Abstract
Elevated serum ferritin is found in a large spectrum of conditions both genetic and acquired, associated or not with iron overload. In this perspective article, Drs. Camaschella and Poggiali examine our current knowledge of the molecular basis of inherited hyperferritinemia. See related article on page 335.
Ferritin has a central role in iron homeostasis since it binds and sequesters intracellular iron. It is a spheric shell with a central cavity where up to 4,500 atoms of iron are oxidized and stored. Ferritin is a multimer composed of 24 H (heavy) and L (light) subunits in variable proportions in different tissues. The two subunits are highly conserved during evolution, but only the H subunit has ferroxidase activity.1 Ferritin is also released in the circulation prevalently as L-ferritin or G-(glycosylated)-subunit through a largely unknown process. Since over recent years serum ferritin measurement has become a routine laboratory test, elevated serum ferritin is a common finding in clinical practice. High serum ferritin is found in a large spectrum of conditions both genetic and acquired, associated or not with iron overload. For this reason a precise diagnosis of hyperferritinemia requires a strategy that includes family and personal medical history, biochemical and eventually genetic or other tests, especially those related to tissue iron measurement.
It is well known that both acute and chronic inflammation, as occurring in infections, autoimmune disorders, chronic renal failure and also cancer – all conditions common in hospitalized patients – are associated with high ferritin levels. Another common process is cytolysis, an event that releases ferritin from the hepatocytes in patients with acute or chronic liver diseases. Very high levels of serum ferritin are found in Still’s disease, where hyperferritinemia is a marker of disease activity. It is biologically of interest that in this condition the glycosylated ferritin levels are lower (< 20%) than in normal subjects, whereas the glycosylated form represents 50–80% of the total ferritin.2 A simple determination of CRP is of help in excluding inflammation when the problem is not clinically evident. In addition, in inflammatory conditions high ferritin is not associated with increased saturation of transferrin that is usually normal or even decreased, according to the high hepcidin production which may lead to anemia of chronic disorders (ACD).3
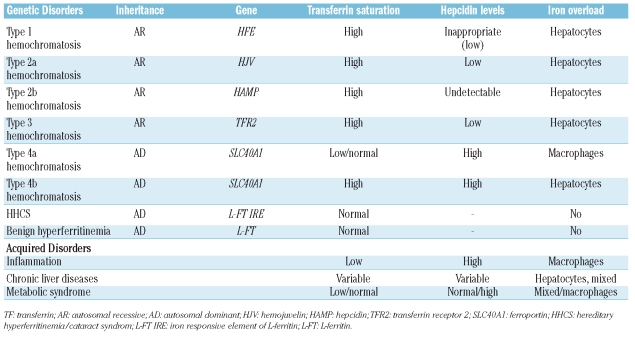
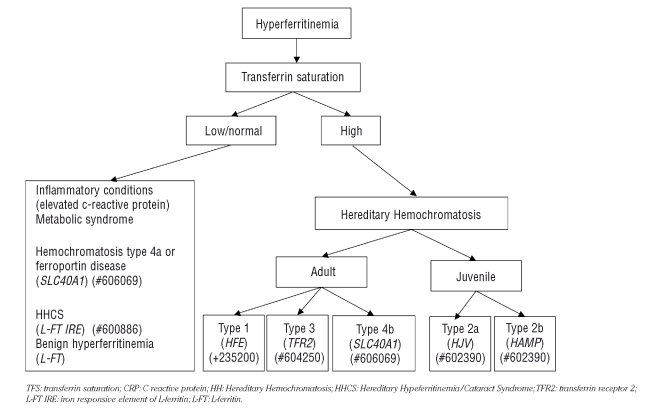
Elevated serum ferritin in association with high transferrin saturation (Table 1 and Figure 1) is usually a sign of iron overload. Increased body iron stores are found in the different forms of hereditary hemochromatosis (Table 1), where serum ferritin levels broadly parallel the entity of iron stores. Type 1 hemochromatosis is the most common form, prevalently expressed in middle age males. In most cases it is due to homozygous C282Y mutation of the HFE gene and in minority of patients to compound heterozygosity for both C282Y and H63D mutations or homozygosity for the latter mutation. Type 2 hemochromatosis, the juvenile form, are very rare due to mutations of hemojuvelin or of hepcidin and type 3 hemochromatosis which is characterized by mutations of TFR2. All these autosomal recessive disorders have inappropriately low hepcidin levels4 with consequent excessive iron release to the plasma from enterocytes and macrophages. For this reason transferrin saturation is high (>45%, but usually >60% up to 100% in the juvenile forms); its increase antedates the increase of serum ferritin and is associated with high levels of Non Transferrin Bound Iron (NTBI), the toxic moiety of iron, responsible for liver damage and eventually damage to the heart, pancreas and pituitary gland.
Table 1.
