An elevated echocardiography-determined tricuspid regurgitant jet velocity predicts high systolic pulmonary artery pressure and early mortality in adults with sickle cell disease. The study provides evidence for independent associations of elevated jet velocity with hemolysis and oxygen desaturation in children and adolescents with sickle cell disease.
Keywords: sickle cell disease, pulmonary hypertension, hemolysis, oxygen saturation, tricuspid regurgitant jet velocity, children
Abstract
Background
Elevation of echocardiography-determined tricuspid regurgitant jet velocity predicts high systolic pulmonary artery pressure and early mortality in adults with sickle cell disease. The definition, prevalence and clinical correlates of elevated jet velocity have not been established in pediatric patients. The present study tested the hypotheses that elevated jet velocity affects 10% of pediatric patients, is associated with both hemolysis and hypoxia, and has clinical correlates with acute chest syndrome, stroke, transfusion requirement and abnormal 6-minute walk test results.
Design and Methods
A prospective multicenter study of 310 patients aged 3–20 years old with sickle cell disease under basal conditions and 54 matched controls was conducted. A hemolytic index was generated by principal component analysis of the levels of lactate dehydrogenase, aspartate aminotransferase and bilirubin and reticulocyte count.
Results
Elevated jet velocity (defined as ≥2.60 m/sec based on the mean±2 SD in controls) occurred in 32 patients (11.0%) including one child of 3 years old. After adjustment for hemoglobin concentration, systolic blood pressure and left ventricular diastolic function, a 2 SD increase in the hemolytic index was associated with a 4.5-fold increase in the odds of elevated jet velocity (p=0.009) and oxygen saturation ≤98% with a 3.2-fold increase (p=0.028). Two or more episodes of acute chest syndrome had occurred in 28% of children with elevated jet velocity compared to in 13% of other children (p=0.012), more than ten units of blood had been transfused in 39% versus 18% (p=0.017) and stroke had occurred in 19% versus 11% (p=0.2). The distance walked in 6-minute walk tests did not differ significantly, but oxygen saturation declined during the tests in 68% of children with elevated jet velocity compared to in 32% of other children (p=0.0002).
Conclusions
According to a pediatric-specific definition the prevalence of elevated jet velocity in this population of young patients with sickle cell disease was 11%. The study provides evidence for independent associations of elevated jet velocity with hemolysis and oxygen desaturation. Further investigations should address whether elevated jet velocity may indicate future complications and whether early intervention is beneficial.
Introduction
Echocardiographic estimation of pulmonary artery pressure by measuring the tricuspid valve regurgitant jet velocity has been validated as a useful screening method for pulmonary hypertension in adult patients with sickle cell disease.1 A jet velocity of 2.5 m/sec or more, which corresponds to a systolic pulmonary artery pressure of 30 mmHg or more, has been used for research purposes to define elevated pulmonary artery pressure in adults with sickle cell disease and the prevalence is about 30%.1–3 Even though this definition includes mild elevations in pulmonary artery pressure, adult sickle cell disease patients with a regurgitant jet velocity of 2.5 m/sec or more have an increased risk of mortality.1,4,5 The prevalence and natural history of elevated jet velocity in children with sickle cell disease at steady state are largely unknown. A recent review of published studies comprising over 600 children combined indicated that the prevalence of a jet velocity of 2.5 m/sec or more is about 30%.6 Most studies were not prospective and some of the children were evaluated during a vasoocclusive crisis or other exacerbation of sickle cell disease. These studies were, therefore, likely biased toward patients with more severe illness.
Pulmonary hypertension may develop in most forms of hereditary and chronic hemolytic anemia7–10 suggesting that there is a clinical syndrome of hemolysis-associated pulmonary hypertension. Nevertheless, an association between hemolysis and pulmonary hypertension in sickle cell disease has been questioned because, in most studies thus far, not all markers of hemolysis have had significant associations with estimated pulmonary artery pressure. For example, in a recent study of 62 children and adolescents with hemoglobin SS or Sβ° thalassemia,11 reticulocyte count had a significant association with jet velocity but hemoglobin, lactate dehydrogenase and bilirubin concentrations did not. Humans exposed to chronic hypoxia have a tendency to develop pulmonary hypertension.12 Patients with sickle cell disease may experience chronic hypoxia due to anemia, upper airway obstruction, chronic hemoglobin oxygen desaturation and repeated episodes of vasoocclusive pain crisis or acute chest syndrome.13–18 Hypoxia might, therefore, be a factor in the development of pulmonary hypertension in patients with sickle cell disease.
