At present, there is still some uncertainty as to whether lenalidomide should be used to treat patients who have myelodysplastic syndrome (MDS) with deletion del(5q).1 On the one hand lenalidomide has emerged as a promising new option in the therapy of myelodysplastic syndromes (MDS), being most effective in low-risk MDS patients who have an isolated deletion del(5q).1–3 On the other hand it is important to determine whether lenalidomide might increase the risk that the myelodysplasia could progress to acute myeloid leukemia, a circumstance inducing the refusal of marketing authorization for lenalidomide by the European Medicines Agency ( EMEA). Here, we present the clinical course and the results of cytogenetic follow-up including fluorescence in situ hybridization (FISH) on selected CD34+ progenitor cells of 3 patients with isolated deletion del(5)(q13q33) who were treated with lenalidomide. Two of the patients initially presented with erythrocyte transfusion-dependent MDS from diagnosis (UPN1, UPN2). One patient (UPN3) had refractory anemia with excess blasts >10% (RAEB 2), and was in complete hematologic remission after allogeneic hematopoietic stem cell transplantation (HSCT) but was threatened with an imminent relapse, as predicted by an increase of endogeneous CD34+ cells that still had the del(5q) leukemic abnormality.
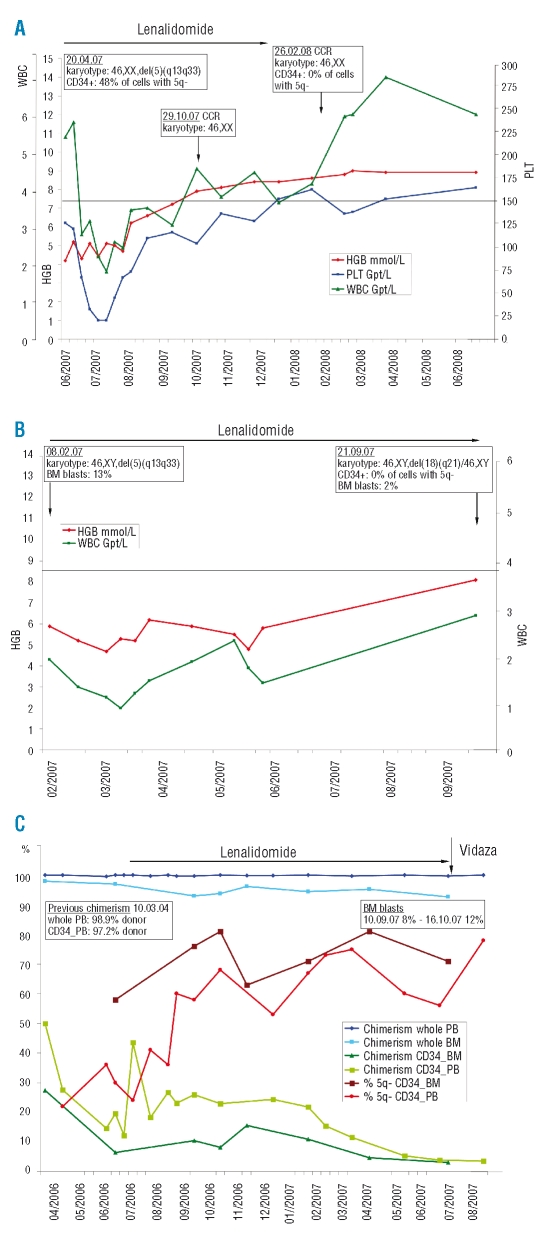
Briefly, the individual details and results were the following: UPN1: Patient UPN1 achieved transfusion independence, and hemoglobin returned to within the normal range two months after the start of lenalidomide therapy (Figure 1A). The 5q- progenitor cells were eradicated. Also, parallel histology did not detect any more features of MDS in the biopsy specimen. Therefore, lenalidomide was stopped. The patient is still in complete hematologic remission. UPN2: Like UPN1, patient UPN2 responded to lenalidomide with an increase in hemoglobin level (Figure 1B) and did not need further transfusions. In addition, the bone marrow blasts decreased to 2%. Cytogenetic investigation seven months after the start of lenalidomide therapy revealed that the 5q- cells had been eradicated from the CD34+ subset. However, an unrelated clone (46,XY,del(18)(q21)) had emerged. The deletion 18q was also confirmed by FISH in the del(5q) negative CD34+ cells. UPN3: Because of the allogeneic setting, patient UPN3 was a special case of lenalidomide administration. The aim was to eradicate the minimal residual disease (MRD) after an abrupt drop in the percentage of donor cell chimerism in the CD34+ cell subset following allogeneic hematopoietic stem cell transplantation (HSCT) (Figure 1C). A dramatic decrease of donor cell chimerism in the CD34+ subset foreshadows hematologic relapse after allogeneic HSCT.4,5 In patient UPN3 lenalidomide transiently suppressed the del(5q) stem cells, delaying relapse for about 18 months (Figure 1C). Subsequent hematologic relapse occurred, with bone marrow blasts reappearing. Additionally, karyotype analysis revealed clonal evolution among the aberrant recipient cells, with a new unbalanced translocation der(6)t(1;6) in addition to the deletion del(5)(q13q33). The patient successfully underwent a second HSCT.
Figure 1.
