TAM receptors (Tyro3, Axl and Mer) are expressed in hematopoietic tissues, but their roles in hematopoiesis are largely unknown. This study shows that Axl and Mer play an important role in regulating erythropoiesis.
Keywords: Axl, Mer, erythropoiesis, hematopoiesis
Abstract
Background
TAM receptors (Tyro3, Axl and Mer) are expressed in hematopoietic tissues. The roles of the three receptors in hematopoiesis are, however, largely unknown. We investigated the role of TAM receptors in regulating erythropoiesis.
Design and Methods
Single and double mutant mice for Axl and Mer were used in the study. Cellularity of bone marrow and spleen, hematologic parameters, flow cytometry analysis of erythroid cell maturation, erythropoietic response to acute hemolytic anemia, bone marrow transplantation and the expression of erythropoisis were analyzed to evaluate the function of Axl and Mer in erythropoiesis.
Results
Axl and Mer, but not Tyro3, were constitutively expressed in developing erythroid cells. Mice lacking Axl and Mer (Axl−/−Me−/−) had impaired erythropoiesis in bone marrow and expanded splenic erythropoiesis. We found an inhibition of differentiation at the transition from erythroid progenitors to proerythroblasts in Axl−/−Mer−/− mice. These mice exhibited a low rate of erythropoietic response to acute anemia induced by phenylhydrazine. Bone marrow transplantation studies showed that the impaired erythropoiesis in Axl−/−Mer−/− mice is erythroid cell-autonomous. TAM receptors may influence erythropoiesis through the regulation of GATA-1 erythropoietin receptor and EpoR expression in erythroid progenitors. Notably, mice lacking single Axl or Mer exhibited normal erythropoiesis in steady-state conditions.
Conclusions
Axl and Mer play an important role in regulating erythropoiesis. This finding provides a novel insight into the mechanism of erythropoiesis.
Introduction
Mammalian erythropoiesis occurs sequentially in the embryonic yolk sac, fetal liver, spleen, and adult bone marrow during development.1 The earliest cells committed to erythropoiesis, as defined by in vitro clonogenic assays, are the slowly proliferating erythroid burst-forming units (BFU-E) arising from megakaryocyte-erythroid progenitors. The BFU-E then differentiate into progenitors with more limited proliferative capacity, termed erythroid colony-forming units (CFU-E).2 The CFU-E undergo three to five divisions, giving rise to several morphologically defined stages of maturing erythroblasts including proerythroblasts, basophilic erythroblasts, polychromatophilic erythroblasts, and orthochromatophilic erythroblasts that become hemoglobinized and extrude their nuclei to form reticulocytes and red cells. The differentiation of erythroid cells depends on extrinsic signals mediated by cytokines and microenvironmental factors through their specific cell-surface receptors. Erythropoietin and its receptor are essential for the differentiation of erythroid cells from CFU-E to basophilic erythroblasts. Analysis of mice carrying targeted mutation of genes has revealed the importance of various factors, such as GATA-1,3,4 GATA-2,5 signal transducer and activator of transcription (STAT)1,6 STAT3,7 and STAT5a/b,8,9 in erythropoiesis. Erythropoietin receptor (EpoR) signaling activates STAT1,10 STAT3 and STAT57 transcription factors and induces the expression of GATA-1 in erythroid cells.8
The TAM subfamily of receptor tyrosine kinases has three members: Tyro3, Axl, and Mer.11 These three receptors have similar ectodomains consisting of two immunoglobulin-like domains and two fibronectin type III repeats, and cytoplasmic regions that contain an intrinsic protein tyrosine kinase domain.12 The TAM receptor tyrosine kinases are widely expressed in various mammalian tissues such as immune, reproductive, and hematopoietic tissues.13,14 Genetic studies using gene-targeting mutations have provided direct insights into the physiological functions of the TAM receptor tyrosine kinases in these locations.15–19 The product of growth arrest-specific gene 6 (Gas6), and protein S (a blood anticoagulant cofactor) are biological ligands of TAM receptors.20 The Gas6/Axl system regulates cell survival, proliferation, migration, adhesion and phagocytosis.12 Gas6 knockout mice were protected from both venous and arterial thrombosis,21 and this protection was afforded through impaired stabilization of platelet aggregation.22 A very recent study demonstrated that Gas6 plays a role in regulating erythropoiesis by enhancing erythropoietin receptor signaling.23 The functional mechanism of Gas6 in erythropoiesis remains to be clarified.
Although TAM receptors are expressed in hematopoietic tissues,24–26 their functions in regulating hematopoiesis remain to be clarified. We have recently demonstrated that TAM receptors cooperatively regulate megakaryocytopoiesis.27 Considering that erythroid cells and megakaryocytes have common precursors (megakaryocyte-erythroid progenitors), we speculated that TAM receptors may participate in regulating erythropoiesis. Here, by investigating erythropoiesis in mice mutant for TAM receptors, we found that Axl and Mer, but not Tyro3, are co-expressed in differentiating erythroid cells, and regulate the differentiation of erythroid cells additivitely. These findings provide novel evidence of a role for TAM receptors in regulating erythropoiesis.