Genetic and acquired disorders associated with hyperferritinemia (not associated with anemia).
Figure 1.
Diagnostic diagram of hyperferritinemia not associated with anemia or liver disorders.
Very high levels of ferritin may be found in hemochromatosis type 4 or ferroportin disease which does not result from impaired hepcidin but rather from heterozygous mutations of the hepcidin receptor ferroportin.5 The disease is autosomal dominant and has a variable clinical phenotype. The true ferroportin disease (hemochromatosis type 4a in Table 1) is due to mutant proteins that are not correctly targeted to the cell surface and is characterized by macrophage iron accumulation, low/normal transferrin saturation and iron-restricted erythropoiesis.6 A second form (hemochromatosis type 4b), due to mutant ferroportins that reach the cell surface but are resistant to hepcidin-induced internalization,7 is characterized by hepatocyte iron accumulation and high transferrin saturation, as in hemochromatosis. The distinction is clinically relevant, because the true ferroportin disease seems not to be associated with clinical complications. These observations strengthen the relevance of assessing serum ferritin always in the context of transferrin saturation (Figure 1).
Ferritin is increased in iron overload secondary to chronic blood transfusions irrespective of the reason and also in the so called iron loading anemias, which include beta-thalassemia syndromes8 and congenital sideroblastic or dyserythropoietic anemias,9 characterized by high levels of ineffective erythropoiesis and low hepcidin production. All these conditions are usually well known from the patient’s history and may be diagnosed by specific tests. Several methods such as magnetic resonance imaging (MRI)10 or SQUID11 have become available to non-invasively assess hepatic iron concentration: however, serial ferritin determinations remain a useful guide to assess iron overload and to monitor iron chelation therapy in these patients.12
Moderate increase of serum ferritin with normal/high transferrin saturation and mild/moderate liver iron accumulation may be common in chronic liver disorders, such as alcoholic liver disease and chronic viral hepatitis. These conditions require attention because iron may amplify the toxic effect of alcohol and viruses, accelerating the evolution towards fibrosis and cirrhosis. In porphyria cutanea tarda hyperferritinemia is a sign of iron overload (in some cases due to HFE mutations)13 and, like alcohol and chronic hepatitis, is a trigger of porphyria attacks.
In the metabolic syndrome (variable combination of hypertension, diabetes, hypertrigliceridemia, obesity, steatohepatitis or fatty liver) moderate elevation of ferritin is common, but usually ferritin levels are disproportionately high in comparison with iron stores,14 whereas transferrin saturation is not increased.
In recent years, new forms of inherited hyperferritinemia not associated with iron overload have been identified, adding new elements for differential diagnosis. A rare dominant trait is hereditary hyperferritinemia/cataract syndrome (HHCS), due to mutation in the IRE element of the 5’ untranslated region of L-ferritin mRNA.15–17 The mutation results in lack of repression of L-ferritin translation that becomes independent of iron availability and of iron regulatory protein regulation and occurs also in iron deficiency. Thus in HHCS, the high serum ferritin levels reflect an increased synthesis of the L-ferritin, but not of total body iron, since the L subunit does not participate in iron oxidation and storage. The condition is benign, the only clinical manifestation being early-onset bilateral cataract. Except for eye surgery, no treatment is required: the few patients who have been treated with phlebotomy developed anemia without changes in serum ferritin levels.15
In this issue of the journal, Kannengiesser et al.18 report a novel genetic dominant hyperferritinemia, that appears to have a benign course in the absence of iron overload (indicated as benign hyperferritinemia in Table 1 and Figure 1). These authors have collected a large number of samples from both familial (n=25) and isolated (n=66) subjects with unexplained hyperferritinemia. They defined as unexplained a condition of high serum ferritin (>200 ng/mL in women and >300 ng/mL in males) with transferrin saturation <45%, no excessive tissue iron (as determined by liver biopsy or MRI), serum iron <25 μmoles/L and absence of mutations in the IRE sequence of L-ferritin promoter. In this series of patients they found a single novel mutation (p.Thr30Ile) in the coding sequence of L-ferritin in half the familial and in a few of the isolated cases, but not in >500 normal controls. This finding is remarkable because mutations of L-ferritin are extremely rare and associated either with HHCS15–17 or a neurological disorder known as neuroferritinopathy.19 Although there is no proof that the mutation is the direct cause of the elevated ferritin levels (the mutation could still be only in disequilibrium with the causative one) the threonine residue at position 30, in the N-terminus of the A α-helix of L-ferritin is evolutionary conserved. In EBV-transformed cell lines from patients the ferritin content was normal, excluding an increased synthesis. The authors speculate that the mutated subunits probably do not have altered function, but since in the 8 patients whose sera was available the proportion of glycosylated L-ferritin was higher (90–100%) than normal, the abnormality could rely on an increased L-ferritin secretion, a process that should occur through the endoplasmic reticulum, but whose molecular mechanisms remain largely unknown. Ferritin levels typically fluctuate over time in these subjects, but at present no specific symptoms/abnormalities have been associated with this mutation. Much remains to be clarified and several other cases of hyperferritinemia still remain unexplained. However, another diagnostic option is now available for selected patients. Since a single mutation is responsible for the phenotype, a simple molecular test could easily be developed for the molecular diagnosis of the L-ferritin p.Thr30Ile mutation. Although no relevant clinical data seem to be associated with this sequence abnormality, the precise diagnosis of this new entity is relevant to reassure patients, and to avoid useless and expensive investigations.