We conducted a multicenter study to determine prospectively the prevalence of elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease at baseline and to test the hypothesis that markers of hemolysis and hypoxia are both independently associated with increased jet velocity in patients with sickle cell disease.
Design and Methods
Study hypotheses
The prospectively determined study hypotheses were: (i) that elevated tricuspid regurgitant jet velocity occurs in 10–20% of children with sickle cell disease; (ii) that systolic blood pressure, hemolysis, hypoxia and degree of anemia are independent risk factors; (iii) that elevated jet velocity is associated with increased need for blood transfusions, risk of stroke, and risk of acute chest syndrome and with a shorter distance walked in the 6-minute walk test.
Enrollment of participants
The study was prospectively designed to enroll 600 patients with sickle cell disease and 100 controls and the present report presents the results of an analysis conducted shortly after the mid-point. Patients (n=310) 3 to 20 years of age with sickle cell disease confirmed by hemoglobin electrophoresis or high performance liquid chromatography were recruited at the Children’s National Medical Center and Howard University Hospital, Washington DC, and University of Michigan, Ann Arbor Michigan. A 10% prevalence of elevated tricuspid regurgitant jet velocity was hypothesized and the analysis of 310 patients provided the power to observe this prevalence with a 95% confidence interval of 6.5% to 14.5%. Controls (n = 54), who were matched by age, sex and ethnicity to every sixth patient recruited at each institution, included relatives and acquaintances of participants, and could not have been hospitalized or have presented to the emergency room with an acute illness in the previous 3 weeks. Children with sickle cell trait (n=12) and hemoglobin C trait (n=5) qualified as controls. Patients were invited to participate on a consecutive basis, as they presented for routine outpatient care; no attempt was made to select them by known or perceived risk factors. At least 3 weeks had to have elapsed since hospitalization, emergency department or clinic visit for acute chest syndrome, a pain crisis, infection or other sickle cell disease-related complication. The study was approved by the Institutional Review Board of each participating institution and written informed consent was obtained for all participants.
Clinical evaluation
The medical history was recorded on a standard form designed for this study and in general did not distinguish between active and past problems. An unencouraged 6-minute walk test was conducted according to the guidelines of the American Thoracic Society.19 Complete blood count and reticulocyte count were measured by a Beckman Coulter LH 750 Analyzer (Fullerton, CA, USA) at Howard University and the University of Michigan, and by a Sysmex 2100QC (Sysmex America, Inc., Mundelein, IL, USA) at the Children’s National Medical Center. Serum biochemistry was evaluated by a Beckman Coulter Unicel DXC800 at Howard University, by a RXL 2 Max, Model 973626 (Dade-Behring, Inc., Dover, DE, USA) at the Children’s National Medical Center and by a Siemens Advia 2400 (Deerfield, IL, USA) at the University of Michigan. Pulse oximetry was measured by a Criticare Model 506 Series (Waukesha, WI, USA) at Howard University, a Welch Allyn instrument (Beaverton, OR, USA) or a SureSigns VS3 No. 3000 (Philips Medical System, Andover, MA, USA) at the Children’s National Medical Center and by a Masimo Rad 8 Signal Extraction Pulse Oximeter at the University of Michigan.
Echocardiography
Transthoracic echocardiography was performed using a Philips Sonos 5500/7500 or iE33 (Philips Medical Systems, Best, Holland), Acuson Sequoa (Siemans Medical Systems, Mountain View, CA, USA), or General Electric VIVID 7 or VIVID I (General Electric, Milwaukee, WI, USA). All images were recorded digitally and subsequently reviewed on an offline digital work station. Cardiac images were obtained, measurements were performed, and the studies were interpreted according to the guidelines of the American Society of Echocardiography.20
Tricuspid regurgitation was assessed in the parasternal long and short-axes, and apical four-chamber views.21 To standardize across the spectrum of ages and body sizes, left ventricular dimensions was expressed as a standard deviation below or above the mean for body surface area (z-score) and left ventricular mass was indexed to body surface area.22 Left ventricular diastolic function was assessed by measuring the peak velocities of the mitral inflow E wave23 and the tissue Doppler E wave at the basilar segments of the left ventricular free wall and interventricular septum. Left atrial (and left ventricular filling) pressures were estimated by calculating the ratio of the mitral inflow E wave to the tissue Doppler E wave.24 Based on the mean ± 2 SD in the controls of this study, peak regurgitant jet velocities of 2.60 m/sec or more and mitral valve E/tissue Doppler E ratios of more than 9.22 were taken to be elevated. The echocardiograms were centrally reviewed. The study was designed to consider right-sided cardiac catheterization for participants found to have a jet velocity of 3.0 m/sec or more. Only one patient had a jet velocity in this range and the parents refused catheterization.
