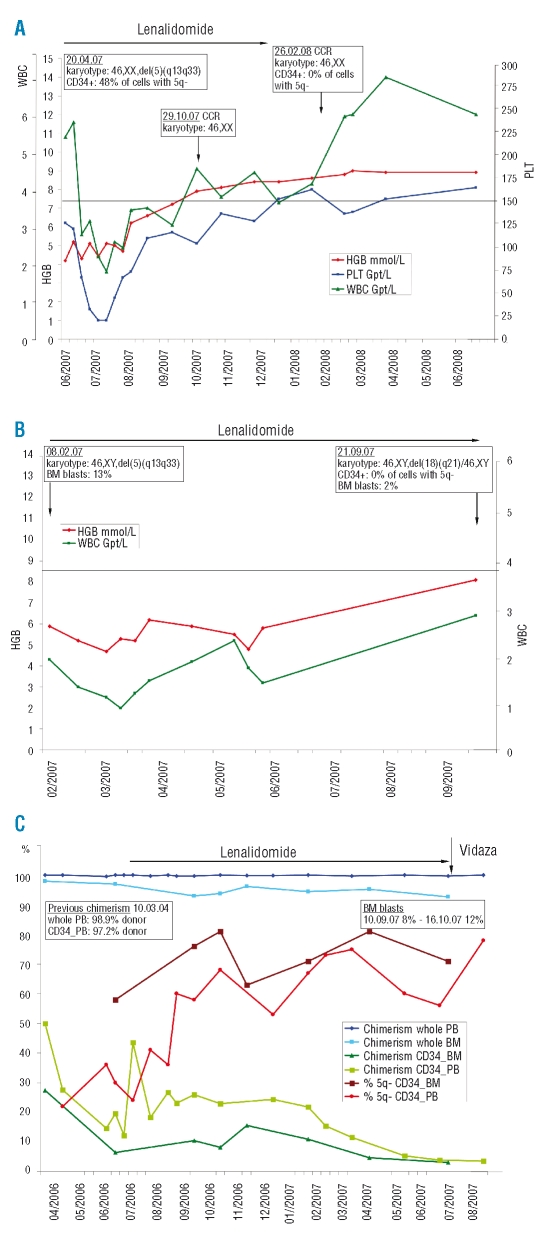
Figure 1.
(A) Response to lenalidomide in a 66 year old woman (UPN1) diagnosed with 5q- syndrome in September 2005. Bone marrow investigation in April 2007 showed all metaphases had deletion 5q (5q-chromosome). In addition, 48% of CD34+ stem cells were 5q-. Lenalidomide (Celgene Co., 10 mg daily on 21 days of every 28-day cycle for six months) was started 21 months after diagnosis in June 2007. The figure depicts the clinical course (hemoglobin level – HGB, platelet count –PLT, white blood cell count - WBC). Treatment resulted in rapid recovery of all three hematopoietic lineages. Four months after the start of lenalidomide (October 2007) complete cytogenetic remission (CCR) was observed and was confirmed at the stem cell level (CD34+ cells) after a further four months (February 2008), two months after the therapy had been discontinued. (B) Clinical response to lenalidomide of a 69 year old man (UPN2) with a diagnosis of RBC transfusion-dependent MDS in September 2004 which transformed to refractory anemia with excess blasts >10% (RAEB 2) in August 2005. Because of progressive neutropenia, thrombocytopenia and increased transfusion need, lenalidomide therapy (Celgene Co., 5 mg daily for 21 days of every 28-day cycle) was instituted 29 months after diagnosis in February 2007 until the time of writing. Lenalidomide abolished transfusion requirement, inducing red cell recovery with a rise in hemoglobin to nearly normal, and eradication of the del(5q) clone, including the stem cells, while white blood cell recovery was prolonged. The proportion of myeloblasts in the bone marrow (BM blasts) returned to 2%. (C) Response to lenalidomide delaying imminent relapse six years after allogeneic hematopoietic stem cell transplantation (HSCT) in a 54 year old patient who had MDS with refractory anemia in 1998, and progression to RAEB >10% blasts one year later. The data depicts the clinical course after first detection of an abrupt donor cell regression in the CD34+ subset about six years after HSCT. Preceding subset analyses documented a complete donor chimerism, also seen in the CD34+ subset (five analyses were made between May 2003, day 1136, and March 2004, day 1437; data not shown). Besides the course of chimerism the figure depicts the reappearance of cells with deletion 5q in the CD34+ subset (whole blood, chimerism whole PB; bone marrow, chimerism whole BM; CD34+ cells isolated from peripheral blood, chimersim CD34_PB, % 5q- CD34_PB; CD34+ cells isolated from bone marrow, chimerism CD34_BM, % 5q- CD34_BM). Due to the risk of relapse, lenalidomide therapy (Celgene Co., 10 mg daily for 21 days of every 28-day cycle) was started for one year. Subset chimerism then decreased dramatically to 5% donor cells. Lenalidomide was stopped, and treatment with Vidaza (5-azacytidine, Pharmion Co., Germany, 4 courses: 75 mg/m2d, s.c. on days 1–7, repeated on day 28) was started. In October 2007, 12% bone marrow blasts confirmed hematologic relapse. Karyotype analysis revealed a clonal evolution within the aberrant recipient cells with an unbalanced translocation der(6)t(1;6) additional to the deletion del(5)(q13q33).