Design and Methods
Animals
Mice singly mutant for Axl or Mer were provided by Dr. Lemke (Salk Institute for Biological Studies, La Jolla, CA. USA), and were progeny of the original colony with a genetic background of 50% 129/sv × 15% C57BL/6. Double mouse knockouts were produced by cross-mating of the single mutant mice. The wild-type controls were the littermates of the mutant mice. All animals were handled in compliance with the guidelines for the care and use of laboratory animals established by the Chinese Council on Animal Care.
Flow cytometry analysis and sorting of erythroid cells
Flow cytometry analysis was performed as described previously.9 Briefly, freshly prepared single-cell suspensions of bone marrows and spleens were pre-incubated in phosphate-buffered saline (PBS)/0.5% bovine serum albumin (BSA) containing mouse IgG (eBioscience, San Diego, CA, USA) to block Fc receptors at 4°C for 10 min. The cells were then immunostained with phycoerythrin (PE)-conjugated anti-Ter119 (1:200) (eBioscience) and fluorescein isothiocyanate (FITC)-conjugated anti-CD71 (1:200) (eBioscience) antibodies for 20 min at 4°C. Propidium iodide was used to exclude dead cells from analysis. In order to sort different stages of erythroblasts, red cells were lysed for 2 min in a lysis buffer (0.15 M NH4Cl, 0.1 mM EDTA, buffered with KHCO3 to pH 7.5) before the immunostaining. R1-R5 cells were sorted with a FACS MoFlo system (DakoCytomatio, Carpinteria, CA, USA) with reduced pressure, and their purity was estimated by re-analysis using cytometry.
Histological analysis
Spleens and femora were fixed in 4% buffered formalin for 48 h. Femora were decalcified in 350 mM EDTA solution (pH 7.4) for 1–2 weeks at 4°C. After embedding in paraffin, serial sections of 5 μm in thickness were sliced with a microtome (E. Leitz®, Wetzlar, Germany). All sections were stained with hematoxylin and eosin after deparaffinization, and examined under a light microscope (X71, Olympus).
BFU-E and CFU-E assays
The procedures used were based on a previous description.6 Briefly, single-cell suspensions of bone marrows and spleens were prepared from 10-week old wild-type and mutant mice, and red cells were removed by lysis. Live cells were counted by trypan blue exclusion. Bone marrow cells (2×104) and spleen cells (1×105) were cultured in triplicate in 12-well plates with 1 mL Iscove’s modified Dulbecco’s medium (IMDM, Gibco) containing 0.9% methylcellulose (Sigma, St Louis, MO, USA) and 1 IU/mL of recombinant erythropoietin (R&D Systems, Minneapolis, MN, USA). Cells were grown in a humidified incubator at 37°C with 5% CO2. The CFU-E containing eight or more cells were counted on day 2, and BFU-E containing 100 or more cells were counted on day 7.
Erythropoietic responses to anemia stress
Mice were injected intraperitoneally on days 0 and 1 with phenylhydrazine solution in PBS. The two groups of mice of each genotype were challenged with different doses (low dose, 25 μg/g body weight; high dose, 50 μg/g) of phenylhydrazine each injection. Blood was collected from the tail vein on days 0, 3, 6, and 9 for measurement of the hematocrit. Ten mice in each group were used in the experiments
Apoptosis and proliferation of erythroid cells
Freshly isolated bone marrow cells were immunostained with PE-conjugated anti-Ter119 and FITC-conjugated anti-CD71 antibodies. For apoptosis analysis, the cells were further incubated with biotin-conjugated annexin-V (eBioscience) according to the manufacturer’s instructions. Different fractions of erythroblasts were gated and analyzed for their apoptosis rate by FACS. For cell cycle analysis, the cells were further stained with propidium iodide for karyotype analysis. Data were analyzed by Modfit software.
Bone marrow transplantation
Bone marrow cells were collected from Axl−/−Mer−/− mice and wild-type littermates. Recipients were lethally irradiated using a single dose of 8.5 Gy 60Co, and injected with 5×106 donor bone marrow cells per mouse through a tail vein. At 6 months after transplantation, bone marrow and spleen cells of the recipients were analyzed for the profiles of erythroid cells. The stromal cells and erythroid cells were isolated and subjected to genotype analysis by polymerase chain reaction (PCR). Primers for the genotyping are listed in Online Supplementary Table S1.
Statistical analyses
Data are presented as mean ± standard error of mean (SEM) for n given determinations. Student’s t tests were used to determine differences between groups. One-way ANOVA was used to calculate the statistical significance for multiple comparisons of means of different groups, Dunnett’s correction was applied to compare genotypes (e.g. Axl−/−, Mer−/− or Axl−/−Mer−/−) to the wild-type control group. All calculations were performed with SPSS version 11.0 statistical software. A p value less than 0.05 was considered statistically significant.