Statistical analysis
Statistical calculations were made by STATA 10.0 (College Station, TX, USA). Continuous variables were assessed for normality and skewed variables were transformed by the method that most closely approximated normality. Student’s t test and the Kolmogorov-Smirnov non-parametric test were used to compare continuous variables between patients with sickle cell disease and control subjects, and Pearson’s χ2 test was used to compare dichotomous variables. Bonferroni adjustments were made for multiple comparisons and for interim analysis. A logistic regression model of tricuspid regurgitant jet velocity less than 2.60 m/sec versus 2.60 m/sec or more was employed to assess the independent associations of prospectively chosen variables with elevated jet velocity.
Principle component analysis was performed to compute a new variable – a hemolytic index – that explained the maximum variance among reticulocyte count and age- and site-adjusted values for lactate dehydrogenase, aspartate aminotransferase and total bilirubin concentrations.25 Principal component analysis is useful for studying underlying mechanisms reflected in individual biological measurements.26 The hemolytic index is a normalized factor of the four hemolytic variables with mean of 0 and SD of 1.56. Because of the different reference ranges for the four markers of hemolysis among the three research sites, the computation of the hemolytic index was stratified by research site. The hemolytic index explained 61–64% of total variance of the four factors. It had correlations of r=0.82–0.90 with age-adjusted lactate dehydrogenase concentration, 0.74–0.88 with age-adjusted aspartate aminotransferase concentration, 0.76–0.82 with age-adjusted total bilirubin concentration and 0.57–0.77 with reticulocyte count.
Results
Clinical characteristics of sickle cell disease patients and control participants
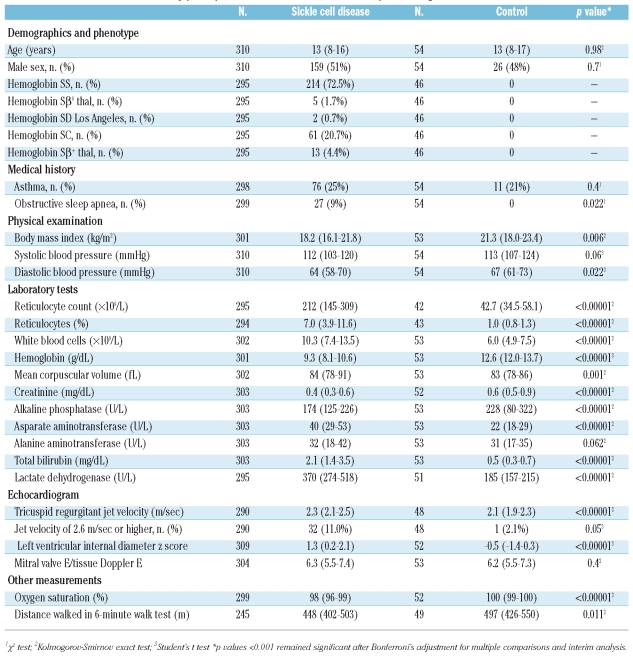
Significant differences were observed in systolic blood pressure, measures of hemolysis (reticulocyte count, lactate dehydrogenase, aspartate aminotranserase, total bilirubin), creatinine, hemoglobin oxygen saturation, 6-minute walk test results and left ventricular internal diameter z-score, a reflection of cardiac output. Only 5% of patients versus 4% of controls had a mitral valve E/tissue Doppler E ratio greater than 9.22, a reflection of elevated left atrial pressure (p=0.6) (Table 1).
Table 1.
Clinical characteristics of study participants. Results are median and interquartile range unless otherwise indicated.
