The findings of this study indicate that Shwachman-Diamond syndrome neutrophils have aberrant chemoattractant-induced F-actin properties that might contribute to the impaired neutrophil chemotaxis present in this syndrome.
Keywords: SBDS gene, Shwachman-Diamond syndrome, actin cytoskeleton, fMLP
Abstract
Shwachman-Diamond syndrome is a hereditary disorder characterized by pancreatic insufficiency and bone marrow failure. Most Shwachman-Diamond syndrome patients have mutations in the SBDS gene located at chromosome 7 and suffer from recurrent infections, due to neutropenia in combination with impaired neutrophil chemotaxis. Currently, the role of the actin cytoskeleton in Shwachman-Diamond syndrome neutrophils has not been investigated. Therefore, we performed immunofluorescence for SBDS and F-actin on human neutrophilic cells. Additionally, we examined in control neutrophils and cells from genetically defined Shwachman-Diamond syndrome patients F-actin polymerization and cytoskeletal polarization characteristics upon chemoattractant stimulation. These studies showed that SBDS and F-actin co-localize in neutrophilic cells and that F-actin polymerization and depolymerization characteristics are altered in Shwachman-Diamond syndrome neutrophils as compared to control neutrophils in response to both fMLP and C5a. Moreover, F-actin cytoskeletal polarization is delayed in Shwachman-Diamond syndrome neutrophils. Thus, Shwachman-Diamond syndrome neutrophils have aberrant chemoattractant-induced F-actin properties which might contribute to the impaired neutrophil chemotaxis.
Introduction
Neutrophils play an important role in host defense against pathogens. To perform their cytotoxic and phagocytic functions, neutrophils are equipped with efficient machinery to detect and migrate towards these pathogens. Upon encountering chemotactic stimuli, like complement factors (i.e. C5a) or bacterial metabolites (i.e. fMLP), neutrophils will rapidly polymerize their F-actin cytoskeleton, polarize and migrate in the direction of the chemotactic source.1,2
Several hematologic syndromes are characterized by impaired neutrophil chemotaxis. Patients with mutations in Rac2, an essential signaling component that is required for actin remodeling, display impaired neutrophil chemotaxis and subsequently suffer from recurrent infections.3–6 Also patients suffering from Shwachman-Diamond syndrome (SDS) display neutrophil chemotaxis defects.7–11
SDS is characterized by exocrine pancreas defects and neutropenia, often accompanied by thrombocytopenia and anemia.8,12,13 Most SDS patients have mutations in the SBDS gene located at chromosome 7, which is currently the only known affected gene in SDS.14 The earliest studies on chemotaxis defects in SDS patients were published in the late 1970s11,15 and were confirmed in later studies.7–9 At the cellular and molecular level, Stepanovic et al. showed that directed, but not random movement of SDS neutrophils is impaired and Wessels et al. showed in Dictyostelium Discoideum that GFP-SBDS was localized in the pseudopod during chemotaxis. This suggests a role for SBDS in the polarization and migration process.10,16 Nevertheless, the molecular defects contributing to impaired chemotaxis in human SDS neutrophils remained unexplored.
Our previous study revealed that neutrophil chemotaxis towards C5a, IL-8 and PAF was disturbed in most SDS patients.8 To investigate the underlying molecular defect, we examined human neutrophils and PLB-985 cells for SBDS co-localization with the F-actin cytoskeleton and subsequently examined chemoattractant-induced F-actin polymerization dynamics in SDS neutrophils. We observed that SBDS co-localizes with F-actin and Rac2 in cellular protrusions and that F-actin dynamics are disturbed. Also, cellular F-actin polarization, which is a prerequisite for directional movement is delayed in SDS neutrophils.
This is the first study revealing a molecular defect, namely defective F-actin cytoskeletal remodeling, which may contribute to impaired chemotaxis in SDS patients.