Results
Axl and Mer are expressed in developing erythroid cells
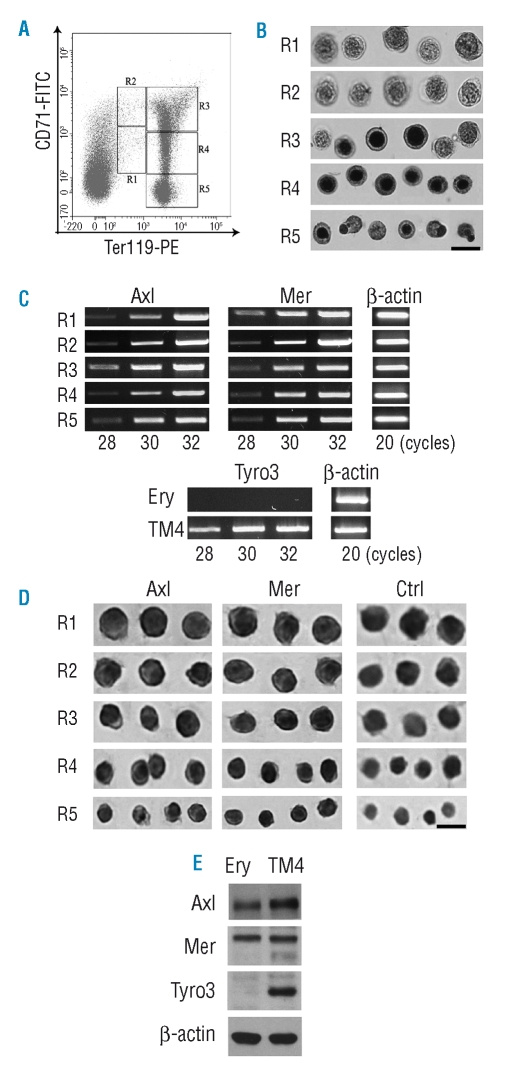
To clarify the expression pattern of TAM receptors in erythroid cells, we isolated erythroblasts of different stages from the bone marrows of 10-week old mice by FACS sorting. The combination of labeling for CD71 and Ter119 allows identification of five district populations of erythroid cells in the bone marrow, defined by their staining pattern: CD71medTer119med, CD71highTer119med, CD71highTer119high, CD71medTer119high, and CD71low-Ter119high referring to regions 1–5 (R1 to R5) in Figure 1A. The R1 to R5 cells correspond, respectively, to erythroid progenitors, proerythroblasts, basophilic erythroblasts, polychromatophilic erythroblasts, and orthochromatophilic erythroblasts. The five populations of erythroid cells were sorted after immunostaining with antibodies against CD71 and Ter119. Based on re-analysis of the sorted cells by flow cytometry, the purity of R1 to R5 cells was 93.6%, 80.3%, 92.5%, 87.1% and 82.0%, respectively. The cells were characterized by benzidine staining and their morphology. During differentiation, erythroid cells become smaller, their chromosomal DNA condenses, their cellular hemoglobin concentration increases, they exit the cell cycle and they extrude nuclei. Benzidine stains for hemoglobin, a hallmark of terminal erythroid differentiation. The morphology of the sorted cells was consistent with the results of the benzidine staining (Figure 1B). All R1 and R2 cells were benzidine-negative, R3 cells were a mix of benzidine-negative and benzidine-positive cells. R4 and R5 cells were all benzidine-positive, indicating that each fraction of cells corresponded to the expected developmental stage.
Figure 1.
Expression of TAM receptors in erythroid cells. (A) FACS density plots of bone marrow erythroid cells. After staining for Ter119 and CD71, the erythroid cells of mouse bone marrow can be distinguished into five distinct populations (R1 to R5) by flow cytometry. (B) The five populations of erythroid cells were sorted by a FACS instrument. The characteristics of the sorted R1 to R5 cells were evaluated by staining with benzidine and their morphology. Representative cells from more than one field are shown. Scale bar = 10 μm. (C) Analysis of the expression of TAM receptors by RT-PCR. Different numbers of PCR cycles were performed to ensure a range of linear amplification. The TM4 cell line was used as a positive control for Tyro3 expression. β-actin was used to control the quality of RNA. (D) Immunocytochemistry for Axl, Mer and Tyro3 proteins on the five populations of erythroid cells. Tyro3 protein was not detected in erythroid cells (data not shown). Representative cells are shown in each region. Scale bar = 10 μm. (E) Western blotting analysis of Axl, Mer and Tyro3 in bone marrow erythroid cells (Ery). TM4 cells were used as controls, and β-actin was used to monitor the loading of samples.