Prevalence of elevated tricuspid regurgitant jet velocity
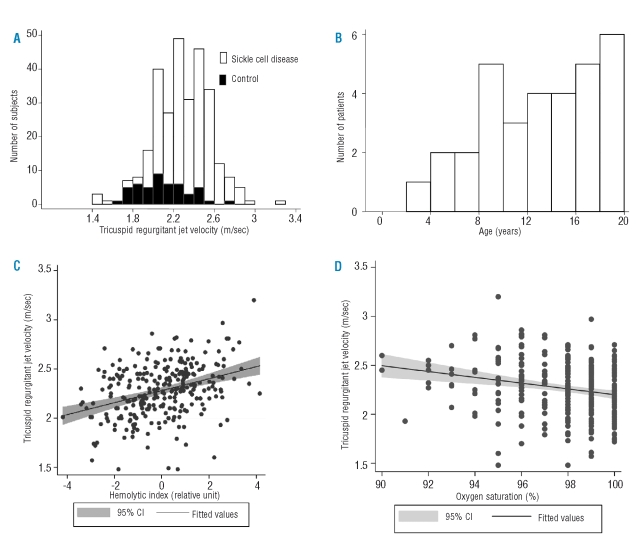
The jet velocity could not be measured in 20 (6.5%) of the patients with sickle cell disease and in 6 (11.1%) of the controls (p=0.2). Among those in whom it could be measured, the jet velocity was significantly higher in the patients with sickle cell disease (Table 1, Figure 1A). Based on the mean ± 2SD jet velocity in controls, the upper limit of normal was 2.59 m/sec. Eleven percent (95% confidence interval of 7.3% to 15.8%) of patients with sickle cell disease had elevated jet velocities of 2.60 m/sec or higher. Figure 1b shows the frequency distribution of ages of the patients with elevated jet velocity and that one patient had elevated jet velocity at 3 years of age.
Figure 1.
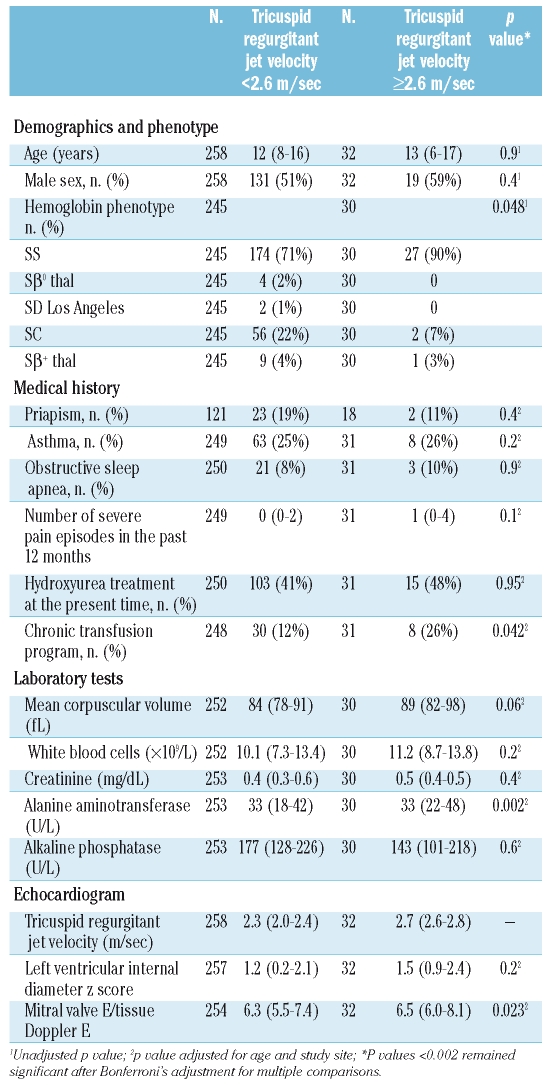
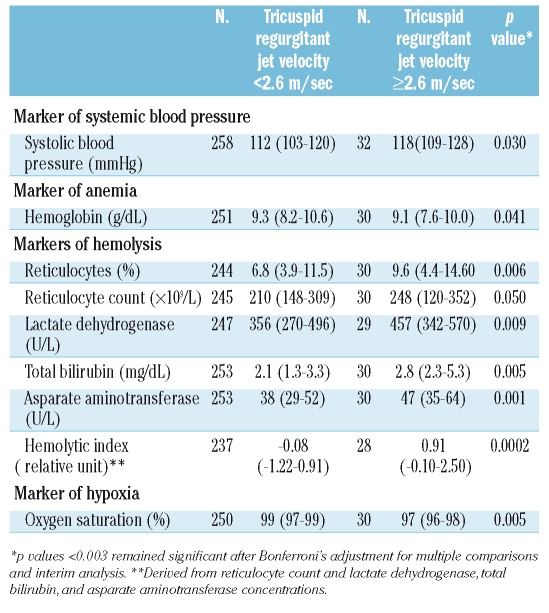
(A) Distribution of tricuspid regurgitant jet velocity in sickle disease patients and controls. (B) Distribution of ages in sickle cell disease patients with elevated jet velocity. (C) Correlation of jet velocity and the hemolytic index in sickle cell disease patients (N=290, r=0.35, p<0.0001). (D) Correlation of jet velocity and oxygen saturation in sickle cell disease patients (N=287, r=−0.20, p=0.001)
Clinical variables according to jet velocity category in patients with sickle cell disease
The proportion of patients with severe sickling phenotypes (hemoglobins SS, Sβ° thal or SD Los Angeles) was higher in the patients with elevated jet velocity. Histories of asthma, sleep apnea, severe pain episodes in the past year and hydroxyurea therapy did not differ significantly. The echocardiogram measurement of mitral valve E/tissue Doppler E ratio was higher (Table 2).