Design and Methods
Neutrophil isolation and cell culture
Blood was obtained after informed consent of healthy volunteers (26–43 years) and SDS patients (2–29 years; all with mutations 183–184 A>CT [K62X]/IVS2 258+2T>C [C84fs]). The study was approved by the Medical Ethics Committees of the AMC Hospital and the institutional review board and was in accordance with the Declaration of Helsinki. Neutrophils were isolated as described.8
PLB-985 and GFP-Rac2 PLB-98517 cells were cultured in RPMI/10% FCS/penicillin (200 μg/mL)/streptomycin (200 μg/mL)/L-glutamine (4 mM). PLB-985 cells were differentiated with 0.5% dimethylformamide (DMF; Sigma-Aldrich) for 5–7 days.
Cellular spreading and immunofluorescence
Neutrophils or PLB-985 cells in Hepes medium (132 mM NaCl, 20 mM Hepes, 6mM KCL, 1 mM MgSO4, 1.2 mM K2HPO4, 1 mM CaCl2, 5 mM Glucose, 2.5% human albumin (Cealb; Sanquin reagents)) were seeded on fibronectin-coated coverslips and allowed to adhere for 30 min. Cells were stimulated with 20nM fMLP, fixed with 4% paraformaldehyde/PBS and processed for immunofluorescence staining as described.18 Antibodies used: SBDS (rabbit polyclonal), Phalloidin-Alexa488 or BODIPY conjugated. Pictures were taken with a Zeiss LSM510 microscope with a Zeiss 65x oil lens and processed with LSM 510 software.
F-actin polymerization and cellular polarization
Neutrophils (2×106/mL) in Hepes medium were stimulated with 100nM fMLP or 40nM C5a. At indicated time points 2×105cells were harvested and fixed with buffer 1 (Intraprep Permeabilization Reagent Kit; Immunotech), permeabilized with buffer 2 and stained with Phalloidin-Alexa488 (Molecular Probes, Invitrogen). Cells were analyzed on LSRII (BD Biosciences) flow cytometer for F-actin content.
To determine F-actin polarization, neutrophils were embedded with Mowiol 4–88 and analyzed by confocal microscopy. For each experiment at least 50 cells per time-point were analyzed in a blind fashion.
Results and Discussion
SBDS co-localizes with F-actin and Rac2 in human neutrophils
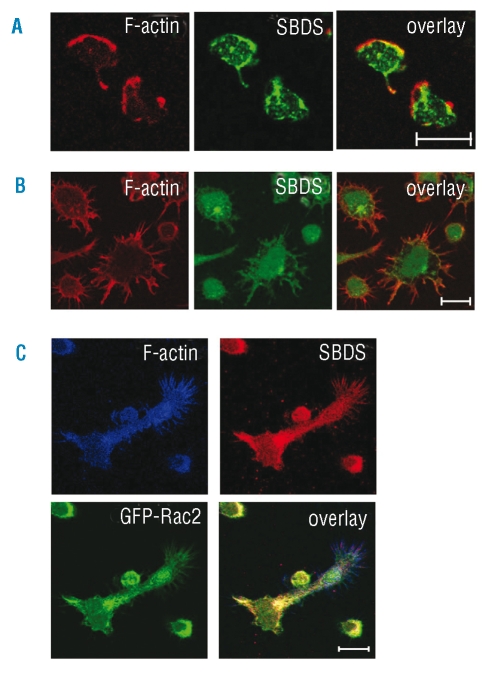
To investigate a possible link between SBDS and the actin cytoskeleton, we performed immunofluorescence stainings in neutrophils and PLB-985 cells, with an anti-SBDS antibody that we have generated and which specifically recognized SBDS (Online Supplementary Figure S1A/B). In resting human neutrophils we observed that SBDS is localized prominently to the nucleus and to a lesser extent to the cytoplasm (Online Supplementary Figure 1C). However, in these resting cells there is not much polymerized F-actin cytoskeleton detectable (data not shown) and therefore we set out to investigate SBDS in relation to the F-actin cytoskeleton in activated neutrophilic cells. Human peripheral blood neutrophils were allowed to adhere to fibronectin and were subsequently stimulated with fMLP. These activated cells displayed F-actin enriched cellular protrusions in which also SBDS is located (Figure 1A). Also in non-transfected, differentiated neutrophilic PLB-985 cells, F-actin and SBDS showed co-localization under adherent conditions (Figure 1B). These data in human neutrophillic cells are consistent with studies in the slime mold Dictyostelium discoideum, in which GFP-SBDS localization to the pseudopods was observed only under chemotactic conditions.16 Moreover, it suggests that SBDS pseudopod localization is an evolutionary conserved process dependent on chemotaxis.
