Mitogen-activated protein kinase (MAPK) cascades serve as important signaling pathways in all eukaryotic cells, as they link the perception of external stimuli to cellular responses. Since the discovery of CONSTITUTIVE TRIPLE RESPONSE1 (CTR1), a Raf-like MAPK kinase kinase (MAPKKK), there has been intense debate whether MAPK modules are involved in the signal transduction of the plant hormone ethylene. Although the discussion continues, evidence in support of this idea has accumulated. The Arabidopsis (Arabidopsis thaliana) MAPKs MPK3 and MPK6 seem to play a central role in the regulation of the ethylene response pathway by promoting the stabilization of EIN3, a key transcriptional regulator of the ethylene-induced transcriptional machinery. Other reports, however, place the MPK3/6-containing MAPK module in ethylene biosynthesis rather than in the signaling pathway. In this Update, we discuss the recent findings on MAPK cascades in ethylene signaling and/or biosynthesis.
CTR1, A RAF-LIKE MAPKKK INVOLVED IN THE ETHYLENE RESPONSE PATHWAY
Ethylene is one of the five classical plant hormones, first discovered at the beginning of the 20th century (Neljubow, 1901; Abeles et al., 1992). Its various well-described physiological effects range from the inhibition of elongation growth to the promotion of fruit ripening (Schaller and Kieber, 2002). Many components of the signaling pathway, which link the perception of the hormone by five endoplasmic reticulum-localized receptors to the expression of ethylene-responsive genes, have been cloned and characterized (Kendrick and Chang, 2008). One of the first components discovered was CTR1, a Raf-like MAPKKK (Kieber et al., 1993; Huang et al., 2003) acting downstream of the receptors. CTR1 turned out to be a negative regulator of ethylene signaling because ctr1 mutant alleles exhibited a constitutive ethylene response phenotype (Kieber et al., 1993; Huang et al., 2003). In the absence of ethylene, CTR1 interacts with the ethylene receptors ETR1 and ERS1, thereby actively suppressing the ethylene signal response (Clark et al., 1998; Huang et al., 2003). After the binding of ethylene to the receptors, CTR1 becomes inactivated and the signaling cascade is initiated. Because the discovery of CTR1 to be a central component in the ethylene signaling pathway, a question arose whether a prototypical MAPK module, which might include downstream MAPK kinases (MAPKKs) and MAPKs, could be involved in ethylene signal transduction (Kieber et al., 1993; Bleecker and Kende, 2000). However, for a long period of time, no supporting evidence was found to underpin this hypothesis.
A MAPK MODULE IN THE ETHYLENE RESPONSE OR BIOSYNTHETIC PATHWAY?
The first hint that MAPK activity might indeed play a role in the ethylene response pathway was published by Novikova et al. (2000). They reported that a protein with similarities to a MAPK was activated by exogenous treatment of Arabidopsis plants with ethylene. A few years later, a study performed by Ouaked et al. (2003) confirmed these findings and showed that ethylene (in the form of 1-aminocyclopropane-1-carboxylic acid [ACC], a precursor of ethylene in the biosynthesis pathway) is able to activate the Medicago MAPKK SIMKK and two MAPKs, namely SIMK and MMK3, in vitro. Additionally, the Arabidopsis MAPK MPK6, one of the best-studied Arabidopsis MAPKs (Desikan et al., 2001; Asai et al., 2002; Menke et al., 2004; Bush and Krysan, 2007; Dozci et al., 2007; Takahashi et al., 2007; Cho et al., 2008; Ren et al., 2008), was found to be inducibly activated in wild-type plants after ethylene treatment and constitutively active in ctr1 mutants. Because the activation of MPK6 by ethylene was dependent on ETR1 and CTR1, but not on EIN2 (a protein similar to Nramp metal ion transporters) and EIN3 (a key transcription factor in ethylene signaling), the MAPK module was placed downstream of CTR1 and upstream of EIN2. Furthermore, the SIMKK-MPK6 module appeared to act as a positive regulator in the ethylene response pathway and to be suppressed by CTR1 (Ouaked et al., 2003). This was the first publication ever, to our knowledge, to report an inhibiting function of one MAPK element (CTR1 as a MAPKKK) on another (MPK6 as a MAPK).