Table 2.
Clinical characteristics of sickle cell cases according to tricuspid regurgitant jet velocity category. Results are median and interquartile range unless otherwise indicated.
Potential risk factors for pulmonary hypertension according to jet velocity category
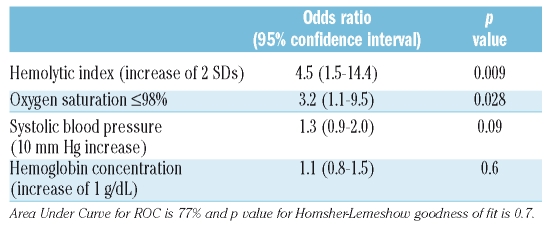
We prospectively hypothesized that systolic blood pressure, hemoglobin concentration and markers of hemolysis and hypoxia would be associated with elevated jet velocity (Table 3). Systolic blood pressure was higher in the patients with elevated jet velocity (p=0.030). Hemoglobin concentration, a potential inverse marker of both hemolysis and hypoxia, was lower (p=0.041). Each of the markers of hemolysis was higher in the patients with elevated jet velocity. The most significant association was with the hemolytic index (Figure 1c; p<0.0001), derived by principal component analysis from the reticulocyte count and the concentrations of lactate dehydrogenase, aspartate aminotransferase and bilirubin. The hemoglobin oxygen saturation measured by pulse oximetry was significantly lower in the patients with elevated jet velocity (Figure 1d; p=0.001).
Table 3.
Distribution of markers prospectively hypothesized to be associated with pulmonary hypertension according to tricuspid regurgitant jet velocity category. Results are median and interquartile range; analyses adjusted for age and study site.
Logistic regression analyses of elevated tricuspid regurgitant jet velocity
In a logistic regression analysis of jet velocity that included four variables chosen prospectively at study design, the hemolytic index and an oxygen saturation of 98% or less had significant independent associations with elevated jet velocity while systolic blood pressure and hemoglobin concentration were not associated (Table 4). In a logistic regression analysis that included these same variables plus a measure of left ventricular diastolic dysfunction (mitral E/tissue Doppler E ratio), the hemolytic index and oxygen saturation of 98% or less continued to have significant associations with elevated jet velocity but neither hemoglobin concentration (p=0.5) nor mitral E/tissue Doppler E ratio (p=0.2) had significant associations (full analysis not shown).
Table 4.
Independent associations of prospectively chosen clinical variables with elevated tricuspid regurgitant jet velocity in logistic regression analysis.