Figure 1.
SBDS co-localizes with F-actin and Rac2 in cellular extensions of neutrophils. (A) Peripheral Blood neutrophils (B) differentiated PLB-985 cells and (C) differentiated PLB-985 GFP-Rac2 cells were allowed to adhere to fibronectin-coated glass coverslips and stimulated with 20 nM fMLP for several minutes. After adhesion and induced spreading, cells were fixed and stained for SBDS and F-actin. SBDS co-localizes with F-actin and Rac2 in cellular extensions. White bar indicates 10 μM.
Since Rac2 is an essential signaling component for actin remodeling, we studied SBDS localization in GFP-Rac2 PLB-985 cells generated previously in our laboratory,17 which were treated in a similar manner as human neutrophils. These cells revealed that SBDS co-localizes with both GFP-Rac2 and F-actin-enriched cellular regions (Figure 1C). This is an interesting observation, since both Rac2 and SBDS deficient neutrophils display chemotaxis defects, which in the Rac2 deficient neutrophils is due to both diminished F-actin polymerization and impaired cell orientation and polarization under chemotactic conditions.3,6 It is noteworthy that Rac2 deficiency results in a clinically more severe phenotype than SDS due to combined adhesion and NADPH oxidase defects,3 while SBDS deficient neutrophils only display impaired chemotaxis properties.8 Nevertheless, our co-localization data raise the interesting possibility that SBDS and Rac2 functions in chemotaxis may be inter-dependent in neutrophils, although further studies are required to investigate this in more detail. In conclusion, under adherent and/or migratory conditions SBDS protein co-localizes with F-actin and Rac2 in cellular protrusions in neutrophilic cells.
Chemoattractant-induced F-actin polymerization characteristics in Shwachman-Diamond syndrome neutrophils are disturbed
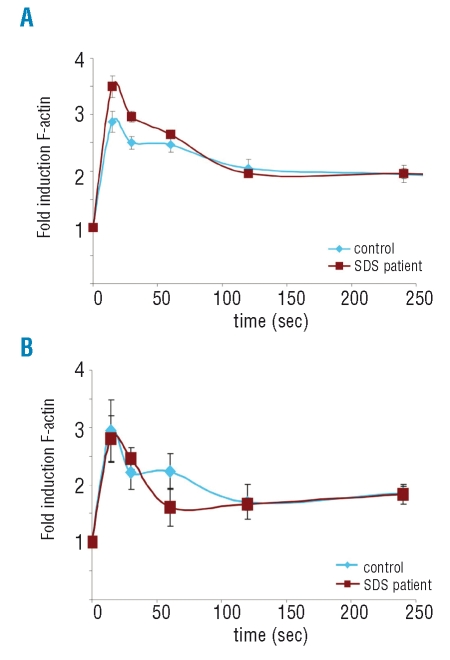
To study F-actin polymerization characteristics in SDS patient cells, we determined fMLP and C5a-induced F-actin polymerization at various time points by flow cytometric analysis in the neutrophil population. Prior to chemoattractant stimulation, the basal level of F-actin polymerization was similar in control and patient neutrophils. Rapidly after fMLP stimulation (15 seconds) the amount of F-actin in the neutrophils increased dramatically. Thereafter, F-actin is rapidly depolymerized, with a delay (plateau phase) between 30 and 60 seconds after fMLP stimulation in the control neutrophils. These observations are similar to studies previously published by others on the characteristics of F-actin polymerization in neutrophils.19 In the SDS neutrophils, the fMLP-induced F-actin polymerization was consistently higher (Figure 2A). Moreover, we observed that the delay in depolymerization between 30 to 60 seconds after fMLP stimulation was not pronounced in SDS neutrophils. C5a stimulation of SDS neutrophils resulted in a similar induction of F-actin polymerization as compared to control cells (Figure 2B). However, the plateau phase in F-actin depolymerization after stimulation was completely absent. To gain more insight into the underlying mechanisms future studies that will investigate the possible affected signaling pathways involved in regulating F-actin (de)polymerization in SDS neutrophils are required. Nevertheless, based on our observations it can be concluded that fMLP and C5a-induced F-actin polymerization characteristics in SDS neutrophils are disturbed.
Figure 2.
C5a and fMLP-induced actin polymerization in SDS neutrophils. (A) Average fold induction in F-actin content in the control and SDS neutrophils after (A) 100 nM fMLP stimulation (n=7) and (B) 40 nM C5a stimulation (n=4). Error bar indicates SEM. Blue line represents control samples and the dark red line indicates SDS patient samples.
F-actin polarization in Shwachman-Diamond syndrome neutrophils is delayed
Besides F-actin polymerization, neutrophil polarization plays an important role in chemotaxis. Immediately after chemoattractant stimulation, neutrophils will polarize their actin cytoskeleton to participate in directed movement towards the chemoattractant. To investigate this response at the level of individual cells, we analyzed control and SDS-patient neutrophils by confocal microscopy. In resting state, neutrophils have low to undetectable levels of F-actin (Figure 3A, left panel) Chemoattractant stimulation induces within seconds a cortical F-actin ring that will rapidly thereafter start polarizing in several directions (Figure 3A, middle panel). Next, neutrophils will become polarized in one direction (Figure 3A, right panel). Resting control neutrophils contained a faint ring of F-actin. Within 15 to 30 seconds after fMLP stimulation most neutrophils displayed a cortical F-actin ring and/or multi-or bipolarized F-actin enrichments (Figure 3B). After 60 seconds of fMLP stimulation, approximately half of the control neutrophils were clearly polarized in one direction and at later time points, more neutrophils became uni-polarized at the expense of the bi- or multi-polarized cells.
Figure 3.
Intracellular polarization of the actin cytoskeleton is delayed in Shwachman-Diamond syndrome neutrophils (A) Representative pictures of neutrophils stained with phalloidin-Alexa488. We discriminate 3 F-actin situations, (1) non-polarized and non-polymerized F-actin (left panel), (2) polymerized F-actin in a cortical ring or not clearly polarized (middle panel) and (3) polymerized F-actin in a polarized fashion (right panel) (B-C) Average percentage of cells with the polarized F-actin phenotype described above (B) control (n=4) and (C) SDS patient (n=4). Error bar indicates SEM.
Similar to control cells, SDS neutrophils contained a faint ring of F-actin prior to fMLP stimulation and displayed a cortical F-actin ring and bi- or multi-polarized cells 15 to 30 seconds after fMLP stimulation (Figure 3C). However, the amount of uni-polarized cells only slowly increased thereafter. In contrast to the control neutrophils, approximately half of the population was uni-polarized 360 seconds after fMLP stimulation. Hence, the SDS neutrophils display a delayed cellular polarization of the F-actin cytoskeleton. Our observations provide a molecular explanation that is consistent with previously published studies which showed that SDS neutrophils have impaired directed movement towards fMLP and more lateral pseudopod formation in the presence of fMLP as compared to control neutrophils.10 This lack of orientation probably contributes to random migration at the expense of directed migration and could be related to altered F-actin polymerization and polarization dynamics as we observed in our studies.
