evaluated at our outpatient clinic. Tuberk Toraks. 2008;56(1):30–6. [PubMed]
- 9.Practice Committee of the American Society for Reproductive Medicine. Smoking and Infertility. Fertil Steril 2004;81(4):1181–6. doi:10.1016/j.fertnstert.2003.11.024. [DOI] [PubMed]
- 10.Richthoff J, Elzanaty S, Rylander L, Hagmar L, Giwercman A. Association between tobacco exposure and reproductive parameters in adolescent males. Int J Androl. 2008;31(1):31–9. [DOI] [PubMed]
- 11.Coutts SM, Fulton N, Anderson RA. Environmental toxicant-induced germ cell apoptosis in the human fetal testis. Hum Reprod. 2007;22(11):2912–18. doi:10.1093/humrep/dem300. [DOI] [PubMed]
- 12.Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126(9):1131–4. [DOI] [PubMed]
- 13.Kim SK, Yoon YD, Park YS, Seo JT, Kim JH. Involvement of the Fas-Fas ligand system and active caspase-3 in abnormal apoptosis in human testes with maturation arrest and Sertoli cell-only syndrome. Fertil Steril. 2007;87(3):547–53. doi:10.1016/j.fertnstert.2006.07.1524. [DOI] [PubMed]
- 14.McCarty KS Jr, Miller LS, Cox EB, et al. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 1985;109:716–21. [PubMed]
- 15.Budwit-Novotny A, McCarty KS, Cox EB, et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–25. [PubMed]
- 16.Sailer BL, Jost LK, Evenson DP. Mammalian sperm DNA susceptibility to in situ denaturation associated with the presence of DNA strand breaks as measured by the terminal deoxynuleatidyl transferase assay. J Androl. 1995;16:80–7. [PubMed]
- 17.Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod. 1998;13:896–900. doi:10.1093/humrep/13.4.896. [DOI] [PubMed]
- 18.Shen HM, Ong CN. Detection of oxidative and damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med. 2000;28:529–36. doi:10.1016/S0891-5849(99)00234-8. [DOI] [PubMed]
- 19.Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–7. doi:10.1530/ror.0.0040031. [DOI] [PubMed]
- 20.Stringer KA, Tobias M, O'Neill HC, Franklin CC. Cigarette smoke extract-induced suppression of caspase-3-like activity impairs human neutrophil phagocytosis. Am J Physiol Lung Cell Mol Physiol. 2007;292(6):L1572–9. doi:10.1152/ajplung.00325.2006. [DOI] [PubMed]
- 21.Rajpurkar A, Jiang Y, Dhabuwala CB, Dunbar JC, Li H. Cigarette smoking induces apoptosis in rat testis. J Environ Pathol Toxicol Oncol. 2002;21(3):243–8. [PubMed]
- 22.Soares SR, Melo MA. Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol. 2008;20(3):281–91. [DOI] [PubMed]
- 23.Tesarik J, Ubaldi F, Rienzi L, Martinez F, Lacobelli M, Mendoza C. Caspase-dependent and independent DNA fragmentation in Sertoli and germ cells from men with primary testicular failure: relationship with histological diagnosis. Hum Reprod. 2004;19:254–61. doi:10.1093/humrep/deh081. [DOI] [PubMed]
- 24.Lin WW, Lamb DJ, Wheeler TM, Abrams J, Lipshultz LI, Kim ED. Apoptotic frequency is increased in spermatogenic maturation arrest and hypospermatogenic states. J Urol. 1997;58:1791–3. doi:10.1016/S0022-5347(01)64130-2. [DOI] [PubMed]
- 25.Takagi S, Itoh N, Kimura M, Sasao T, Tsukamoto T. Spermatogonial proliferation and apoptosis in hypospermatogenesis associated with nonobstructive azoospermia. Fertil Steril. 2001;76:901–7. doi:10.1016/S0015-0282(01)02732-7. [DOI] [PubMed]
- 26.Rajpurkar A, Dhabuwala CB, Jiang Y, Li H. Chronic cigarette smoking induces an oxidant antioxidant imbalance in the testis. J Environ Pathol Toxicol Oncol. 2000;19(4):369–73. [PubMed]
- 27.Rajpurkar A, Li H, Dhabuwala CB. Morphometric analysis of rat testis following chronic exposure to cigarette smoke. J Environ Pathol Toxicol Oncol. 2000;19(4):363–8. [PubMed]
- 28.Wenger RH, Katschinski DM. The hypoxic testis and post-meiotic expression of PAS domain proteins. Semin Cell Dev Biol. 2005;16(4–5):547–53. doi:10.1016/j.semcdb.2005.03.008. [DOI] [PubMed]
- 29.Collin O, Bergh A. Leydig cells secrete factors which increase vascular permeability and endothelial cell proliferation. Int J Androl. 1996;19(4):221–8. doi:10.1111/j.1365-2605.1996.tb00466.x. [DOI] [PubMed]
- 30.Ergün S, Kiliç N, Fiedler W, Mukhopadhyay AK. Vascular endothelial growth factor and its receptors in normal human testicular tissue. Mol Cell Endocrinol. 1997;131(1):9–20. doi:10.1016/S0303-7207(97)00082-8. [DOI] [PubMed]
- 31.Hwang GS, Wang SW, Tseng WM, Yu CH, Wang PS. Effect of hypoxia on the release of vascular endothelial growth factor and testosterone in mouse TM3 Leydig cells. Am J Physiol Endocrinol Metab. 2007;292(6):E1763–9. doi:10.1152/ajpendo.00611.2006. [DOI] [PubMed]
- 32.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci USA. 1998;95(26):15809–14. doi:10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed]