Abstract
The control of foliar isoprene emission is shared between the activity of isoprene synthase, the terminal enzyme catalyzing isoprene formation from dimethylallyldiphosphate (DMADP), and the pool size of DMADP. Due to limited in vivo information of isoprene synthase kinetic characteristics and DMADP pool sizes, the relative importance of these controls is under debate. In this study, the phenomenon of postillumination isoprene release was employed to develop an in vivo method for estimation of the DMADP pool size and to determine isoprene synthase kinetic characteristics in hybrid aspen (Populus tremula × Populus tremuloides) leaves. The method is based on observations that after switching off the light, isoprene emission continues for 250 to 300 s and that the integral of the postillumination isoprene emission is strongly correlated with the isoprene emission rate before leaf darkening, thus quantitatively estimating the DMADP pool size associated with leaf isoprene emission. In vitro estimates demonstrated that overall leaf DMADP pool was very large, almost an order of magnitude larger than the in vivo pool. Yet, the difference between total DMADP pools in light and in darkness (light-dependent DMADP pool) was tightly correlated with the in vivo estimates of the DMADP pool size that is responsible for isoprene emission. Variation in in vivo DMADP pool size was obtained by varying light intensity and atmospheric CO2 and O2 concentrations. From these experiments, the in vivo kinetic constants of isoprene synthase were determined. In vivo isoprene synthase kinetic characteristics suggested that isoprene synthase mainly operates under substrate limitation and that short-term light, CO2, and O2 dependencies of isoprene emission result from variation in DMADP pool size rather than from modifications in isoprene synthase activity.
Biogenic isoprene is a key player in the photochemistry of the troposphere (Shallcross and Monks, 2000; Monson and Holland, 2001; Guenther et al., 2006) and also serves an important protective role in plant responses to heat and oxidative stresses (Sharkey and Singsaas, 1995; Singsaas et al., 1997; Loreto et al., 2001; Behnke et al., 2007). Despite the importance of biogenic isoprene, the mechanisms of isoprene formation and emission are still under lively discussion (Loreto et al., 2001; Rosenstiel et al., 2003; Sanadze, 2004; Sharkey et al., 2008). Resolving the controlling steps of isoprene synthesis is of key significance for constructing qualitatively new predictive models able to simulate isoprene emissions in stressed plants and the emission responses to global modifications in CO2 and temperature (Monson et al., 2007; Grote and Niinemets, 2008). Due to existing uncertainties in isoprene emission mechanisms, it has even been suggested that pressing global change issues cannot be currently simulated on the basis of existing knowledge (Loreto et al., 2007; Monson et al., 2007).
There is a consensus that isoprene synthase, the terminal chloroplastic enzyme catalyzing the formation of isoprene from its substrate dimethylallyldiphosphate (DMADP), is the basic site of control of isoprene emission by different environmental and endogenous factors (Kuzma and Fall, 1993; Schnitzler et al., 1997; Loreto et al., 2004; Sharkey et al., 2005). However, currently there is a major debate of whether the responses of isoprene emission to light and atmospheric CO2 and O2 concentrations result from rapid allosteric modifications in the activity of isoprene synthase (Fall and Wildermuth, 1998; Logan et al., 2000; Wolfertz et al., 2003; Nogués et al., 2006) or whether these emission responses to environment are controlled by substrate availability (Rosenstiel et al., 2002, 2006; Zimmer et al., 2003; Magel et al., 2006; Monson et al., 2007). Substrate limitation of isoprene emission may arise if isoprene synthase has a relatively large Km value and the concentrations of DMADP vary with environmental factors. Synthesis of DMADP requires a large amount of reductive and energetic equivalents (NADPH and ATP), and there is evidence that DMADP pool size scales positively with light availability (Brüggemann and Schnitzler, 2002; Rosenstiel et al., 2002), suggesting that substrate level control is principally possible.
Discrimination between the synthase and substrate level controls on isoprene emission is currently seriously hampered by available methods for the estimation of isoprene synthase kinetic constants and DMADP pool size that are necessarily destructive. There are currently major uncertainties with in vitro estimates of Km values of isoprene synthase, with estimates ranging from 0.5 to 9 mm (Kuzma and Fall, 1993; Silver and Fall, 1995; Schnitzler et al., 1996; Lehning et al., 1999; Wolfertz et al., 2004). Furthermore, DMADP pool sizes obtained by different in vitro methods from plants sampled under similar conditions span over 2 orders of magnitude (Fisher et al., 2001; Brüggemann and Schnitzler, 2002; Nogués et al., 2006; Wiberley et al., 2008), complicating consensus theory development.
Another major limitation of in vitro DMADP pool size estimations is that most methods assess the whole leaf DMADP pool size. However, in addition to the chloroplastic methylerythritol phosphate (MEP) pathway, DMADP is also synthesized via the mevalonate-dependent pathway located in the cytosol. While there is some cross talk between the two pathways (Hemmerlin et al., 2003), the exchange of isoprenoid precursors between the two pathways is slow and plant isoprene emission mainly relies on the DMADP pool synthesized in chloroplasts (Disch et al., 1998; Eisenreich et al., 2001). There are currently two encouraging methods for separation of chloroplastic and cytosolic pools of DMADP: nonaqueous fractionation of leaf material (Rosenstiel et al., 2002) and 13C labeling of the chloroplast pool by fumigating leaves with 13CO2 (Loreto et al., 2004). While the nonaqueous fractionation apparently successfully separated the different DMADP pools at midday, changes in the activity of fraction marker enzymes may limit the use of this technique in other situations (Rosenstiel et al., 2002, for discussion of the problems). The 13C-labeling technique requires assumptions about the labeling time kinetics and further assumes that the recovery of DMADP is the same for both cytosolic and chloroplastic pools (Rosenstiel et al., 2002, for problems of recovery of DMADP in different leaf fractions). As isoprene of 13C-fumigated leaves does not become 100% labeled even after long-term fumigation (Delwiche and Sharkey, 1993; Karl et al., 2002), the 13C-labeled DMADP pool may not fully reflect the entire DMADP pool that is associated with isoprene synthesis. These difficulties with both methods can partly explain large discrepancies in estimated chloroplastic pool sizes of DMADP and the distribution of total leaf DMADP pool size between chloroplasts and cytosol (Rosenstiel et al., 2002; Loreto et al., 2004).
In this study, we used the phenomenon of postillumination isoprene release (Monson and Fall, 1989) to develop an in vivo method for the estimation of DMADP pool size and to determine isoprene synthase kinetic characteristics in vivo in hybrid aspen (Populus tremula × Populus tremuloides) leaves. The method is fast and determines the DMADP pool that is directly associated with isoprene synthesis. We further compared this method with a widely used in vitro method of total leaf DMADP pool size determination (Fisher et al., 2001) both in the light and in the dark after cessation of isoprene emission and tested the assumption that the differences in total DMADP pool size between light and dark account for the DMADP pool responsible for isoprene emissions in light (Falbel and Sharkey, 2005). In vivo characteristics of isoprene synthase were further derived from isoprene emission measurements concurrently with in vivo DMADP pool size. These data collectively suggest that isoprene production mainly operates in substrate-limited conditions, and the short-term dependencies of isoprene emission on light and atmospheric CO2 and O2 concentrations result from changes in DMADP pool size.