In the studies reported here we have addressed the molecular properties of the F-actin cytoskeleton in SDS neutrophils and showed that F-actin polymerization and polarization properties are disturbed as compared to control neutrophils. It remains to be further investigated how the SBDS protein is involved in regulating the F-actin cytoskeleton. Several studies have shown that SBDS is able to bind to ribosomal RNA and has a role in ribosome maturation.16,20–22 The ribosome or RNA-related function of SBDS does not have to exclude a possible role in chemotaxis or F-actin cytoskeletal dynamics. Several studies have shown that Zipcode-Binding-Protein (ZBP) family members, mRNA binding proteins, are required to transport mRNAs to specific cellular locations. β-actin mRNA has been shown to be transported to the cell periphery and locally translated, and it was proposed that this contributed to actin remodeling in the migration process.23 Perhaps SBDS could play a related function for β-actin mRNA transport required for F-actin remodeling and polymerization.
Based on our immunofluorescence studies, another interesting possibility that deserves further investigation is that SBDS and Rac2-mediated F-actin regulation might be inter-dependent. Future protein-protein interaction studies, which were beyond the scope of this study, could reveal whether there is a direct interaction between members of the Rac2 and/or other signaling pathways and the SBDS protein.
Altogether, we have shown here for the first time that neutrophils from SDS patients have altered F-actin polymerization and polarization properties, which may well contribute to the observed impaired neutrophil chemotaxis in these patients.
Supplementary Material
Footnotes
Funding: support was provided by Landsteiner Foundation for Bloodtransfusions Research (LSBR 0519) and Stichting tot Steun (2005-50). Acknowledgments: we thank E. Mul and F. van Alphen for helpful confocal microscope instructions and Dr. P. van Hennik for helpful suggestions. We thank all members in the lab and especially Drs. A.Verhoeven, D.Roos and T. van den Berg for helpful suggestions and discussions.
Authorship and Disclosures
CO and TWK designed experiments. CO performed the experiments and analyzed data. TWK collected patient material. CO and TWK drafted the manuscript. The authors reported no potential conflict of interest.
References
- 1.Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–86. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- 2.Zigmond SH. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977;75:606–16. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc Natl Acad Sci USA. 2000;97:4654–9. doi: 10.1073/pnas.080074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carstanjen D, Yamauchi A, Koornneef A, Zang H, Filippi MD, Harris C, et al. Rac2 regulates neutrophil chemotaxis, superoxide production, and myeloid colony formation through multiple distinct effector pathways. J Immunol. 2005;174:4613–20. doi: 10.4049/jimmunol.174.8.4613. [DOI] [PubMed] [Google Scholar]
- 5.Chodniewicz D, Zhelev DV. Chemoattractant receptor-stimulated F-actin polymerization in the human neutrophil is signaled by 2 distinct pathways. Blood. 2003;101:1181–4. doi: 10.1182/blood-2002-05-1435. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–14. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 7.Cipolli M, D’Orazio C, Delmarco A, Marchesini C, Miano A, Mastella G. Shwachman’s syndrome: pathomorphosis and long-term outcome. J Pediatr Gastroenterol Nutr. 1999;29:265–72. doi: 10.1097/00005176-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Kuijpers TW, Alders M, Tool AT, Mellink C, Roos D, Hennekam RC. Hematologic abnormalities in Shwachman Diamond syndrome: lack of genotype-phenotype relationship. Blood. 2005;106:356–61. doi: 10.1182/blood-2004-11-4371. [DOI] [PubMed] [Google Scholar]