Total RNA was extracted from the sorted erythroblasts, and the expression of TAM receptors was analyzed by reverse transcriptase-PCR. RNA for Axl and Mer was detected in all five populations of erythroid cells (Figure 1C). In contrast, no signal for Tyro3 was observed in these cells. TM4, a Sertoli cell line that positively expresses Tyro3 gene, was used as a control for amplification of Tyro3. The presence of Axl and Mer proteins in developing erythroid cells was demonstrated by immunocytochemistry. Consistent with the results at the mRNA level, all stages of erythroid cells could be positively immunostained for Axl and Mer proteins (Figure 1D), but were negative for Tyro3 (data not shown). In order to confirm the presence of TAM receptors in erythroid cells, we isolated Ter119+ cells from bone marrow by sorting and performed western blotting assays. In agreement with the results of immunocytochemical staining, Axl and Mer proteins, but not Tyro3, were detected in the erythroid cells of the bone marrow (Figure 1E). All three receptors were detected by western blotting in the control TM4 cells.
Axl−/−Mer−/− mice display differentiation inhibition of erythroid progenitors
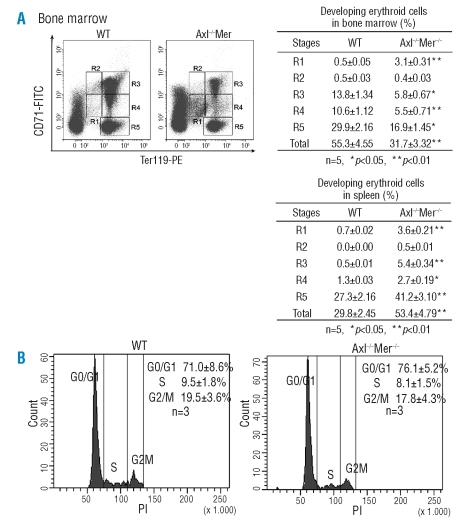
During analysis of the phenotypes of mice mutant for TAM receptors, we found impaired erythropoiesis in Axl−/−Mer−/− mice (Online Supplementary results). To understand which stages of erythroid cells were impaired during erythropoiesis in Axl−/−Mer−/− mice, we first quantified erythroid progenitors of the mutant mice using clonogenic assays for BFU-E and CFU-E. The results of these investigation are presented in Online Supplementary Table S2. Surprisingly, although a significant reduction in erythroid cells was observed in Axl−/−Mer−/− bone marrow, the number of CFU-E progenitors was increased by 1.8-fold compared with the number in wild-type controls. There was, however, no significant difference in the number of earlier progenitors (BFU-E) between bone marrow from Axl−/−Mer−/− and wild-type animals. Notably, Axl−/−Mer−/−spleen-derived BFU-E and CFU-E were increased 31-fold and 104-fold, respectively, compared to in the wild-type controls. The results suggest that Axl and Mer play a role in regulating the development of erythroid progenitors.
Thereafter, we analyzed different stages of erythroblasts in Axl−/−Mer−/− bone marrow and spleen by flow cytometry. After immunostaining for CD71 and Ter119, five erythroid cell populations (R1 to R5) were defined in the bone marrows (Figure 2A). The majority of erythroid cells were Ter119high (R3-R5) in wild-type bone marrow; the ratios of R3-R5 cells to total bone marrow cells were 13.8%, 10.6% and 29.9% respectively. A few erythroid progenitors (R1 cells) were identified. The ratio of R1 cells to total bone marrow cells in wild-type mice was only 0.5%. The erythroid cell profiles in Axl−/−Mer−/− bone marrow were strikingly different. A 6-fold increased ratio (3.1%) of R1 cells was observed in Axl−/−Mer−/− bone marrow. However, the percentages of more differentiated erythroid cells (R3-R5) in Axl−/−Mer−/− bone marrow were reduced significantly by about 2-fold compared to in the wild-type controls. The flow cytometry profile for Axl−/−Mer−/− spleens showed that the percentage of R1 cells was, on average, increased 5-fold compared to that in the wild-type controls (3.6% vs. 0.7%) (Figure 2B). Although the percentage of more differentiated erythroblasts (R3-R5) was also increased in Axl−/−Mer−/−spleens, the ratio of erythroid progenitors to differentiated erythroblasts was markedly increased. These results demonstrate an accumulation of R1 cells in both bone marrow and spleen of Axl−/−Mer−/− mice.
Figure 2.
Flow cytometry assessment of erythroid cell maturation. (A) Erythroid cell profiles at different developmental stages in the bone marrow and spleen. Left panels: representative FACS density plots, and right panels: quantitative comparison of erythroid cells. R1, erythroid progenitors; R2, proerythroblasts; R3, basophilic erythroblasts; R4, late basophilic and polychromatophilic erythroblasts; and R5, orthochromatophilic erythroblasts. (B) Cell cycles of the erythroid progenitors (R1) in bone marrows. The data are mean ± SEM.
The increased erythroid progenitors and decreased differentiating erythroblasts in Axl−/−Mer−/− mice could occur through different mechanisms, including a potentially higher growth rate of the progenitors, elevated apoptosis of developing erythroblasts and inhibition of the differentation of progenitors into erythroblasts. In order to distinguish these possibilities, we examined the apoptosis rate of the erythroblasts. We found that the apoptosis rates of the different populations of erythroid cells were not significantly different in Axl−/−Mer−/− bone marrows and spleens from those in controls (data not shown). In order to analyze the growth rate of the erythroid progenitors, the R1 cells were gated and a karyotype analysis was performed. This analysis showed that cell cycling was comparable between the erythroid progenitors from the bone marrow of wild-type and Axl−/−Mer−/− mice (Figure 2C). Taken together, the results suggest that the differentiation of erythroid cells is inhibited at the transition of erythroid progenitors (R1) to proerythroblasts (R2), which results in the accumulation of erythroid progenitors and the reduction of erythroblasts in the bone marrow from Axl−/−Mer−/− mice.