RESULTS
Transient Kinetics of Isoprene Emission after Switching Off the Light
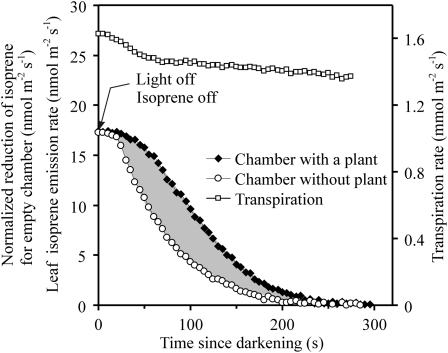
After light was turned off, isoprene emission from hybrid aspen leaves declined under current experimental conditions essentially to zero within 250 to 300 s (Fig. 1), demonstrating that the dominant part of isoprene emission was supported by light. As the experiments with the isoprene flow through the empty chamber demonstrated, the time dependence of isoprene emission was driven both by the finite size of the chamber (i.e. the amount of isoprene that was emitted before the transient and that was still present in the chamber during the transient due to limited air turnover time) and by the release of isoprene from leaves in darkness (Fig. 1). The difference between the integrals of the curves with and without the leaf provides the total amount of postillumination isoprene release from the leaves and quantitatively corresponds to the in vivo DMADP pool size responsible for isoprene emission that was present in the leaves before the transient. Transpiration rate measurements together with leaf energy balance calculations conducted simultaneously with isoprene emission measurements indicated that there were only minor reductions in stomatal openness of about 10% and in leaf temperature of less than 1.5°C during the light/dark transient (Fig. 1).
Figure 1.
Sample recordings of postillumination isoprene emission rate (chamber with hybrid aspen, clone 200) and normalized system transient response (chamber without the plant). The transient response of the system was assessed using an artificial isoprene source. First, a steady-state isoprene flow was established, then isoprene flow was turned off and the system transient response was recorded. This system-specific response curve (the shape of which is independent of isoprene concentration of the isoprene source) was scaled to given leaf isoprene emission rates at steady-state conditions before switching off the light (i.e. the empty-chamber response was normalized with respect to leaf isoprene emission rate at 0 s). DMADP pool size was calculated as the area between the curves with and without the plant, as shown by the shaded area. Transpiration rate measurements together with leaf energy balance calculations demonstrated that there was a small decrease in leaf temperature of approximately 1.4°C (from 29.1°C to 27.7°C) corresponding to the initial drop of leaf transpiration rate (from 1.6 to 1.45 mmol m−2 s−1) and a further approximately 10% reduction in stomatal conductance (from 120 to 110 mmol m−2 s−1) after switching off the light.
After the postillumination measurements, light was turned on again. In all cases, the initial CO2 uptake and isoprene emission rates recovered to the previous level, indicating that no irreversible changes had occurred in the plant during the transient (data not shown).
Isoprene Emission Rate and in Vivo DMADP Pool Size under Various Environmental Conditions
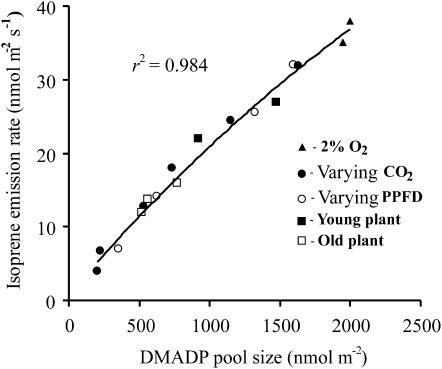
The rate of isoprene emission from aspen leaves was stabilized at different light (150–550 μmol m−2 s−1) and atmospheric concentrations of CO2 (390–1,400 μmol mol−1) and O2 (21% versus 2%) and in plants with leaves of different developmental stages, while leaf temperature was maintained at 28°C to 30°C. The steady-state isoprene emission rate increased with increasing quantum flux density and decreased with increasing CO2 and O2 concentrations and was lower in young than in mature leaves, agreeing with numerous previous observations (data not shown; Monson and Fall, 1989; Loreto and Sharkey, 1990; Sharkey et al., 1991; Monson et al., 1994, for environmental effects on isoprene emission). In each of our experiments, postillumination isoprene release and corresponding in vivo DMADP pool size were estimated as in Figure 1, and overall variation in in vivo DMADP pool size in response to the environmental and ontogenetic changes was between approximately 100 and 2,000 nmol m−2 (Fig. 2).
Figure 2.
The rate of isoprene emission in hybrid aspen leaves is dependent on the in vivo DMADP pool size measured at 28°C to 30°C. The DMADP pool size was calculated as the integral of the postillumination isoprene emission (see Fig. 1 for sample postillumination isoprene emission kinetics) for different combinations of environmental conditions before terminating the illumination. The standard conditions in the experiments were 21% O2, 390 μmol mol−1 CO2, and incident quantum flux density of 550 μmol m−2 s−1. In experiments with varying CO2 (black symbols), CO2 concentration was increased from 390 μmol mol−1 (highest emission rate for the given treatment) to 1,600 μmol mol−1 (lowest emission rate). In experiments with varying light, light was reduced from 550 to 150 μmol m−2 s−1 (lowest emission rate observed for the given treatment). In all individual experiments (n = 17), steady-state isoprene emission rates at specific cuvette conditions were established before measuring the decay kinetics.
In all cases, the estimated in vivo DMADP pool size increased with increasing isoprene emission rate before the measurements (Fig. 2), indicating that integrated postilluminatory isoprene release did scale with the isoprene emission rate before the measurements and that the gasometric estimates of DMADP pool size derived this way reflected steady-state isoprene emission levels.
In Vivo Versus in Vitro DMADP Pool Sizes
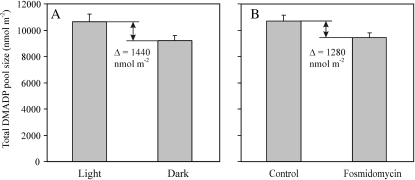
The total pool size of DMADP determined by the chemical method (in vitro) in the light varied between 8,100 and 12,600 nmol m−2 (average ± se = 10,500 ± 600 nmol m−2; Fig. 3A), being thus approximately an order of magnitude larger than the gasometric (in vivo) estimates. In all cases, the DMADP pool size was smaller in darkness immediately after the cessation of isoprene synthesis (Fig. 1 for emission kinetics; at most 5–6 min since the darkening), with total pool size varying between 7,500 and 10,500 nmol m−2 (average ± se = 9,180 ± 370 nmol m−2; the means between light and darkness are statistically significant at P < 0.001 according to a paired t test).
Figure 3.
Comparison of in vitro estimates of total leaf DMADP pool sizes in light and in darkness (A) and during light and after feeding for 45 to 50 min with the chloroplastic MEP pathway inhibitor fosmidomycin (20 μm) through the leaf petiole (B) in aspen. The DMADP pool in the light was estimated after steady-sate isoprene emission rates were achieved, while the dark pool was estimated when isoprene emission reached zero. n = 62 (31 pairs of leaves measured in light and in darkness) for A and n = 5 for B. The chemical estimation of total leaf DMADP pool size follows Fisher et al. (2001). Error bars denote se.
As with the darkness versus light contrast, in leaves in which isoprene emission had been inhibited by the MEP pathway inhibitor fosmidomycin, the total pool size was reduced in fosmidomycin-inhibited leaves numerically to a very similar extent, by about 15% (Fig. 3B).
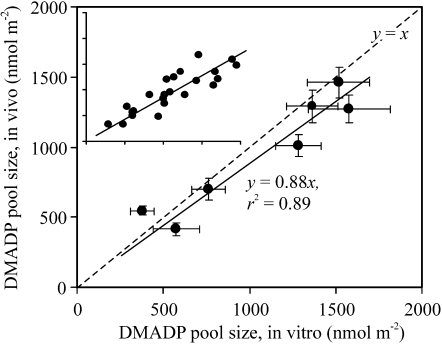
Total DMADP pool size determined chemically was weakly if at all related to the in vivo pool size (y = 0.096x for the in vivo versus in vitro relationship, with r2 = 0.39, P = 0.09). By contrast, strong positive correlations (P < 0.001) between the difference in light versus dark in vitro DMADP pool sizes and in vivo pool size were observed, with only approximately 12% departure from the 1:1 regression line (Fig. 4), suggesting that the difference between the in vitro light and dark pools corresponds to the DMADP pool responsible for isoprene synthesis. Overall, the chemical estimates of the DMADP pool size responsible for isoprene emission were characterized by greater variance. Average coefficient of variation (sample sd per sample mean in percentage) calculated for treatment means was 29.6% for chemical estimations versus 17.2% for gasometric estimations (these means are significantly different at P < 0.01 according to a paired t test).
Figure 4.
Relationship between in vitro and in vivo DMADP pool sizes responsible for isoprene emission. In vivo pool size was determined gasometrically as shown in Figure 1, while in vitro pool size was found as the difference between the total pool sizes in light and in darkness immediately after switching off the light (see Fig. 3). The variation in DMADP pool sizes was obtained using leaves of different age (n = 7 for the main panel). For each leaf age class, four to six replicate estimations of in vitro and in vivo pool sizes were conducted (n = 31). The inset shows all measurements (y = 0.85x, r2 = 0.73, P < 0.001). Error bars denote se of replicate experiments for each leaf age class. Dashed lines show 1:1 dependence.
In Vivo Kinetic Characteristics of Isoprene Synthase
Provided that gasometric estimates of DMADP pool size correspond to the DMADP pool responsible for isoprene formation, the data reported in Figure 2 represent an in vivo kinetic response curve of the isoprene synthase reaction. For the observed variation of 100 to 2,000 nmol m−2 in in vivo DMADP pool size, isoprene emission rates varied between 2 and 37 nmol m−2 s−1. The small scatter of data points around the regression line underscores the high reliability of the method. As for nonconstant isoprene synthase activity, varying values of isoprene emission rate at any given DMADP pool size are expected, the small scatter also suggests the constancy of isoprene synthase activity in these experiments.
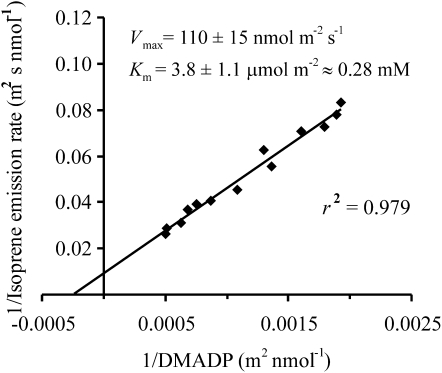
The measured data formed a slightly hyperbolic segment of a kinetic curve (Fig. 2) describing the emission rate (V) as dependent on the DMADP pool size (SD). The maximum capacity of isoprene synthase (Vmax) of 110 nmol m−2 s−1 and Km of 3.8 μmol m−2 was estimated from the Lineweaver-Burk plot (Fig. 5). Using an appropriate conversion factor between area-based and volume-based units, a Km for isoprene synthase of 0.28 mm was obtained. Similar estimates were obtained from other enzyme kinetic plots. For instance, the Woolf-Hanes plot (SD versus SD/V axes) provided a Km value of 0.24 mm, suggesting that a robust estimate was derived from these data.
Figure 5.
Estimation of in vivo isoprene synthase kinetic characteristics at 28°C to 30°C from the Lineweaver-Burk plot (data in Fig. 2). Assuming that isoprene synthesis follows Michaelis-Menten kinetics, the dependence of emission rate, V, on the DMADP pool size (SD) is given as V = VmaxSD/(Km + SD), where Vmax is the maximum reaction velocity and Km is the Michaelis-Menten constant. Thus, the intercept of the Lineweaver-Burk plot is equal to −1/Km, while the slope is equal to Km/Vmax. The error estimates of Vmax and Km were derived from the se of the regression slope and intercept.
DISCUSSION
Phenomenology of Postillumination Isoprene Emission
The phenomenon of postillumination isoprene emission has been known for some time (Monson and Fall, 1989), but the reasons for and implications of such a postillumination release of isoprene have not been addressed. As in Monson and Fall (1989), we observed that the postillumination release of isoprene can continue for a relatively long time, for minutes, before complete cessation of isoprene emission (Fig. 1).
It may be suggested that postillumination isoprene emission relies on an isoprene pool nonspecifically stored in leaf gas, liquid, and lipid phases. Such nonspecific storage, in particular in leaf liquid and lipid phases, has been shown to significantly contribute to emissions of strongly lipid-soluble monoterpenes (Niinemets and Reichstein, 2002) and water-soluble compounds such as methanol (Harley et al., 2007; Hüve et al., 2007). Examination of the physicochemical characteristics of isoprene suggests that the effect of nonspecific storage on isoprene postillumination emission must be small. First, isoprene has a high air-liquid phase partition coefficient (Henry's law constant; Niinemets and Reichstein, 2003); thus, isoprene is preferentially distributed in the gas phase and is not significantly stored in the leaf liquid phase. Second, the octanol/water partition coefficient (Kow) for isoprene that characterizes the partitioning of compound between lipid and liquid phases is only approximately 260 at 25°C (Niinemets and Reichstein, 2003). This is approximately 2 orders of magnitude less than the Kow for nonoxygenated monoterpenes (Copolovici and Niinemets, 2005). Direct measurements of foliar isoprene concentrations further indicate that nonspecifically stored isoprene pools in emitting species are approximately 2 orders of magnitude less than monoterpene concentrations in monoterpene-emitting species (Loreto et al., 1998). This evidence implies that the bulk of the isoprene emitted in darkness results from de novo isoprene synthesis from the immediate isoprene precursor DMADP.
It follows that to understand the postillumination isoprene emissions, the crucial question is the origin of DMADP for the isoprene synthesis in darkness. Previously, postillumination isoprene emission has been explained to reflect the de novo synthesis of DMADP (Monson and Fall, 1989). However, the chloroplastic MEP pathway of isoprene synthesis is dependent upon the energetic cofactors ATP and NADPH, generated by the photosynthetic electron transport chain in the light. After turning out the light, NADP reduction stops faster than ATP production that is made by a stored proton gradient (Sharkey et al., 1986). Consequently, the production of glyceraldehyde phosphate (GAP), which relies both on ATP and NADPH, stops faster than the production of ribulose-1,5-bisphosphate (RuBP) from GAP, which depends only on ATP (Sharkey et al., 1986). Thus, GAP availability will limit the first step of the MEP pathway and reducing power will limit both the second step and the penultimate step of the pathway. This suggests that other metabolites in the MEP pathway contribute less than GAP to de novo DMADP production once the light is turned off. Overall, the pools of both cofactors, ATP and NADPH, are low, and these pools are rapidly exhausted in darkened leaves (Pearcy et al., 1997, for review). In fact, the dark levels of NADPH, ATP, and GAP can support photosynthetic CO2 fixation for only a few seconds if CO2 is made readily available (Laisk et al., 1984). Given this evidence, we conclude that the amount of DMADP synthesized in chloroplasts after leaf darkening is very small and can contribute to the postillumination isoprene emission that lasts on average for approximately 250 s (Fig. 1) only to a minor extent. In light of this evidence, we conclude that the postillumination isoprene emission results from a DMADP pool present in leaves before darkening.
Estimation of DMADP Pool Size from Postillumination Emission Measurements
Given that postillumination isoprene emission relies mainly on a DMADP pool present in leaves before darkening, our major expectation was that the amount of isoprene emitted after darkening scales with the steady-state isoprene emission rate. This was indeed observed in our study. Larger steady-state isoprene emission rates in response to increased light, reduced CO2 or O2 concentrations, or in younger fully mature leaves were in all cases associated with higher integrated postillumination isoprene emission rates (Fig. 2). As discussed above, the contributions of the nonspecifically stored isoprene pool as well as the de novo synthesis of DMADP to postillumination isoprene emission are essentially negligible, suggesting that the total integrated postillumination isoprene emission provides an estimate of DMADP pool size that participates in isoprene emission before darkening. However, for this to be true, the third necessary condition is that the activity of isoprene synthase remains constant during the postillumination process. This is a relevant condition given that it has been previously hypothesized that the light dependence of isoprene emission results from light-driven modifications in the activity of isoprene synthase (Silver and Fall, 1995).
Changes in enzyme activity can result from transcription/translation-type regulation of protein synthesis, processes evidently too slow to control the postillumination isoprene emission rate. Faster regulation of synthase activity can result from changes in temperature, pH, or allosteric inhibitors. There was a reduction of leaf temperature on the order of about 1.5°C after the light was turned off, but this reduction was clearly too small to cause the almost complete cessation of the emission. As for pH, isoprene synthase has a broad pH optimum around 7.7 to 8.2 (Lehning et al., 1999), while chloroplast stroma pH changes in response to the light/dark transitions at most by 0.4 units (Oja et al., 1986). Therefore, significant alteration of the isoprene synthase activity during the postillumination process is unlikely. Furthermore, direct measurements of the activity of isoprene synthase extracted from illuminated and darkened leaves indicate a constant enzyme activation state (Wildermuth and Fall, 1998).
Taken together, this evidence suggests that the integral of the postillumination isoprene emission is a measure of the DMADP pool size participating in light-dependent isoprene production. This in vivo method for the estimation of DMADP pool size in intact leaves is analogous to the method in photosynthesis research for the measurement of the CO2 acceptor, RuBP pool size from postillumination CO2 uptake. After the light is turned off, CO2 fixation declines not immediately and CO2 uptake continues mainly at the expense of RuBP accumulated in chloroplasts during the light, while a smaller component of the postillumination CO2 fixation is due to some RuBP synthesized in the dark at the expense of ATP and hexose phosphates produced in the light (Ruuska et al., 1998). The excellent coincidence between RuBP pools determined by the postillumination CO2 fixation and by direct biochemical measurements confirms that postillumination CO2 provides a reliable estimate of RuBP pool size (Ruuska et al., 1998).
In Vivo Versus in Vitro DMADP Pool Sizes
In our study, we found very high bulk DMADP pools, 8,000 to 13,000 nmol m−2 (Fig. 3), using the in vitro method. These very high pools actually confirm other observations of whole leaf DMADP contents (Fisher et al., 2001; Wiberley et al., 2008) that may even extend up to 100 μmol m−2 (Wiberley et al., 2008). However, these massive whole leaf DMADP pools were not related to isoprene emission at all (Wiberley et al., 2008; this study), and the correlation with the in vivo gasometrically estimated DMADP pool size in our study was very scattered. In fact, in leaves, DMADP is synthesized via the light-dependent MEP pathway in chloroplasts and via the light-independent cytosolic mevalonate-dependent pathway. While there is cross talk between the two pathways, mainly through the DMADP precursor isopentenyl diphosphate (Rodríguez-Concepción and Boronat, 2002), the rate of the exchange of isopentenyl diphosphate between the cytosolic and chloroplastic pools is slow. As the result, the fraction of cytosolic intermediates penetrating into chloroplasts is estimated to be only 1% to 2% (Disch et al., 1998; Eisenreich et al., 2001). Thus, to understand the dependence of isoprene emission on substrate availability, it is pertinent to estimate not the bulk leaf DMADP contents but the DMADP pool size that is associated with isoprene emission.
In fact, biochemical estimations of DMADP pool size demonstrate that DMADP concentrations are lower in the darkness than in the light (Brüggemann and Schnitzler, 2002; Rosenstiel et al., 2002; Falbel and Sharkey, 2005), and it has been suggested that the difference between light and dark pool sizes of DMADP characterizes the DMADP pool responsible for isoprene synthesis in the light (Falbel and Sharkey, 2005). In our study, this difference coincided with the gasometric estimate of in vivo DMADP pool size (Fig. 4), suggesting that both methods characterize the DMADP pool responsible for isoprene formation in the leaves.
The overall size of the DMADP pool responsible for isoprene formation appeared to be only 10% to 15% of the total leaf DMADP pool (Fig. 3). This percentage is somewhat smaller than that previously reported by Fisher et al. (2001) and Rosenstiel et al. (2002), but in their study, extended darkening periods of up to 12 h were used for dark measurements, while we took the dark measurement immediately after cessation of isoprene emission (at most 5–6 min after darkening; Fig. 1 for dark emission kinetics). Furthermore, inhibition with fosmidomycin until cessation of isoprene emission further provided a similar percentage of 10% to 15% of the DMADP pool responsible for isoprene synthesis (Fig. 3B), further confirming that the DMADP pool responsible for isoprene synthesis is small relative to the total leaf DMADP pool. In addition, the data of Nogués et al. (2006) and Behnke et al. (2007) for poplar plants measured under similar conditions using pulse 13C labeling to detect the chloroplastic pool of DMADP practically coincide with our results of in vivo DMADP pools responsible for isoprene formation of approximately 0.2 to 2 μmol m−2 (Fig. 2).
However, while both in vivo and in vitro methods provided similar estimates of the DMADP pool size responsible for isoprene emission, the chemical method was characterized by larger variability, which is not surprising given that this estimate is the difference between two large values. In addition, the chemical method is necessarily destructive; thus, inherent leaf-to-leaf variability (Fig. 4, inset) complicates studies of environmental effects on light-dependent DMADP pool size and isoprene emission (Brüggemann and Schnitzler, 2002; Rosenstiel et al., 2002; Falbel and Sharkey, 2005). By contrast, with the gasometric in vivo method, pertinent DMADP pool sizes can be repeatedly estimated in the same leaves, making it possible to conduct rigorous experiments for testing the role of the light-dependent DMADP pool on the control of isoprene emission.
In Vivo Isoprene Synthase Kinetics
We related the in vivo DMADP pool size determined under different light, CO2, and O2 conditions and for leaves of different age to the steady-state rate of isoprene emission just before the darkening, constructing the kinetic curve of isoprene synthase with respect to its substrate DMADP in intact leaves (Fig. 2). This allowed us to derive the isoprene synthase kinetic constants Km and Vmax in vivo (Fig. 5). This method for the derivation of enzyme kinetic characteristics in intact leaves is analogous to the method for the estimation of Rubisco Km for RuBP in vivo (Laisk et al., 2001, for details). In fact, for isoprene, this method is even cleaner, as the reaction product rapidly escapes the leaf, while for Rubisco kinetics, the reaction product 3-phosphoglyceric acid accumulates, progressively inhibiting the reaction during the postillumination process.
We derived a Vmax value of 110 nmol m−2 s−1, which is similar to values reported in the literature (Lehning et al., 2001; Wolfertz et al., 2004). The Km value derived was 3.8 μmol m−2, corresponding to 0.28 mm when converted to chloroplast volume. In early studies, high Km values on the order of 8 to 9 mm were observed (Kuzma and Fall, 1993; Silver and Fall, 1995). Such very high Km values would require unrealistically high chloroplastic DMADP pools for normal functioning. In fact, these values have been suggested to result from an underestimation of isoprene synthase activity in in vitro measurements due to inactivation of the enzyme during the isolation procedure (Wildermuth and Fall, 1998). Sanadze (2004) further suggested that the high Km values reflect the loss of activating cofactors normally present in chloroplasts or the loss of enzyme native conformation during purification. In fact, there was a positive dependence between the degree of isoprene synthase “purification” and its Km(DMADP) (Sanadze, 2004).
In more recent studies, Km values around 0.5 to 0.6 mm have been determined, assuming that all leaf DMADP is in the chloroplastic compartment (Schnitzler et al., 1996; Lehning et al., 1999; Wolfertz et al., 2004). Given that the bulk of DMADP is in the cytosolic compartment, according to our study 85% to 90%, and is not participating in isoprene emission, we suggest that our estimated Km of 0.28 mm represents the true in vivo Km for isoprene synthase.
While it was initially suggested that light-dependent modifications in isoprene emission are driven by modifications in isoprene synthase activity (Fall and Wildermuth, 1998), our data confirm the more recent understanding that substrate availability (i.e. DMADP pool size) controls the emissions (Niinemets et al., 1999; Zimmer et al., 2000; Rosenstiel et al., 2006; Monson et al., 2007). The control of isoprene emissions by DMADP has also been extended to other factors such as CO2 (Funk et al., 2004; Rosenstiel et al., 2006; Monson et al., 2007). As the DMADP pool responsible for isoprene emission was mostly in the linear part of the kinetic curve for different O2 and CO2 concentrations as well (Fig. 2), our study supports the suggestion that changes in substrate availability are responsible for the O2 and CO2 dependence of isoprene emission.
In summary, this evidence strongly suggests that the integral of postillumination isoprene emission is a true measure of the DMADP pool associated with isoprene production in intact leaves. The applied postillumination isoprene emission measurements provide a convenient, fast, and reliable technique for the estimation of a DMADP pool that is directly associated with isoprene emission in intact leaves. The DMADP pool responsible for isoprene emission is small relative to the whole leaf DMADP pool, the bulk of which is presumably located in the cytosol. The in vivo Km value of aspen isoprene synthase is smaller than previously reported in the literature. Nevertheless, isoprene production still operates mostly under substrate limitation at different light intensities and external CO2 and O2 concentrations. The maximum capacity of isoprene synthase, Vmax, exceeds four to five times the actual reaction rates; thus, these data suggest that substrate rather than synthase activity is responsible for the short-term light, CO2, and O2 dependencies of isoprene emission.
MATERIALS AND METHODS
Plants and Growth Conditions
One-year-old seedlings of hybrid aspen (Populus tremula × Populus tremuloides, clone 200; Vahala et al., 2003, for description of the genotype) were grown in plastic pots containing 4 kg of a peat:sand mixture (1:1) in a Percival AR-95 HIL growth chamber (CLF PlantClimatics) under a photosynthetic photon flux density of 500 μmol m−2 s−1 with a 14-h light period. Air temperature was kept at 25°C during the day and 20°C during the night, while relative air humidity was 60% ± 2%. Plants were watered daily to field capacity with distilled water and once per weak with a combined nutrient solution containing macroelements according to the Knopp recipe and microelements according to the Hoagland recipe.
Description of the Gas-Exchange System
The gas-exchange measurements were conducted with cylindrical glass chambers of either 3 or 8 L depending on plant size. The bottom of the chambers was composed of two well-fitted glass plates with perforations for plant stem, gas input and output ports, a temperature sensor, and a fan, while the chamber edges were polished for a perfect seal. All tubing and connections were made of Teflon and stainless steel. The gas flow rate through the measurement chamber was 8.5 L min−1 for the 8-L chamber and 3 L min−1 for the 3-L chamber. This resulted in the response time constant of the system of 0.017 s−1 (response half-time of approximately 42 s). The chambers were hermetically sealed and operated only under slight overpressure of a few millibars to avoid uncontrolled leakage of air. A fan inside the chamber ensured high turbulence and uniform air mixing. The chambers were illuminated with four 50-W halogen lamps, with a regulatory unit providing dynamic control of light intensity between 0 and 550 μmol m−2 s−1. Temperature inside the chambers could be controlled between room temperature of 22°C and 30°C using an infrared heating body. Temperature in the chamber was continuously monitored by a NTC thermistor (model ACCАСС-001; RTI Electronics). Leaf temperature calculated from leaf energy balance was within ±1°C of the air temperature in the chamber.
Ambient air passing through a large buffer volume of 100 L was used in most experiments. For CO2 concentrations above the ambient level, CO2 was added into the gas stream using a capillary mixer (Laisk and Oja, 1998, for details). For preparation of CO2 concentrations below the ambient, CO2 was first removed by KOH, then CO2 was added by the mixer and air was rehumidified to the desired dew point. In experiments with low O2 concentrations, N2 and O2 from pressure bottles were dynamically mixed and humidified and the required CO2 was set.
Measurement of CO2 and Water Exchange and Isoprene Emission Rates
The entire seedling or the uppermost portion of the seedling with approximately 10 fully expanded mature leaves (15–35 d after bud burst, except where noted) was enclosed in the gas-exchange chamber. CO2 and water vapor concentrations were measured with an infrared analyzer (LI-COR 6262; Li-Cor), while isoprene concentration was continuously monitored with a Fast Isoprene Analyzer equipped with an ozone generator (Hills Scientific). The isoprene measurement is based on the chemiluminescence principle and is highly sensitive only to isoprene with several orders of magnitude lower sensitivity to other volatile hydrocarbons such as monoterpenes (Hills and Zimmerman, 1990). The analyzer was frequently calibrated with standard gas containing 5.74 μL L−1 isoprene in N2. Due to the humidity sensitivity of the isoprene analyzer, a custom-made humidifier was set before the analyzer to keep constant the humidity of air before entering the analyzer. Gas concentrations of incoming and outgoing air were measured interchangeably, and gas-exchange rates were calculated according to a standard protocol (von Caemmerer and Farquhar, 1981).