(A) Response to lenalidomide in a 66 year old woman (UPN1) diagnosed with 5q- syndrome in September 2005. Bone marrow investigation in April 2007 showed all metaphases had deletion 5q (5q-chromosome). In addition, 48% of CD34+ stem cells were 5q-. Lenalidomide (Celgene Co., 10 mg daily on 21 days of every 28-day cycle for six months) was started 21 months after diagnosis in June 2007. The figure depicts the clinical course (hemoglobin level – HGB, platelet count –PLT, white blood cell count - WBC). Treatment resulted in rapid recovery of all three hematopoietic lineages. Four months after the start of lenalidomide (October 2007) complete cytogenetic remission (CCR) was observed and was confirmed at the stem cell level (CD34+ cells) after a further four months (February 2008), two months after the therapy had been discontinued. (B) Clinical response to lenalidomide of a 69 year old man (UPN2) with a diagnosis of RBC transfusion-dependent MDS in September 2004 which transformed to refractory anemia with excess blasts >10% (RAEB 2) in August 2005. Because of progressive neutropenia, thrombocytopenia and increased transfusion need, lenalidomide therapy (Celgene Co., 5 mg daily for 21 days of every 28-day cycle) was instituted 29 months after diagnosis in February 2007 until the time of writing. Lenalidomide abolished transfusion requirement, inducing red cell recovery with a rise in hemoglobin to nearly normal, and eradication of the del(5q) clone, including the stem cells, while white blood cell recovery was prolonged. The proportion of myeloblasts in the bone marrow (BM blasts) returned to 2%. (C) Response to lenalidomide delaying imminent relapse six years after allogeneic hematopoietic stem cell transplantation (HSCT) in a 54 year old patient who had MDS with refractory anemia in 1998, and progression to RAEB >10% blasts one year later. The data depicts the clinical course after first detection of an abrupt donor cell regression in the CD34+ subset about six years after HSCT. Preceding subset analyses documented a complete donor chimerism, also seen in the CD34+ subset (five analyses were made between May 2003, day 1136, and March 2004, day 1437; data not shown). Besides the course of chimerism the figure depicts the reappearance of cells with deletion 5q in the CD34+ subset (whole blood, chimerism whole PB; bone marrow, chimerism whole BM; CD34+ cells isolated from peripheral blood, chimersim CD34_PB, % 5q- CD34_PB; CD34+ cells isolated from bone marrow, chimerism CD34_BM, % 5q- CD34_BM). Due to the risk of relapse, lenalidomide therapy (Celgene Co., 10 mg daily for 21 days of every 28-day cycle) was started for one year. Subset chimerism then decreased dramatically to 5% donor cells. Lenalidomide was stopped, and treatment with Vidaza (5-azacytidine, Pharmion Co., Germany, 4 courses: 75 mg/m2d, s.c. on days 1–7, repeated on day 28) was started. In October 2007, 12% bone marrow blasts confirmed hematologic relapse. Karyotype analysis revealed a clonal evolution within the aberrant recipient cells with an unbalanced translocation der(6)t(1;6) additional to the deletion del(5)(q13q33).
Taken together, our results confirm that lenalidomide is effective in MDS patients with single del(5q), moreover in UPN1 and UPN2 the del(5q) CD34+ stem cells were eradicated. This has not been previously reported, and possibly suggests that short-term lenalidomide could cure a subset of del(5q) MDS patients. However, long-term follow-up will be needed to exclude the persistence and repopulation of disseminated 5q- stem cells. Given that lenalidomide suppresses the del(5q) clone, a prerequisite for response is that normal residual stem cells are present.1 The best responder (UPN1) fitted into this category, with only 48% of CD34+ stem cells harboring the deletion 5q before the start of therapy.
Despite the eradication of the del(5q) stem cells, patient UPN2 developed an unrelated aberrant clone; however, there have been no signs of any disease progression so far.
This is in contrast to patient UPN3, whose del(5q) CD34+ cells were not eradicated. This patient had a longer history of MDS with excess blasts and was a different case because of the allogeneic setting. Lenalidomide exerts immunomodulatory effects, which might have caused the donor T cells to only transiently control the del(5q) CD34+ cells. The documented clonal progression is attributable to selection pressure favoring a more proliferative subclone unresponsive to lenalidomide.
Long-term lenalidomide therapy might support clonal selection. Hence, close monitoring of bone marrow cytology and cytogenetics, possibly including selected CD34+ cells, is imperative in these patients.
Acknowledgments
we thank Barbara Brocard, Ulrike Fitze, Jeannette Mundt, Anne Richter, Manuela Neumann, Heidrun Zengler, and Cornelia Grosse for their technical assistance and Michael Kramer for his assistance in preparing the figures.
References
- 1.Cazzola M. Myelodysplastic syndrome with isolated 5q deletion (5q- syndrome). A clonal stem cell disorder characterized by defective ribosome biogenesis. Haematologica. 2008;93:967–72. doi: 10.3324/haematol.13377. [DOI] [PubMed] [Google Scholar]
- 2.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of Lenalidomide in Myelodysplastic Syndromes. N Engl J Med. 2005;352:549–57. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 3.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the Myelodysplastic Syndrome with Chromosome 5q Deletion. N Engl J Med. 2006;355:1456–65. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 4.Mattsson J, Uzunel M, Tammik L, Aschan J, Ringdén O. Leukemia lineage-specific chimerism analysis is a sensitive predictor of relapse in patients with acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplantation. Leukemia. 2001;15:1976–85. doi: 10.1038/sj.leu.2402311. [DOI] [PubMed] [Google Scholar]
- 5.Thiede C, Lutterbeck K, Oelschlägel U, Kiehl M, Steudel Ch, Platzbecker U, et al. Detection of relapse by sequential monitoring of chimerism in circulating CD34+ cells. Ann Hematol. 2002;81(Suppl 2):S27–8. [PubMed] [Google Scholar]