Potential clinical correlates of pulmonary hypertension according to jet velocity category
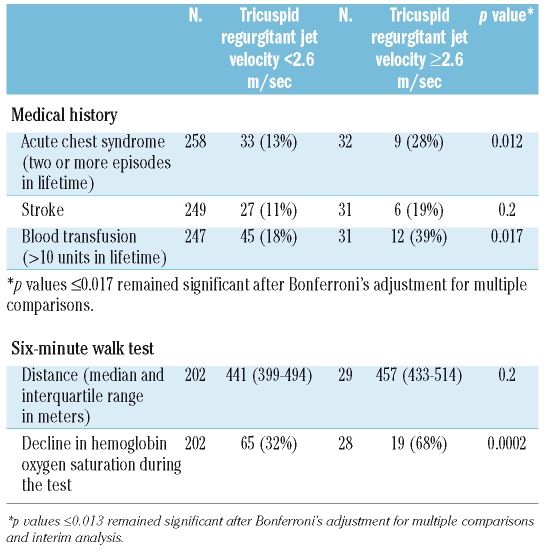
We prospectively hypothesized that elevated tricuspid regurgitant jet velocity would be associated with increased frequency of acute chest syndrome and stroke, and increased need for blood transfusions. Patients with elevated jet velocity had a medical history of higher numbers of acute chest syndrome episodes (p=0.012) and blood transfusions (p=0.017). A history of stroke was given by 19% of the patients with elevated jet velocity compared to 11% of those with normal jet velocity (p=0.2). We also hypothesized that elevated jet velocity would be associated with a shorter distance walked in 6 minutes, but this did not turn out to be the case. However, a decline in hemoglobin oxygen saturation during the 6-minute walk was greater in the children with elevated jet velocity (p=0.0002) (Table 5).
Table 5.
Prospectively chosen potential clinical correlates of pulmonary hypertension according to tricuspid regurgitant jet velocity category. Results in n. (%) unless otherwise stated; analyses adjusted for age and study site.
Discussion
We found the prospectively-determined prevalence of elevated tricuspid regurgitant jet velocity to be 11% among unselected pediatric patients with sickle cell disease under basal circumstances using a definition of 2.6 m/sec or more as derived from the controls. This prevalence is lower than the prevalences in most of the previous studies of pediatric sickle cell disease patients.6,11 The present study was prospective, included children as young as 3 years of age with mild to severe sickle phenotypes, and avoided effects of exercise or acute vasoocclusive crisis, known to be confounding factors for the determination of steady state jet velocity in patients.27 In particular, patients who were hospitalized in the preceding 3 weeks could not be enrolled and this tended to exclude children with frequent or prolonged hospitalizations, a group that may have a higher prevalence of elevated jet velocity. The threshold for elevated jet velocity was 0.1 m/sec higher than in most previous studies. A relatively high proportion of the participants were on hydroxyurea therapy, but this was not associated with elevated jet velocity.
As prospectively hypothesized, the results of this study support independent associations of markers of hemolysis and hypoxia with elevated tricuspid regurgitant jet velocity in children with sickle cell disease. Each of the clinical measurements that are recognized to reflect degree of hemolysis had associations with elevated jet velocity. A hemolytic index, derived by principal component analysis from lactate dehydrogenase, aspartate aminotransferase and bilirubin concentrations and reticulocyte count but not hemoglobin level, correlated with elevated jet velocity with a high degree of statistical significance. This correlation did not appear to merely reflect the degree of anemia, increased blood volume or cardiac output, because the hemolytic index was independently associated with increased odds of elevated jet velocity even after adjustment for hemoglobin concentration. Furthermore, the hemoglobin concentration did not have a significant independent effect on jet velocity in the logistic regression analyses, suggesting against a strong primary role of anemia in addition to the association with hemolysis. This observation is consistent with the view that a hemolytic vasculopathy contributes to pulmonary hypertension in sickle cell disease.28,29
Low hemoglobin oxygen saturation is associated with markers of hemolysis16,30,31 and increased risk of stroke in sickle cell disease.32 In this study, lower oxygen saturation correlated significantly with increased jet velocity even after adjustment for the hemolytic index. This finding is consistent with our study hypothesis that hypoxia itself, in addition to hemolysis, contributes to sickle cell-related pulmonary hypertension.
Left ventricular diastolic dysfunction is associated with mortality in sickle cell disease and may contribute to elevated pulmonary artery pressures,33 and elevated mitral valve E/tissue Doppler E ratio is a marker of left ventricular diastolic dysfunction.24 Only 5.3% of the patients with sickle cell disease had left ventricular diastolic dysfunction, as defined by a mitral valve E/tissue Doppler E ratio of greater than 9.22, and there was not a significant association of this ratio with elevated jet velocity in multivariate logistic regression analysis. Thus, left ventricular diastolic dysfunction seemed to be a relatively minor factor in the development of elevated jet velocity in this study.
As prospectively hypothesized, we obtained histories of significantly increased numbers of acute chest syndrome episodes and units of blood transfused in the patients with elevated jet velocity in this study.
Although almost twice as many patients with elevated jet velocity had a history of stroke, this difference was not statistically significant. About one-third of the children and adolescents in our data set who received more than ten transfusions were being treated for stroke, and it is conceivable that similar mechanisms are at play in cerebral and pulmonary vasculopathy.34 It is also possible that chronic transfusion therapy for stroke may have prevented the development of elevated jet velocity in some patients.