In Vivo Estimation of DMADP Pool Size
Leaf DMADP pool size was estimated using a postillumination transient technique that is based on the observation that after turning off the light, the evolution of isoprene continues in the dark until DMADP is present (see “Discussion” for underlying assumptions). Thus, the integral of isoprene emission after switching off the light corresponds to the steady-state DMADP pool size before the darkening. However, the time-dependent reduction of isoprene concentration in the chamber outlet also depends on the measurement system response (chamber capacity); thus, chamber and plant responses must be first separated. To resolve plant and system responses, we simulated an analog transient isoprene reduction with an empty chamber. For this, isoprene of known concentration was supplied to the chamber. After the system reached a steady state (i.e. isoprene influx and outflux were equal), isoprene supply was abruptly stopped. In this case, the isoprene efflux from the chamber approached zero faster than with the plant in the chamber (Fig. 1). The shape of the decay curve without the plant is constant for a given chamber, and flow rate and is independent of isoprene concentration before the transient. Thus, for comparison with leaf emissions, the empty chamber decay curve can be normalized with respect to the leaf isoprene emission at the time of the transient. The area (integral) of the difference between the two recordings, with the plant and without it (normalized to the initial plant emission) represents the postillumination isoprene emission supported by the DMADP pool accumulated in the light and thus provides the foliar pool size of DMADP responsible for isoprene emission (nmol m−2) under given conditions. All of these measurements were conducted at temperatures of 28°C to 30°C.
The described technique is similar to an established in vivo gasometric method for the measurement of CO2 acceptor, RuBP, pool size in leaves during photosynthesis that is based on analogous assumptions (Laisk et al., 1984, 2001). After the light is turned off, CO2 uptake continues in the dark for a certain time at the expense of the RuBP pool built up during the light period (Laisk et al., 1984, 2001).
Estimation of the Total Pool of DMADP and the Pool Responsible for Isoprene Emission Using in Vitro Methods
Additional experiments were conducted to compare in vivo and in vitro methods of DMADP pool size measurements and to study the correspondence between the gasometrically detected pool and total leaf DMADP pool. In these experiments, we used a smaller thermostatted leaf chamber of 1.2 L and air turnover of about 1 min. Leaf temperature was kept at 30°C. A proton-transfer reaction mass spectrometer (PTR-MS; high-sensitivity; www.ptrms.com; Ionicon Analytik) was used to measure the emission of isoprene. After isoprene emission reached the steady state at a given quantum flux density, the light was switched off and the size of the light-dependent pool of DMADP was determined as described above. The light was then switched on again, and the leaf was kept under the same conditions as before until the same steady-state level of isoprene emission was achieved. Three discs, each of 1.8 cm2, were then quickly, within 5 to 6 s, taken from one half of the leaf and put immediately in liquid N2. The light was then switched off, and the isoprene emission in the darkness was monitored continuously. When isoprene emission in darkness reached zero, three discs again were taken from the other side of the leaf within 5 to 6 s and put in liquid nitrogen. In all cases (different leaf ages and light conditions), at least five replicate analyses in light and darkness were made. As mechanical damage such as wounding can result in modifications in isoprene emissions, with moderate increases in the grass Phragmites australis (Loreto et al., 2006) or decreases in the vine Pueraria lobaria and the herb Mucuna deeringeniana (Loreto and Sharkey, 1993) being reported, we also checked for the effect of leaf disc removal on isoprene emissions from poplar. In all cases, isoprene emission rates after taking the discs reached the same values as before the sampling. Analogously to our study, twig cutting did not affect isoprene emissions in the tree Quercus robur (Kreuzwieser et al., 2002), and sampling for leaf discs did not alter the other MEP pathway isoprenoid contents in P. tremula (Niinemets et al., 2003).
We used the method of Fisher et al. (2001) to estimate the pool of DMADP in the leaves. This method is based on acid hydrolysis of DMADP in crude leaf extracts and quantitative detection of isoprene released into the head space. Leaf discs of approximately 100 mg fresh mass were homogenized in liquid nitrogen with a pestle and mortar and quantitatively transferred to ice-cold reaction vials. As in the original paper by Fisher et al. (2001), 500 μL of 8 m H2SO4 was added, and the samples were incubated for 1 h at 30°C in 10-mL vials (CTC Headspace; CTC Analytics) and air-tight closed with an UltraClean 18-mm screw cap with a polytetrafluoroethylene septum (Agilent Technologies). The screw cap had custom-made openings for rapid connection to the PTR-MS through a valve and a T-shaped tube (Agilent Technologies). At the end of the incubation, the incubation vial was rapidly (in less than 1 s) switched to the PTR-MS, and the isoprene signal was recorded at 10-ms resolution. The time-integrated signal was used to calculate the amount of isoprene produced by acid hydrolysis of DMADP. As several products form from DMADP under acid hydrolysis (Fisher et al., 2001), calibration of the method was conducted using aqueous solutions of DMADP (Echelon Biosciences) as described by Fisher et al. (2001).
We estimated the light-dependent pool size of DMADP from the difference between the DMADP pool sizes in illuminated leaves and in the darkness just after cessation of isoprene emission.
Leaf fresh mass-to-area ratio and dry-to-fresh mass ratio were estimated separately using three to five discs taken from neighboring leaves of the same age. Preliminary experiments demonstrated that the errors in fresh and dry mass estimation were less than 2% to 3%.
Analysis of the Effect of Fosmidomycin on in Vitro DMADP Pool Size
An additional series of experiments was conducted to evaluate the influence of the MEP pathway inhibitor fosmidomycin (Invitrogen) on the total DMADP concentration in the leaves. Samples were taken from plants adapted to standard ambient conditions with petioles kept in water and after 45 to 50 min in 20 μm fosmidomycin solution. By this time, we observed essentially full inhibition of isoprene emission (to less than 1% of the initial value). Although the percentage of fosmidomycin inhibition can be somewhat higher (e.g. approximately 10% residual emission was observed after 30 min of feeding by Loreto et al. [2004]), very low residual emissions similar to our study have been reported by others (Sharkey et al., 2001, after 40 min of feeding).
In Vivo Kinetic Characteristics of Isoprene Synthase
The standard conditions for in vivo DMADP pool size measurements were fixed temperature of 28°C to 30°C, O2 concentration of 21%, CO2 concentration of 390 μmol mol−1, and quantum flux density of 550 μmol m−2 s−1. Additional experiments were conducted under 2% O2, varying CO2 (390–1,600 μmol mol−1) and light (150–550 mmol m−2 s−1), and in plants with leaves of different age to develop an extensive set of pairs of DMADP pool size and steady-state emission rate before the transient. These paired values corresponding to individual experiments (n = 17) were further employed to determine in vivo values of the Michaelis-Menten constant (Km; nmol m−2) and Vmax (nmol m−2 s−1) of isoprene synthase using the Lineweaver-Burk plot (double reciprocal plot). To compare our estimates of Km values expressed per unit of leaf area with the chemical estimations of Km that are expressed per unit of chloroplast volume, the conversion factor between area-based and volume-based units (m2 leaf area/m3 chloroplast volume) was determined according to Wolfertz et al. (2004). In these calculations, we used the measurements of leaf fresh mass per area (110 g m−2) and leaf liquid volume fraction (0.85) and used the same chloroplast-to-leaf liquid volume ratio as assumed by Wolfertz et al. (2004).