Footnotes
The Author’s research work described here was supported by E.U. Contract N. LSHM-CT-2006-037296.
References
- 1.Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Fautrel B. Adult-onset Still disease. Best Pract Res Clin Rheumatol. 2008;22:773–92. doi: 10.1016/j.berh.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12:107–11. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Camaschella C. Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders. Blood. 2005;106:3710–7. doi: 10.1182/blood-2005-05-1857. [DOI] [PubMed] [Google Scholar]
- 5.De Domenico I, Ward DM, Nemeth E, Vaughn MB, Musci G, Ganz T, Kaplan J. The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci USA. 2005;102:8955–60. doi: 10.1073/pnas.0503804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drakesmith H, Schimanski LM, Ormerod E, Merry-weather-Clarke AT, Viprakasit V, Edwards JP, et al. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood. 2005;106:1092–7. doi: 10.1182/blood-2005-02-0561. [DOI] [PubMed] [Google Scholar]
- 7.Schimanski LM, Drakesmith H, Merryweather-Clarke AT, Viprakasit V, Edwards JP, Sweetland E, et al. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood. 2005;105:4096–102. doi: 10.1182/blood-2004-11-4502. [DOI] [PubMed] [Google Scholar]
- 8.Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, Nemeth E. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92:583–8. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 9.Tamary H, Shalev H, Perez-Avraham G, Zoldan M, Levi I, Swinkels DW, et al. Elevated growth differentiation factor 15 expression in patients with congenital dyserythropoietic anemia type I. Blood. 2008;112:5241–4. doi: 10.1182/blood-2008-06-165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–61. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 11.Fischer R, Piga A, Harmatz P, Nielsen P. Monitoring long-term efficacy of iron chelation treatment with biomagnetic liver susceptometry. Ann N Y Acad Sci. 2005;1054:350–7. doi: 10.1196/annals.1345.043. [DOI] [PubMed] [Google Scholar]
- 12.Angelucci E, Barosi G, Camaschella C, Cappellini MD, Cazzola M, Galanello R, et al. Italian Society of Hematology practice guidelines for the management of iron overload in thalassemia major and related disorders. Haematologica. 2008;93:741–52. doi: 10.3324/haematol.12413. [DOI] [PubMed] [Google Scholar]
- 13.Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, Obando J, Di Bisceglie A, Tattrie C, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology. 1998;27:1661–9. doi: 10.1002/hep.510270627. [DOI] [PubMed] [Google Scholar]
- 14.Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, et al. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117:1155–63. doi: 10.1016/s0016-5085(99)70401-4. [DOI] [PubMed] [Google Scholar]
- 15.Cazzola M. Role of ferritin and ferroportin genes in unexplained hyperferritinaemia. Best Pract Res Clin Haematol. 2005;18:251–63. doi: 10.1016/j.beha.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Hetet G, Devaux I, Soufir N, Grandchamp B, Beaumont C. Molecular analyses of patients with hyperferritinemia and normal serum iron values reveal both L-ferritin IRE and 3 new ferroportin (Slc11A3) mutations. Blood. 2003;102:1904–10. doi: 10.1182/blood-2003-02-0439. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari F, Foglieni B, Arosio P, Camaschella C, Daraio F, Levi S, et al. Microelectronic DNA chip for hereditary hyperferritinemia cataract syndrome, a model for large-scale analysis of disorders of iron metabolism. Hum Mut. 2006;27:201–8. doi: 10.1002/humu.20294. [DOI] [PubMed] [Google Scholar]
- 18.Kannengiesser C, Jouanolle A-N, Hetet G, Mosser A, Muzeau F, Henry D, Bardou-Jacquet E, et al. A new missense mutation in the L ferritin coding sequence associated with elevated levels of glycosylated ferritin in serum and absence of iron overload. Haematologica. 2009;94:335–9. doi: 10.3324/haematol.2008.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levi S, Cozzi A, Arosio P. Neuroferritinopathy: a neurodegenerative disorder associated with L-ferritin mutation. Best Pract Res Clin Haematol. 2005;18:265–76. doi: 10.1016/j.beha.2004.08.021. [DOI] [PubMed] [Google Scholar]