In contrast to our hypothesis and results of studies in adults, the elevated jet velocity in the children and adolescents in this study was not associated with a shorter distance covered in the 6-minute walk test. The reason for this lack of association is not clear, but it may be that limitation in the 6-minute walk test occurs only after systolic pulmonary artery pressure has been elevated for a certain length of time. Another potentially adverse physiological parameter related to the 6-minute walk test, a decrease in oxygen saturation of hemoglobin during the walk, was significantly more common in the patients with elevated jet velocity. Other studies have shown that oxygen desaturation during the 6-minute walk test is associated with mortality in patients with primary pulmonary hypertension35 and reflects pulmonary disease severity in those with secondary pulmonary hypertension.36
The associations of high jet velocity with a history of acute chest syndrome or blood transfusions and with oxygen desaturation during the 6-minute walk test suggest that children with elevated jet velocity may be at high risk of increased complications in later decades of life. Further investigations are, therefore, needed to clarify the clinical consequences of elevated jet velocity in children and adolescents, and to determine whether early intervention may prevent morbidity and early mortality.
Footnotes
Funding: supported in part by grant nos. 2 R25 HL003679-08 and 1 R01 HL079912-02 from NHLBI, by Howard University GCRC grant no 2MOI RR10284-10 from NCRR, NIH, Bethesda, MD, and by the intramural research program of the National Institutes of Health (USA).
Authorship and Disclosures
CPM participated in designing the study, data collection and preparing the manuscript; CS participated in data collection and preparing the manuscript; AC participated in data collection and preparing the manuscript; SR participated in data collection and preparing the manuscript; GE participated in data collection and preparing the manuscript; ND participated in data collection; OO participated in data collection and preparing the manuscript; DD participated in data collection and preparing the manuscript; MN participated in data analysis and preparing the manuscript; GJK participated in designing the study and preparing the manuscript; MTG participated in designing the study and preparing the manuscript; OLC participated in designing the study, data collection and preparing the manuscript; VG participated in designing the study, data collection, data analysis and prepared the manuscript.
The authors reported no potential conflicts of interest.
References
- 1.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 2.Ataga KI, Sood N, De Gent G, Kelly E, Henderson AG, Jones S, et al. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117:665–9. doi: 10.1016/j.amjmed.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Castro O, Gladwin MT. Pulmonary hypertension in sickle cell disease: mechanisms, diagnosis, and management. Hematol Oncol Clin North Am. 2005;19:881–96. doi: 10.1016/j.hoc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, et al. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134:109–15. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 5.De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ. Pulmonary hypertension associated with sickle cell disease: clinical and laboratory endpoints and disease outcomes. Am J Hematol. 2008;83:19–25. doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- 6.Kato GJ, Onyekwere OC, Gladwin MT. Pulmonary hypertension in sickle cell disease: relevance to children. Pediatr Hematol Oncol. 2007;24:159–70. doi: 10.1080/08880010601185892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayag-Barin JE, Smith RE, Tucker FC., Jr Hereditary spherocytosis, thrombocytosis, and chronic pulmonary emboli: a case report and review of the literature. Am J Hematol. 1998;57:82–4. doi: 10.1002/(sici)1096-8652(199801)57:1<82::aid-ajh15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Collins FS, Orringer EP. Pulmonary hypertension and cor pulmonale in the sickle hemoglobinopathies. Am J Med. 1982;73:814–21. doi: 10.1016/0002-9343(82)90763-x. [DOI] [PubMed] [Google Scholar]
- 9.Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, et al. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001;97:3411–6. doi: 10.1182/blood.v97.11.3411. [DOI] [PubMed] [Google Scholar]
- 10.Heller PG, Grinberg AR, Lencioni M, Molina MM, Roncoroni AJ. Pulmonary hypertension in paroxysmal nocturnal hemoglobinuria. Chest. 1992;102:642–3. doi: 10.1378/chest.102.2.642. [DOI] [PubMed] [Google Scholar]