Axl−/−Mer−/− mice exhibit an impaired erythropoietic response to acute hemolytic anemia
To evaluate the role of TAM receptors in the erythropoietic response to acute hemolytic anemia, we challenged mice with phenylhydrazine to induce hemolytic anemia. Under baseline conditions, all the mutant mice including Axl−/−Mer−/−, Mer−/− and Axl−/−Mer−/− mice had normal hematocrit levels. After a low dose (25 μg/g body weight) of phenylhydrazine, both the mutated mice and the wild-type controls developed anemia (Figure 3A). Although the hematocrit levels were decreased similarly in all the wild-type and mutant mice at day 3 after administration of the phenylhydrazine, the hematocrit of wild-type and Mer−/− mice returned to nearly normal by day 6. In contrast, the hematocrit of Axl−/−Mer−/− and Axl−/− mice did not recover fully until day 9 after phenylhydrazine administration. When given a high dose (50 μg/g body weight) of phenylhydrazine, all the mice developed severe anemia. The wild-type and Mer−/− mice were still capable of recovering normal hematocrit levels on day 9 (Figure 3B). In sharp contrast, the hematocrit dropped to extremely low levels in Axl−/−Mer−/− mice (~13%) and in Axl−/− mice (19%). Notably, 60% (6/10) of Axl−/−Mer−/−mice and 20% (2/10) of Axl−/− mice failed to recover from the anemia and died within 6 days of the administration of phenylhydrazine. The hematocrit of the survivors never recovered, and all remained severly anemic throughout the period of observation (Figure 3B). Notably, Axl−/−Mer−/− mice exhibited a more severely impaired erythropoietic response to acute anemia than did the Axl−/− mice. The results suggest that Axl and Mer additively confer erythropoietic protection against acute anemia and that Axl is more important than Mer.
Figure 3.
Erythropoietic response to phenylhydrazine-induced acute anemia. (A) Hematocrit levels of the mice challenged by a low dose of phenylhydrazine (25 μg/g body weight), (n = 10). (B) Hematocrit levels of the mice challenged with a high dose of phenylhydrazine (50 μg/g), (n≥4). Impaired responses to the induced acute anemia were observed in Axl−/− Mer−/− and Mer−/− mice. (*) p<0.05, (**) p<0.01.
Impaired erythropoiesis in Axl−/−Mer−/− mice is cell autonomous
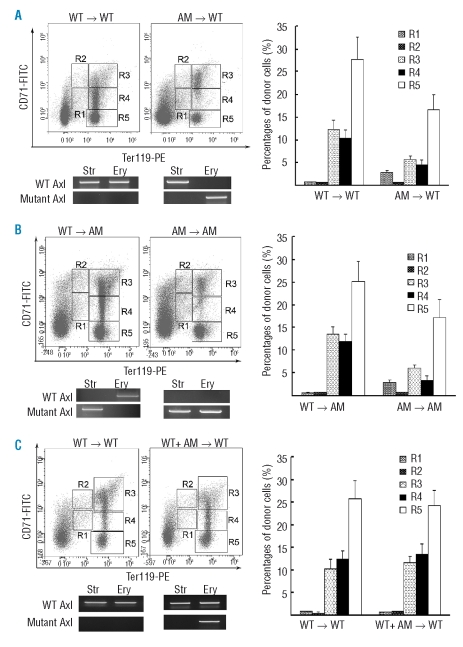
Effective erythropoiesis in bone marrow depends on a specific microenvironment. In order to determine whether loss of Axl and Mer impairs the microenvironment for erythropoiesis and thus results in defective erythropoiesis, we performed bone marrow transplantation experiments. Lethally irradiated Axl−/−Mer−/− mice and wild-type littermates were transplanted with bone marrow cells from either wild-type or Axl−/−Mer−/− mice. The profile of erythroid cells in the reconstituted bone marrows was analyzed 6 months after transplantation. We found that the bone marrows of wild-type mice engrafted with Axl−/−Mer−/− bone marrow cells had impaired erythropoiesis (Figure 4A). Conversely, Axl−/−Mer−/− mice that received wild-type bone marrow cells displayed normal erythropoiesis (Figure 4B). To analyze the origin of microenvironmental and erythroid cells in the reconstituted bone marrows, the genotype of stromal cells and erythroblasts was examined by PCR. By amplifying mutant and wild-type Axl, we found that the reconstituted bone marrows contained host stromal cells and donor erythroid cells (Figure 4A, B, lower panels). Similar analyses were performed on spleens of the engrafted mice. Consistent with the observations in the reconstituted bone marrows, the spleens of the engrafted mice exhibited the phenotype of the donor mice with regards to the size and profile of erythroid cells (data not shown).Taken together, these results demonstrate that the impaired erythropoiesis in Axl−/−Mer−/− mice is cell autonomous.