However, the implications of these findings gave rise to intense debate on account of some conceptual weaknesses in the study of Ouaked et al. (2003) as reported by Ecker (2004). Moreover, in contrast to this study, data provided by Liu and Zhang (2004) indicated that MPK6 is involved in ethylene biosynthesis but not in ethylene signal transduction. They showed that MPK6 targets at least two isoforms of the ACC synthase, namely ACS2 and ACS6 (Liu and Zhang, 2004). ACC synthases convert S-adenosyl-l-Met to ACC (Chae and Kieber, 2005). Extensive biochemical and functional data implied that ACS2 and ACS6 are substrates of a MKK4/5-MPK6 module and that the phosphorylation of ACS2 and ACS6 promotes the enhanced accumulation of the enzymes in vivo, consequently leading to an elevated ethylene production (Liu and Zhang, 2004). These data supported earlier reports that NtSIPK, the tobacco (Nicotiana tabacum) ortholog of AtMPK6, triggers ethylene production in tobacco leaves (Kim et al., 2003). In agreement with these findings, recent investigations have revealed that MPK6 is capable of directly phosphorylating the ACS6 protein in vitro at three Ser residues in the C terminus (Joo et al., 2008). Through this phosphorylation, ACS6 is preserved from 26S proteasome-mediated degradation, accumulates to enhanced protein level, and induces elevated ethylene synthesis. In summary, these data favored the hypothesis that a MAPK module participates in the regulation of ethylene biosynthesis and does not function as a downstream component of CTR1 in ethylene signaling.
THE DEBATE GOES ON
Novel evidence for the involvement of MAPK cascades in ethylene signaling was, however, recently provided by Yoo et al. (2008), who proposed that a MKK9-MPK3/MPK6 module is an integral component of the ethylene signal transduction pathway (Fig. 1A). In a first line of experiments, they showed that MPK3 and MPK6 kinase activity was enhanced in mutant ctr1 protoplasts but inhibited by the expression of constitutively active CTR1 (CTR1a). Secondly, MKK9 and its closest homolog, MKK7, were shown to be capable of activating MPK3 and MPK6 in protoplasts. MPK activation was abolished in mkk9 mutant plants. Intriguingly, two different mkk9 T-DNA insertion alleles displayed insensitivity to low concentrations of ACC. Furthermore, no activation of the expression of ETHYLENE RESPONSE FACTOR (ERF) genes (ERF1 and ERF5) after ethylene treatment was observed in the mkk9 mutants. Ectopic expression of a constitutively active MKK9 (MKK9a) promoted the expression of ERF1 and ERF5 and resulted in plants exhibiting a dwarfed, constitutive ethylene phenotype.
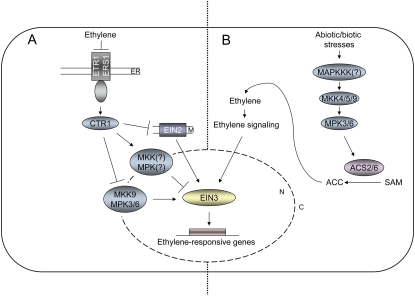
Figure 1.
Proposed models for the involvement of MAPK modules in the ethylene signaling (A) or the ethylene biosynthetic pathway (B). A, Ethylene is perceived by endoplasmic reticulum (ER)-localized ethylene receptors. In the absence of ethylene, ETR1 and ERS1 interact with and activate the Raf-like MAPKKK CTR1. CTR1 represses ethylene signaling by inhibiting the activity of the potential membrane (M)-bound Nramp ion metal transporter-like protein EIN2 and inactivating the MKK9-MPK3/6 module. Additionally, CTR1-dependent signaling, possibly through another MAPK cascade, leads to the phosphorylation of the EIN3 transcription factor on T592 and, thus, to the degradation of EIN3 via the 26S proteasome. After the binding of ethylene, the signaling through the receptor/CTR1 module is inactivated and downstream signaling events are initiated. After translocation of MKK9 from the cytosol (C) to the nucleus (N), the MKK9-MPK3/6 module phosphorylates EIN3 on T174, causing the stabilization of the protein and protecting it from 26S proteasome-mediated degradation. Moreover, EIN2 positively regulates the ethylene-dependent transcriptional responses mediated by EIN3 through an unknown mechanism. B, Diverse abiotic and biotic stresses induce the activation of MAPK modules involving MKK4/5/9 and MPK3/6. The activated MPK3 and MPK6 are capable of phosphorylating two isoforms of the ACC synthase, namely ACS2 and ACS6, at their C terminus. By this phosphorylation, ACS2/6 become stabilized and preserved from degradation via the 26S proteasome. As ACC synthases convert S-adenosyl-l-Met (SAM) to ACC, a precursor of ethylene, the stabilization of the two ACS2/6 isoforms leads to an enhanced ethylene production and activation of the ethylene signaling pathway.
The inhibition of hypocotyl elongation in MKK9a-expressing etiolated seedlings was not suppressed in etr1-1 mutants or by the application of Ag+, an antagonist of ethylene perception, but it was suppressed in the ein2 mutant. Thus, the MKK9-MPK3/6 module was placed between CTR1 and EIN2 in ethylene signaling and not in the ethylene biosynthetic pathway.
These results appeared to be supported by the observation that MKK9-GFP was shown to locate to the nucleus after ACC treatment in wild-type protoplasts but showed a cytosolic localization in protoplasts derived from etr1-1 mutants. Furthermore, MKK9a-GFP was located exclusively in the nucleus of ctr1 protoplasts. These data would imply that MKK9 translocates from the cytosol into the nucleus in response to ethylene treatment. However, neither the localization of MKK9-GFP in wild-type protoplasts in the absence of ACC nor the localization of wild-type MKK9-GFP in the ctr1 mutant background were shown in this report, although such experiments would have been very important: if the model proposed by Yoo et al. (2008) is correct, one would expect a predominantly cytoplasmic localization of MKK9-GFP in wild-type protoplasts in the absence of ethylene and a constitutive nuclear localization in ctr1 mutant protoplasts.
Yoo et al. (2008) also propose that MKK9a-activated MPK6 immunoprecipitated from protoplast extracts phosphorylates EIN3 in vitro. However, the presented data do not entirely exclude the possibility that a copurified kinase, other than MPK6, could be responsible for EIN3 phosphorylation in vitro.
Two phosphorylation sites (T174P175 and T592P593) within EIN3, each of which was located in proximity to a conserved MAPK docking motif, were identified in silico. Analyses of EIN3 stability in ein3 protoplasts show that the two phosphorylation sites have opposing functions in the regulation of EIN3 protein stability. T174 appeared to be targeted by MKK9-activated MPK6, leading to the stabilization of EIN3, whereas phosphorylation of T592 promotes EIN3 degradation and may be the target of another CTR1-dependent, yet-unknown phosphorylation cascade. These data show that in vivo phosphorylation and protein stability of EIN3 depends on the activity of MPK6. However, the question whether EIN3 is actually a direct substrate of MPK6 in vivo remains open. A demonstration of the in vivo interaction of MPK6 and EIN3 would have substantiated the hypothesis of a direct phosphorylation of EIN3 by MPK6.
In a recent report, Xu et al. (2008) presented data that, again, at least partly, gave rise to an opposite hypothesis on the function of the MKK9-MPK3/MPK6 module to that proposed by Yoo et al. (2008). As outlined in this work (Xu et al., 2008), the MKK9-MPK3/6 module triggers the production of ethylene but is not part of the ethylene signaling pathway (Fig. 1B). In accordance with the work published by Yoo et al. (2008), dexamethasone-inducible, constitutively active MKK9 (MKK9DD) was able to induce MPK3 and MPK6 kinase activity in vitro and in planta and to up-regulate the transcript amount of ACS2 and ACS6 as well as of several ERF genes. The effect of MKK9DD activity was diminished in MKK9DD/mpk3 plants and almost completely abolished in MKK9DD/mpk6 lines. These results indicate that the realization of the MKK9 function requires both MAPKs, but especially MPK6. In sharp contrast to the data from Yoo et al. (2008), the effects of a dexamethasone-inducible MKK9DD on hypocotyl elongation growth and ERF expression were blocked by the application of Ag+ and aminoethoxyvinylglycine, an inhibitor of ethylene biosynthesis. According to these data, the MKK9-MPK3/MPK6 module does not lie downstream of CTR1 in the ethylene signaling pathway but rather upstream of the ethylene perception in the regulation of ethylene biosynthesis (Liu and Zhang, 2004; Joo et al., 2008; Xu et al., 2008).
PERSPECTIVES
The striking differences between the two recent reports (Xu et al., 2008; Yoo et al., 2008) and also in earlier findings need to be clarified by additional experiments to provide a definite answer to this long-debated issue. However, if the MKK9-MPK3/6 module or any other MAPK cascade module is indeed part of the ethylene signal response pathway (Fig. 1), important new questions arise and will be the subject of future investigations.
First, what is the connection between CTR1 and MKK9? A direct negative regulatory function of CTR1 phosphorylation activity on MKK9 would be the first of its kind for all MAPK cascades ever investigated and appears to be unlikely (Ecker, 2004). However, it is important to note that (1) constitutively active MKK9a, which mimic the phosphorylated state of the kinase, have a positive regulatory function in the ethylene signaling pathway (Yoo et al., 2008); and (2) MKK9 belongs to the class of autophosphorylating kinases (Xu et al., 2008). Therefore, is CTR1 kinase activity actually needed for the regulation of MKK9 by phosphorylation? Or does CTR1 suppress the autophosphorylation activity of MKK9 in the absence of ethylene by, for instance, direct protein-protein interaction that could abolish the nuclear uptake of MKK9? In this case, the constitutive ethylene response phenotype of ctr1 mutants should be, at least partially, suppressed in the ctr1/mkk9 double mutant. Yoo et al. stated that only the phenotype of light-grown (Alonso et al., 1999), but not that of dark-grown, ctr1 seedlings was partly diminished in the ctr1/mkk9 double mutant. This is surprising because the functional, biochemical, and gene expression data indicate a dramatic impact of MKK9 on the hypocotyl growth response in etiolated seedlings on EIN3 protein level and ERF gene expression. However, EIN3 and the ERF transcription factors are known to be crucial components for the realization of the ethylene response in dark-grown seedlings (Chao et al., 1997; Solano et al., 1998). Hence, the model proposed by Yoo et al. may be true only for light-grown plants, while another signaling pathway may be more important in etiolated seedlings. On the other hand, it cannot be excluded that MKK7, the closest homolog of MKK9, partly substitutes for MKK9 in the mkk9 mutant background specifically in etiolated seedlings.
Second, the identity of the MAPK signaling cascade that might mediate the phosphorylation of T592 and, thus, the degradation of the EIN3 transcription factor (Yoo et al., 2008) remains to be determined. This MAPK cascade could also include CTR1, indicating that CTR1 controls ethylene-dependent transcriptional responses via at least two independent signaling pathways with opposing effects on EIN3 protein stability (Yoo et al., 2008). Hence, the MKK9-MPK3/6 module that possibly participates in the ethylene signaling would only partially fill the gap in the still-uncompleted jigsaw puzzle of ethylene signaling.
And last, the function of EIN2 still remains enigmatic. Genetics has clearly positioned this potential membrane-localized protein in the ethylene signaling pathway between CTR1 and EIN3 (Roman et al., 1995; Chao et al., 1997). However, to date, it is still unclear how EIN2 is mechanistically linked to the upstream components of the pathway and to downstream EIN3.