This work was supported by the Human Frontiers of Science Program (https://www.hfsp.org), the Estonian Science Foundation (grant no. 7645), the Estonian Academy of Sciences, and the Estonian Ministry of Education and Science (grant no. SF1090065s07).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ülo Niinemets (ylo.niinemets@emu.ee).
References
- Behnke K, Ehlting B, Teuber M, Bauerfeind M, Louis S, Hänsch R, Polle A, Bohlmann J, Schnitzler JP (2007) Transgenic, non-isoprene emitting poplars don't like it hot. Plant J 51 485–499 [DOI] [PubMed] [Google Scholar]
- Brüggemann N, Schnitzler JP (2002) Diurnal variation of dimethylallyl diphosphate concentrations in oak (Quercus robur) leaves. Physiol Plant 115 190–196 [DOI] [PubMed] [Google Scholar]
- Copolovici LO, Niinemets Ü (2005) Temperature dependencies of Henry's law constants and octanol/water partition coefficients for key plant volatile monoterpenoids. Chemosphere 61 1390–1400 [DOI] [PubMed] [Google Scholar]
- Delwiche CF, Sharkey TD (1993) Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ 16 587–591 [Google Scholar]
- Disch A, Hemmerlin A, Bach TJ, Rohmer M (1998) Mevalonate-derived isopentenyl diphosphate is the biosynthetic precursor of ubiquinone prenyl side chain in tobacco BY-2 cells. Biochem J 331 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Rohdich F, Bacher A (2001) Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci 6 78–84 [DOI] [PubMed] [Google Scholar]
- Falbel TG, Sharkey TD (2005) Determining the DMAPP that is available for isoprene synthesis. Poster 57, Secondary metabolism. In Plant Biology 2005, July 16–July 20, 2005, Seattle, WA. American Society of Plant Biologists, Rockville, MD, no. 625
- Fall R, Wildermuth MC (1998) Isoprene synthase: from biochemical mechanism to emission algorithm. J Geophys Res 103 25599–25609 [Google Scholar]
- Fisher AJ, Rosenstiel TN, Shirk MC, Fall R (2001) Nonradioactive assay for cellular dimethylallyl diphosphate. Anal Biochem 292 272–279 [DOI] [PubMed] [Google Scholar]
- Funk JL, Mak JE, Lerdau MT (2004) Stress-induced changes in carbon sources for isoprene production in Populus deltoides. Plant Cell Environ 27 747–755 [Google Scholar]
- Grote R, Niinemets Ü (2008) Modeling volatile isoprenoid emission: a story with split ends. Plant Biol 10 8–28 [DOI] [PubMed] [Google Scholar]
- Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer PI, Geron C (2006) Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos Chem Phys 6 3181–3210 [Google Scholar]
- Harley P, Greenberg J, Niinemets Ü, Guenther A (2007) Environmental controls over methanol emission from leaves. Biogeosciences 4 1083–1099 [Google Scholar]
- Hemmerlin A, Hoeffler JF, Meyer O, Tritsch D, Kagan IA, Grosdemange-Billiard C, Rohmer M, Bach TJ (2003) Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco Bright Yellow-2 cells. J Biol Chem 278 26666–26676 [DOI] [PubMed] [Google Scholar]
- Hills AJ, Zimmerman PR (1990) Isoprene measurement by ozone-induced chemiluminescence. Anal Chem 62 1055–1060 [Google Scholar]
- Hüve K, Christ MM, Kleist E, Uerlings R, Niinemets Ü, Walter A, Wildt J (2007) Simultaneous growth and emission measurements demonstrate an interactive control of methanol release by leaf expansion and stomata. J Exp Bot 58 1783–1793 [DOI] [PubMed] [Google Scholar]
- Karl T, Fall R, Rosenstiel TN, Prazeller P, Larsen B, Seufert G, Lindinger W (2002) On-line analysis of the 13CO2 labeling of leaf isoprene suggests multiple subcellular origins of isoprene precursors. Planta 215 894–905 [DOI] [PubMed] [Google Scholar]
- Kreuzwieser J, Graus M, Wisthaler A, Hansel A, Rennenberg H, Schnitzler JP (2002) Xylem-transported glucose as an additional carbon source for leaf isoprene formation in Quercus robur. New Phytol 156 171–178 [DOI] [PubMed] [Google Scholar]
- Kuzma J, Fall R (1993) Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiol 101 435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk A, Kiirats O, Oja V (1984) Assimilatory power (postillumination CO2 uptake) in leaves. Plant Physiol 76 723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk A, Oja V (1998) Dynamics of Leaf Photosynthesis: Rapid-Response Measurements and Their Interpretations. CSIRO Publishing, Canberra, Australia
- Laisk A, Oja V, Rasulov B, Rämma H, Eichelmann H, Kasparova I, Pettai H, Padu E, Vapaavuori E (2001) A computer-operated routine of gas exchange and optical measurements to diagnose photosynthetic apparatus in leaves. Plant Cell Environ 25 923–943 [Google Scholar]
- Lehning A, Zimmer I, Steinbrecher R, Brüggemann N, Schnitzler JP (1999) Isoprene synthase activity and its relation to isoprene emission in Quercus robur L. leaves. Plant Cell Environ 22 495–504 [Google Scholar]
- Lehning A, Zimmer W, Zimmer I, Schnitzler JP (2001) Modeling of annual variations of oak (Quercus robur L.) isoprene synthase activity to predict isoprene emission rates. J Geophys Res 106 3157–3166 [Google Scholar]
- Logan BA, Monson RK, Potosnak MJ (2000) Biochemistry and physiology of foliar isoprene production. Trends Plant Sci 5 477–481 [DOI] [PubMed] [Google Scholar]
- Loreto F, Barta C, Brilli F, Nogues I (2006) On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ 29 1820–1828 [DOI] [PubMed] [Google Scholar]
- Loreto F, Centritto M, Barta C, Calfapietra C, Fares S, Monson RK (2007) The relationship between isoprene emission rate and dark respiration rate in white poplar (Populus alba L.) leaves. Plant Cell Environ 30 662–669 [DOI] [PubMed] [Google Scholar]
- Loreto F, Ciccioli P, Brancaleoni E, Cecinato A, Frattoni M (1998) Measurement of isoprenoid content in leaves of Mediterranean Quercus spp. by a novel and sensitive method and estimation of the isoprenoid partition between liquid and gas phase inside the leaves. Plant Sci 136 25–30 [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Pinelli P, Brancaleoni E, Ciccioli P (2004) 13C labeling reveals chloroplastic and extra-chloroplastic pools of dimethylallyl pyrophosphate and their contribution to isoprene formation. Plant Physiol 135 1903–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD (1990) A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta 182 523–531 [DOI] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD (1993) Isoprene emission by plants is affected by transmissible wound signals. Plant Cell Environ 16 563–570 [Google Scholar]
- Magel E, Mayrhofer S, Müller A, Zimmer I, Hampp R, Schnitzler JP (2006) Photosynthesis and substrate supply for isoprene biosynthesis in poplar leaves. Atmos Environ 40 S138–S151 [Google Scholar]