Figure 4.
Transplantation experiments demonstrated a cell-autonomous role of Axl and Mer in erythropoiesis. (A) Wild-type (WT) recipients engrafted with WT and mutant bone marrows. ( B ) Mutant recipients engrafted with WT and mutant bone marrows. Left panels, representative FACS density plots of donor erythroid cells; lower panels, genotyping for stromal (Str) and erythroid (Ery) cells by PCR; right panels, percentages of donor erythroid cells. The analyses were performed on the bone marrow of recipients at 6 months after transplantation. Values are mean ± SEM, (n=5 per group).
Axl and Mer regulate expression of erythropoiesis-related genes
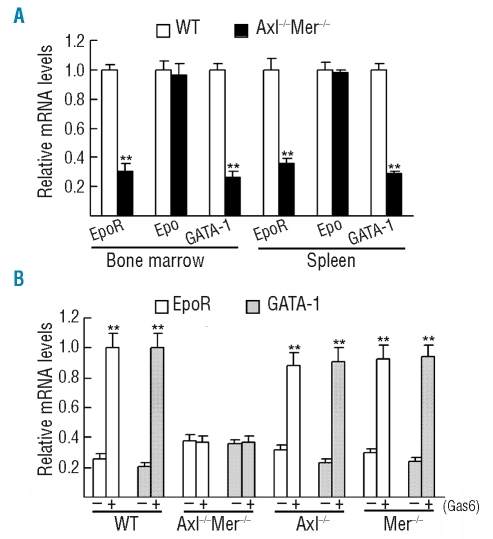
Finally, we tried to determine the molecular mechanism underlying the defect of erythropoiesis in Axl−/−Mer−/− mice. Erythropoietin/EpoR signaling is critical for early erythropoiesis, and several transcription factors such as GATA-1, GATA-2, STAT1, STAT3 and STAT5a/b play important roles in the regulation of erythropoiesis at the erythroid progenitor stage. In order to determine whether the expression of these genes is regulated by TAM receptors, we analyzed the expression of these erythropoiesis-related genes in Axl−/−Mer−/− erythroid progenitors (R1 cells) from bone marrows and spleens by real-time reverse transcriptase-PCR. The results from the bone marrow-derived cells are shown in Figure 5. The same results from spleen-derived cells were similar (data not shown). The relative mRNA levels of EpoR and GATA-1 were significantly down-regulated by about 3.5-fold and 4.2-fold, respectively, in Axl−/−Mer−/− erythroid progenitors (Figure 5A). However, there was no evident difference in the expression of erythropoietin, GATA-2, STAT1, STAT3 and STAT5a between Axl−/−Mer−/− and wild-type erythroid progenitors (data not shown). To further confirm that Axl and Mer regulate the expression of GATA-1 and EpoR, we analyzed the expression of GATA-1 and EpoR in bone marrow-derived erythroid progenitors cultured in vitro in the presence of recombinant Gas6, a common ligand of TAM receptors. We found that Gas6 significantly increased the expression of EpoR and GATA-1 in erythroid progenitors (Figure 5B). In contrast, the Gas6 induction of EpoR and GATA-1 mRNA was completely blocked in Axl−/−Mer−/− cells. Notably, Gas6 can up-regulate the expression of EpoR and GATA-1 in the single mutant (Axl−/−Mer−/−) erythroblasts. The results indicate that Axl and Mer may participate in erythropoiesis through regulating the expression of EpoR and GATA-1.
Figure 5.
Relative mRNA levels analyzed by real-time reverse transcriptase-PCR. (A) Relative expression of the erythropoiesis(Epo)-related genes in the sorted erythroid progenitors (R1) from wild-type and Axl−/−Mer−/− bone marrows and spleens. (B) Sorted R1 cells from bone marrows were cultured in vitro in the presence of Gas6. The relative expression of EpoR and GATA-1 was compared. The data are represented as mean ± SEM of three experiments, (**) p<0.01.
Discussion
Although it is known that TAM receptors are expressed in hematopoietic tissues, their lineage-specific expression patterns and functions during hematopoiesis remain to be clarified. In this study, we show that Ax1 and Mer, but not Tyro3, are co-expressed in all stages of developing erythroid cells, and play a critical role at the early stage of erythropoiesis.
A recent study demonstrated that splenic erythroblasts express all three TAM receptors.23 By contrast, we did not detect the expression of Tyro3 in either bone marrow or splenic erythroid cells. Potential explanations for this discrepancy between the results of the two studies could include the different genetic background of the mice, the fact that the erythroblasts were isolated by different methods and different primer pairs were used. In a baseline state, very few R1 cells can be observed in the bone marrow of adult mice, because they are transiently retained and quickly enter the next stage of erythropoiesis. R1 cells were not, therefore, defined as a population in adult bone marrow and spleen in previous studies.9,28 Since an evident accumulation of this population of cells is observed in Ax1−/−Mer−/− mice, we defined these CD71medTer119med erythroid cells as R1 cells in our present study. In fact, a 6-fold increase in R1 cells and a 2.7-fold decrease in mature red cells was observed in bone marrow from Ax1−/−Mer−/− mice compared to that from wild-type controls. These observations suggest ineffective erythropoiesis in Ax1−/−Mer−/−bone marrow.