Acknowledgments
We thank Felicity de Courcy for proofreading the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. AFGN–Ha2146/5 to K.H.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Klaus Harter (klaus.harter@zmbp.uni-tuebingen.de).
References
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284 2148–2152 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415 977–983 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16 1–18 [DOI] [PubMed] [Google Scholar]
- Bush SM, Krysan PJ (2007) Mutational evidence that Arabidopsis MAP kinase MPK6 is involved in anther, influorescence, and embryo development. J Exp Bot 58 2181–2191 [DOI] [PubMed] [Google Scholar]
- Chae HS, Kieber JJ (2005) Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci 10 291–296 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89 1133–1144 [DOI] [PubMed] [Google Scholar]
- Cho SK, Larue CT, Chevalier D, Wang H, Jinn TL, Zhang S, Walker JC (2008) Regulation of floral organ abscission in Arabidopsis thaliana. Proc Natl Acad Sci USA 105 15629–15634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Larsen PB, Wang X, Chang C (1998) Association of the Arabidopsis CTR1 raf-like kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci USA 95 5401–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Ichimura K, Shinozaki K, Neill SJ (2001) Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol 126 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozci R, Brader G, Pettkó-Szandtner A, Rajh I, Djamei A, Pitzschke A, Teige M, Hirt H (2007) The Arabidopsis mitogen-activated protein kinase kinase MKK3 is upstream of group C mitogen-activated protein kinases and participates in pathogen signaling. Plant Cell 19 3266–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JR (2004) Reentry of the ethylene MPK6 module. Plant Cell 16 3169–3174 [Google Scholar]
- Huang Y, Li H, Hutchison CE, Laskey J, Kieber JJ (2003) Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J 33 221–233 [DOI] [PubMed] [Google Scholar]
- Joo S, Liu Y, Lueth A, Zhang S (2008) MAPK phosphorylation-induced stabilization of ACS6 protein is mediated by the non-catalytic C-terminal domain, which also contains the cis-determinant for rapid degradation by the 26S proteasome pathway. Plant J 54 129–140 [DOI] [PubMed] [Google Scholar]
- Kendrick MD, Chang C (2008) Ethylene signaling: new levels of complexity and regulation. Curr Opin Plant Biol 11 479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72 427–441 [DOI] [PubMed] [Google Scholar]
- Kim CY, Liu Y, Thorne ET, Yang H, Fukushige H, Gassmann W, Hildebrand D, Sharp RE, Zhang S (2003) Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 15 2707–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang S (2004) Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16 3386–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke FL, van Pelt JA, Pieterse CM, Klessig DF (2004) Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell 16 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neljubow D (1901) Über die horizentale Nutation der Stengel von Pisum sativum und einiger anderen Pflanzen. Beih Bot Zentralbl 10 128–139 [Google Scholar]
- Novikova GV, Moshkov IE, Smith AR, Hall MA (2000) The effect of ethylene on MAPKinase-like activity in Arabidopsis thaliana. FEBS Lett 474 29–32 [DOI] [PubMed] [Google Scholar]
- Ouaked F, Rozhon W, Lecourieux S, Hirt H (2003) A MAPK pathway mediates ethylene signaling in plants. EMBO J 22 1282–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, Zhang S (2008) A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad USA 105 5638–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR (1995) Genetic analysis of ethylene signal transduction Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Kieber JJ (2002) Ethylene. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0071, http://www.aspb.org/publications/arabidopsis/
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F, Yoshida R, Ichimura K, Mizoguchi T, Seo S, Yonezawa M, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K (2007) The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19 805–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li Y, Liu H, Lei L, Yang H, Liu G, Ren D (2008) Activation of MAP KINASE KINASE 9 induces ethylene and camalexin biosynthesis, and enhances sensitivity to salt stress in Arabidopsis. J Biol Chem 283 26996–27006 [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Tena G, Xiong Y, Sheen J (2008) Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signaling. Nature 451 789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]