- 11.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Prevalence and risk factors of elevated pulmonary artery pressures in children with sickle cell disease. Pediatrics. 2008;121:777–82. doi: 10.1542/peds.2007-0730. [DOI] [PubMed] [Google Scholar]
- 12.Preston IR. Clinical perspective of hypoxia-mediated pulmonary hypertension. Antioxid Redox Signal. 2007;9:711–21. doi: 10.1089/ars.2007.1587. [DOI] [PubMed] [Google Scholar]
- 13.Samuels MP, Stebbens VA, Davies SC, Picton-Jones E, Southall DP. Sleep related upper airway obstruction and hypoxaemia in sickle cell disease. Arch Dis Child. 1992;67:925–9. doi: 10.1136/adc.67.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rackoff WR, Kunkel N, Silber JH, Asakura T, Ohene-Frempong K. Pulse oximetry and factors associated with hemoglobin oxygen desaturation in children with sickle cell disease. Blood. 1993;81:3422–7. [PubMed] [Google Scholar]
- 15.Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101:846–8. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 16.Setty BN, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003;362:1450–5. doi: 10.1016/S0140-6736(03)14689-2. [DOI] [PubMed] [Google Scholar]
- 17.Homi J, Levee L, Higgs D, Thomas P, Serjeant G. Pulse oximetry in a cohort study of sickle cell disease. Clin Lab Haematol. 1997;19:17–22. doi: 10.1046/j.1365-2257.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 18.Uong EC, Boyd JH, DeBaun MR. Daytime pulse oximeter measurements do not predict incidence of pain and acute chest syndrome episodes in sickle cell anemia. J Pediatr. 2006;149:707–9. doi: 10.1016/j.jpeds.2006.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. Task Force of the Pediatric Council of the American Society of Echocardiography; Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19:1413–30. doi: 10.1016/j.echo.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z Scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008;21:922–34. doi: 10.1016/j.echo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6:359–65. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 23.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 24.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 25.Van Belle G, Lloyd D, Fisher LD, Heagerty PJ, Lumley T. Biostatistics: A Methodology for the Health Sciences. 2nd ed. Hoboken: John Wiley & Sons; 2004. [Google Scholar]
- 26.Genser B, Cooper PJ, Yazdanbakhsh M, Barreto ML, Rodrigues LC. A guide to modern statistical analysis of immunological data. BMC Immunol. 2007;8:27. doi: 10.1186/1471-2172-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machado RF, Mack AK, Martyr S, Barnett C, Macarthur P, Sachdev V, et al. Severity of pulmonary hypertension during vasoocclusive pain crisis and exercise in patients with sickle cell disease. Br J Haematol. 2007;136:319–25. doi: 10.1111/j.1365-2141.2006.06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor JGt, Nolan VG, Mendelsohn L, Kato GJ, Gladwin MT, Steinberg MH. Chronic hyper-hemolysis in sickle cell anemia: association of vascular complications and mortality with less frequent vasoocclusive pain. PLoS ONE. 2008;3:e2095. doi: 10.1371/journal.pone.0002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn CT, Ahmad N. Clinical correlates of steady-state oxyhaemoglobin desaturation in children who have sickle cell disease. Br J Haematol. 2005;131:129–34. doi: 10.1111/j.1365-2141.2005.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato GJ, McGowan V, Machado RF, Little JA, Taylor J, 6th, Morris CR, et al. Lactate dehydrogenase as a bio-marker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–85. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn CT, Sargent JW. Daytime steady-state haemoglobin desaturation is a risk factor for overt stroke in children with sickle cell anaemia. Br J Haematol. 2008;140:336–9. doi: 10.1111/j.1365-2141.2007.06927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachdev V, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49:472–9. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato GJ, Hsieh M, Machado R, Taylor J, 6th, Little J, Butman JA, et al. Cerebrovascular disease associated with sickle cell pulmonary hypertension. Am J Hematol. 2006;81:503–10. doi: 10.1002/ajh.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paciocco G, Martinez FJ, Bossone E, Pielsticker E, Gillespie B, Rubenfire M. Oxygen desaturation on the six-minute walk test and mortality in untreated primary pulmonary hypertension. Eur Respir J. 2001;17:647–52. doi: 10.1183/09031936.01.17406470. [DOI] [PubMed] [Google Scholar]
- 36.Villalba WO, Sampaio-Barros PD, Pereira MC, Cerqueira EM, Leme CA, Jr, Marques-Neto JF, Paschoal IA. Six-minute walk test for the evaluation of pulmonary disease severity in scleroderma patients. Chest. 2007;131:217–22. doi: 10.1378/chest.06-0630. [DOI] [PubMed] [Google Scholar]