The fact that Ax1−/−Mer−/− mice have normal hematologic parameters suggests that compensatory mechanisms of erythropoiesis exist in these animals. A marked splenomegaly and vastly expanded splenic erythropoiesis are observed in Ax1−/−Mer−/−mice. Splenic enlargement in triple mutant mice (Tyro3−/−Ax1−/−Mer−/−) was previously interpreted as a proliferative disorder of lymphocytes.16 Based on this study, the vast splenic erythropoiesis should, at least in part, account for splenomegaly in Ax1−/−Mer−/− mice. The expanded splenic erythropoiesis in Ax1−/−Mer−/− mice could be a compensatory response to the impaired bone marrow erythropoiesis, and contribute to the normal hematologic parameters. Notably, a 5-fold increase in the percentage of R1 cells, but only a 1.5-fold increase in that of the differentiated erythroid cells were observed in spleens from Ax1−/−Mer−/− mice, which suggests that a differentiation inhibition of erythroid progenitors also appears in spleen. In contrast, splenomagaly was not observed in Gas6−/− mice in the previous study,23 which could be explained considering that other ligands (such as protein S) of TAM receptors exist in vivo. It would be useful to investigate whether protein S is involved in erythropoiesis through TAM receptors. In addition, the genetic background of the two models was not identical, which could also have contributed to the phenotypic discrepancy. The normal hematologic parameters may be also partially attributable to a longer lifespan of red blood cells resulting from slower clearance of senescent red cells in these mutant mice. In the model of acute anemia induced by phenyl-hydrazine, there was a delayed response to erythropoietic stress in Ax1−/−Mer−/− mice, confirming that the rate of erythropoiesis is altered because of the loss of Axl and Mer receptors. Notably, although impaired erythropoiesis was not observed in single mutant (Ax1−/− or Mer−/−) mice in baseline conditions, Ax1−/− mice but not Mer−/− mice appeared to have a significantly impaired response to phenylhydrazine-induced erythropoietic stress. The impaired response to phenylhydrazine-induced anemia was more severe in Ax1−/−Mer−/− mice than in Ax1−/− mice. These observations suggest that Axl and Mer additively regulate erythropoiesis, but that Axl is more important than Mer. This suggestion is consistent with the results of a recent study.23 Furthermore, our study demonstrates that the impaired erythropoiesis in Ax1−/−Mer−/− mice is attributable to an inhibition of differentiation from erythroid progenitors to proerythroblasts.
Normal hematopoiesis relies heavily on the bone marrow microenviroment.29 Bone marrow engraftment assays demonstrated that the inefficient erythropoiesis is due to an intrinsic lack of Ax1 and Mer in erythroid cells, but not due to the bone marrow microenviroment. Notably, to maintain a similar genetic background between host and donor mice, we performed transplants between mutant mice and wild-type littermates. We then examined the origin of stromal cells and erythroblasts by genotyping. This approach could be practical in bone marrow transplantation assays.
Hematopoiesis is controlled in large part by lineage-specific transcription factors.30 GATA-1 is highly expressed in erythroid progenitors and serves a critical function in the maturation of committed erythroid precursors.31 In this study, we demonstrated that GATA-1 expression is down-regulated by a lack of TAM receptor signaling. The down-regulation of GATA-1 in Ax1−/−Mer−/− erythroid progenitors could contribute to the inhibition of differentiation of the progenitors. EpoR signaling is essential for the differentiation of erythroid progenitors,32,33 and the expression of the receptor is down-regulated in Ax1−/−Mer−/− erythroid progenitors. The impaired erythropoiesis in Ax1−/−Mer−/− mice could, therefore, also be attributable to the down-regulation of EpoR in erythroblasts lacking Axl and Mer. Furthermore, we demonstrated that recombinant Gas6 up-regulates EpoR and GATA-1 expression in vitro. A recent study showed that Gas6 enhances the activity of erythropoietin in stimulating erythropoiesis.23 The up-regulation of EpoR could be at the basis of Gas6 induction of erythropoietin activity. GATA-1 is an erythropoietin-regulated transcription factor.8 In this study, we found that TAM signaling induces GATA-1 and EpoR expression. The fact that Gas6 up-regulates GATA-1 expression in cultured erythroblasts without erythropoietin suggests that TAM signaling induces GATA-1 expression in a way independent of EpoR activation.
In conclusion, we demonstrated that Axl and Mer play critical roles in mouse erythropoiesis, which reveals a new function of Axl and Mer. Understanding the mechanics of erythropoiesis regulation by TAM receptors could lend to novel therapeutic approaches to erythropoietic disorders.