- 9.Smith OP, Hann IM, Chessells JM, Reeves BR, Milla P. Haematological abnormalities in Shwachman-Diamond syndrome. Br J Haematol. 1996;94:279–84. doi: 10.1046/j.1365-2141.1996.d01-1788.x. [DOI] [PubMed] [Google Scholar]
- 10.Stepanovic V, Wessels D, Goldman FD, Geiger J, Soll DR. The chemotaxis defect of Shwachman-Diamond syndrome leukocytes. Cell Motil Cytoskeleton. 2004;57:158–74. doi: 10.1002/cm.10164. [DOI] [PubMed] [Google Scholar]
- 11.Thong YH. Impaired neutrophil kinesis in a patient with the Shwachman-Diamond syndrome. Aust Paediatr J. 1978;14:34–7. doi: 10.1111/j.1440-1754.1978.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 12.Dror Y, Freedman MH. Shwachman-Diamond syndrome. Br J Haematol. 2002;118:701–13. doi: 10.1046/j.1365-2141.2002.03585.x. [DOI] [PubMed] [Google Scholar]
- 13.Ginzberg H, Shin J, Ellis L, Morrison J, Ip W, Dror Y, et al. Shwachman syndrome: phenotypic manifestations of sibling sets and isolated cases in a large patient cohort are similar. J Pediatr. 1999;135:81–8. doi: 10.1016/s0022-3476(99)70332-x. [DOI] [PubMed] [Google Scholar]
- 14.Boocock GR, Morrison JA, Popovic M, Richards N, Ellis L, Durie PR, et al. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat Genet. 2003;33:97–101. doi: 10.1038/ng1062. [DOI] [PubMed] [Google Scholar]
- 15.Szuts P, Katona Z, Ilyes M, Szabo I, Csato M. Correction of defective chemotaxis with thiamine in Shwachman-Diamond syndrome. Lancet. 1984;1:1072–3. doi: 10.1016/s0140-6736(84)91476-4. [DOI] [PubMed] [Google Scholar]
- 16.Wessels D, Srikantha T, Yi S, Kuhl S, Aravind L, Soll DR. The Shwachman-Bodian-Diamond syndrome gene encodes an RNA-binding protein that localizes to the pseudopod of Dictyostelium amoebae during chemotaxis. J Cell Sci. 2006;119:370–9. doi: 10.1242/jcs.02753. [DOI] [PubMed] [Google Scholar]
- 17.van Bruggen R, Anthony E, Fernandez-Borja M, Roos D. Continuous translocation of Rac2 and the NADPH oxidase component p67(phox) during phagocytosis. J Biol Chem. 2004;279:9097–102. doi: 10.1074/jbc.M309284200. [DOI] [PubMed] [Google Scholar]
- 18.Orelio C, Dzierzak E. Expression analysis of the TAB2 protein in adult mouse tissues. Inflamm Res. 2007;56:98–104. doi: 10.1007/s00011-006-6058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard TH, Oresajo CO. The kinetics of chemotactic peptide-induced change in F-actin content, F-actin distribution, and the shape of neutrophils. J Cell Biol. 1985;101:1078–85. doi: 10.1083/jcb.101.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganapathi KA, Austin KM, Lee CS, Dias A, Malsch MM, Reed R, et al. The human Shwachman-Diamond syndrome protein, SBDS, associates with ribosomal RNA. Blood. 2007;110:1458–65. doi: 10.1182/blood-2007-02-075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menne TF, Goyenechea B, Sanchez-Puig N, Wong CC, Tonkin LM, Ancliff PJ, et al. The Shwachman-Bodian-Diamond syndrome protein mediates translational activation of ribosomes in yeast. Nat Genet. 2007;39:486–95. doi: 10.1038/ng1994. [DOI] [PubMed] [Google Scholar]
- 22.Savchenko A, Krogan N, Cort JR, Evdokimova E, Lew JM, Yee AA, et al. The Shwachman-Bodian-Diamond syndrome protein family is involved in RNA metabolism. J Biol Chem. 2005;280:19213–20. doi: 10.1074/jbc.M414421200. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez AJ, Shenoy SM, Singer RH, Condeelis J. Visualization of mRNA translation in living cells. J Cell Biol. 2006;175:67–76. doi: 10.1083/jcb.200512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.