Supplementary Material
Acknowledgments
we are grateful to Dr. Lemke for kindly providing mutant mice.
Footnotes
Funding: this work was supported by the National Basic Research Program of China (Grant No. 2006CB504001, 2007CB947504), and the National Natural Science Foundation of China (Grant No. 30570678).
Authorship and Disclosures
DH designed the study; HT, SC, HWa and HWu performed the research; HT, SC, HWa, QL and DH analyzed the data; HT and DH wrote the paper.
The authors reported no potential conflicts of interest.
References
- 1.Zon LI. Developmental biology of hematopoiesis. Blood. 1995;86:2876–91. [PubMed] [Google Scholar]
- 2.Gregory CJ, Eaves AC. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978;51:527–37. [PubMed] [Google Scholar]
- 3.Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci USA. 1996;93:12355–8. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch JJ, Watts JA, Vakoc CR, Yao Y, Wang H, Hardison RC, et al. Global regulation of erythroid gene expression by transcription factor GATA-1. Blood. 2004;104:3136–47. doi: 10.1182/blood-2004-04-1603. [DOI] [PubMed] [Google Scholar]
- 5.Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89:3636–43. [PubMed] [Google Scholar]
- 6.Halupa A, Bailey ML, Huang K, Iscove NN, Levy DE, Barber DL. A novel role for STAT1 in regulating murine erythropoiesis: deletion of STAT1 results in overall reduction of erythroid progenitors and alters their distribution. Blood. 2005;105:552–61. doi: 10.1182/blood-2003-09-3237. [DOI] [PubMed] [Google Scholar]
- 7.Kirito K, Uchida M, Yamada M, Miura Y, Komatsu N. A distinct function of STAT proteins in erythropoietin signal transduction. J Biol Chem. 1997;272:16507–13. doi: 10.1074/jbc.272.26.16507. [DOI] [PubMed] [Google Scholar]
- 8.Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–91. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 9.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–73. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 10.Penta K, Sawyer ST. Erythropoietin induces the tyrosine phosphorylation, nuclear translocation, and DNA binding of STAT1 and STAT5 in erythroid cells. J Biol Chem. 1995;270:31282–7. doi: 10.1074/jbc.270.52.31282. [DOI] [PubMed] [Google Scholar]
- 11.Lemke G, Lu Q. Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol. 2003;15:31–6. doi: 10.1016/s0952-7915(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 12.Hafizi S, Dahlback B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. Febs J. 2006;273:5231–44. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- 13.Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–36. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–8. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 16.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–11. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 17.Caraux A, Lu Q, Fernandez N, Riou S, Di Santo JP, Raulet DH, et al. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat Immunol. 2006;7:747–54. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- 18.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–36. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–11. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 20.Taylor IC, Roy S, Yaswen P, Stampfer MR, Varmus HE. Mouse mammary tumors express elevated levels of RNA encoding the murine homology of SKY, a putative receptor tyrosine kinase. J Biol Chem. 1995;270:6872–80. doi: 10.1074/jbc.270.12.6872. [DOI] [PubMed] [Google Scholar]
- 21.Angelillo-Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–21. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 22.Angelillo-Scherrer A, Burnier L, Flores N, Savi P, DeMol M, Schaeffer P, et al. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J Clin Invest. 2005;115:237–46. doi: 10.1172/JCI22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angelillo-Scherrer A, Burnier L, Lambrechts D, Fish RJ, Tjwa M, Plaisance S, et al. Role of Gas6 in erythropoiesis and anemia in mice. J Clin Invest. 2008;118:583–96. doi: 10.1172/JCI30375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neubauer A, Fiebeler A, Graham DK, O’Bryan JP, Schmidt CA, Barckow P, et al. Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood. 1994;84:1931–41. [PubMed] [Google Scholar]
- 25.Crosier PS, Freeman SA, Orlic D, Bodine DM, Crosier KE. The Dtk receptor tyrosine kinase, which binds protein S, is expressed during hematopoiesis. Exp Hematol. 1996;24:318–23. [PubMed] [Google Scholar]
- 26.Graham DK, Bowman GW, Dawson TL, Stanford WL, Earp HS, Snodgrass HR. Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene. 1995;10:2349–59. [PubMed] [Google Scholar]
- 27.Wang H, Chen S, Chen Y, Wang H, Wu H, Tang H, et al. The role of Tyro 3 subfamily receptors in the regulation of hemostasis and megakaryocytopoiesis. Haematologica. 2007;92:643–50. doi: 10.3324/haematol.10939. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Pop R, Sadegh C, Brugnara C, Haase VH, Socolovsky M. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood. 2006;108:123–33. doi: 10.1182/blood-2005-11-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 30.Orkin SH. Development of the hematopoietic system. Curr Opin Genet Dev. 1996;6:597–602. doi: 10.1016/s0959-437x(96)80089-x. [DOI] [PubMed] [Google Scholar]
- 31.Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D’Agati V, et al. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–60. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 32.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–55. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.