- Monson RK, Fall R (1989) Isoprene emission from aspen leaves: influence of environment and relation to photosynthesis and photorespiration. Plant Physiol 90 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Harley PC, Litvak ME, Wildermuth M, Guenther AB, Zimmerman PR, Fall R (1994) Environmental and developmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia 99 260–270 [DOI] [PubMed] [Google Scholar]
- Monson RK, Holland EA (2001) Biospheric trace gas fluxes and their control over tropospheric chemistry. Annu Rev Ecol Syst 32 547–576 [Google Scholar]
- Monson RK, Trahan N, Rosenstiel TN, Veres P, Moore D, Wilkinson M, Norby RJ, Volder A, Tjoelker MG, Briske DD, et al (2007) Isoprene emission from terrestrial ecosystems in response to global change: minding the gap between models and observations. Philos Trans R Soc Lond A 365 1677–1695 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Kollist H, García-Plazaola JI, Becerril JM (2003) Do the capacity and kinetics for modification of xanthophyll cycle pool size depend on growth irradiance in temperate trees? Plant Cell Environ 26 1787–1801 [Google Scholar]
- Niinemets Ü, Reichstein M (2002) A model analysis of the effects of nonspecific monoterpenoid storage in leaf tissues on emission kinetics and composition in Mediterranean sclerophyllous Quercus species. Global Biogeochemical Cycles 16 1110 [Google Scholar]
- Niinemets Ü, Reichstein M (2003) Controls on the emission of plant volatiles through stomata: sensitivity or insensitivity of the emission rates to stomatal closure explained. J Geophys Res 108 4208 [Google Scholar]
- Niinemets Ü, Tenhunen JD, Harley PC, Steinbrecher R (1999) A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus. Plant Cell Environ 22 1319–1336 [Google Scholar]
- Nogués I, Brilli F, Loreto F (2006) Dimethylallyl diphosphate and geranyl diphosphate pools of plant species characterized by different isoprenoid emissions. Plant Physiol 141 721–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja V, Laisk A, Heber U (1986) Light-induced alkalization of the chloroplast stroma in vivo as estimated from the CO2 capacity of intact sunflower leaves. Biochim Biophys Acta 849 355–365 [Google Scholar]
- Pearcy RW, Gross LJ, He D (1997) An improved dynamic model of photosynthesis for estimation of carbon gain in sunfleck light regimes. Plant Cell Environ 20 411–424 [Google Scholar]
- Rodríguez-Concepción M, Boronat A (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids: a metabolic milestone achieved through genomics. Plant Physiol 130 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel TN, Fall R, Monson RK (2006) Substrate versus enzyme controls over isoprene emission from poplar leaves grown at elevated CO2 concentration. Geophysical Research Abstracts 8 01562 [Google Scholar]
- Rosenstiel TN, Fisher AJ, Fall R, Monson RK (2002) Differential accumulation of dimethylallyl diphosphate in leaves and needles of isoprene- and methylbutenol-emitting and nonemitting species. Plant Physiol 129 1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel TN, Potosnak MJ, Griffin KL, Fall R, Monson RK (2003) Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 421 256–259 [DOI] [PubMed] [Google Scholar]
- Ruuska S, Andrews TJ, Badger MR, Hudson GS, Laisk A, Price GD, von Caemmerer S (1998) The interplay between limiting processes in C3 photosynthesis studied by rapid-response gas exchange using transgenic tobacco impaired in photosynthesis. Aust J Plant Physiol 25 859–870 [Google Scholar]
- Sanadze GA (2004) Biogenic isoprene (a review). Russ J Plant Physiol 51 729–741 [Google Scholar]
- Schnitzler JP, Arenz R, Steinbrecher R, Lehning A (1996) Characterization of an isoprene synthase from leaves of Quercus petraea (Mattuschka) Liebl. Bot Acta 109 216–221 [Google Scholar]
- Schnitzler JP, Lehning A, Steinbrecher R (1997) Seasonal pattern of isoprene synthase activity in Quercus robur leaves and its significance for modeling isoprene emission rates. Bot Acta 110 240–243 [Google Scholar]
- Shallcross DE, Monks PS (2000) A role for isoprene in biosphere-climate-chemistry feedbacks? Atmos Environ 34 1659–1660 [Google Scholar]
- Sharkey TD, Chen XY, Yeh S (2001) Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol 125 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F, Delwiche CF (1991) High carbon dioxide and sun/shade effects on isoprene emission from oak and aspen tree leaves. Plant Cell Environ 14 333–338 [Google Scholar]
- Sharkey TD, Seemann JR, Pearcy RW (1986) Contribution of metabolites of photosynthesis to postillumination CO2 assimilation in response to lightflecks. Plant Physiol 82 1063–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL (1995) Why plants emit isoprene. Nature 374 769 [Google Scholar]
- Sharkey TD, Wiberley AE, Donohue AR (2008) Isoprene emission from plants: why and how. Ann Bot (Lond) 101 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Yeh S, Wiberley AE, Falbel TG, Gong D, Fernandez DE (2005) Evolution of the isoprene biosynthetic pathway in kudzu. Plant Physiol 137 700–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver GM, Fall R (1995) Characterization of aspen isoprene synthase, an enzyme responsible for leaf isoprene emission to the atmosphere. J Biol Chem 270 13010–13016 [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD (1997) Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol 115 1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahala J, Keinänen M, Schützendübel A, Polle A, Kangasjärvi J (2003) Differential effects of elevated ozone on two hybrid aspen genotypes predisposed to chronic ozone fumigation: role of ethylene and salicylic acid. Plant Physiol 132 196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153 376–387 [DOI] [PubMed] [Google Scholar]
- Wiberley AE, Donohue AR, Meier ME, Westphal MM, Sharkey TD (2008) Regulation of isoprene emission in Populus trichocarpa leaves subjected to changing growth temperature. Plant Cell Environ 31 258–267 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Fall R (1998) Biochemical characterization of stromal and thylakoid-bound isoforms of isoprene synthase in willow leaves. Plant Physiol 116 1111–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kühnemann F (2004) Rapid regulation of the methylerythritol 4-phosphate pathway during isoprene synthesis. Plant Physiol 135 1939–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kühnemann F, Yeih S, Weise SE (2003) Biochemical regulation of isoprene emission. Plant Cell Environ 26 1357–1364 [Google Scholar]
- Zimmer W, Brüggemann N, Emeis S, Giersch C, Lehning A, Steinbrecher R, Schnitzler JP (2000) Process-based modelling of isoprene emission by oak leaves. Plant Cell Environ 23 585–595 [Google Scholar]
- Zimmer W, Steinbrecher R, Körner C, Schnitzler JP (2003) The process-based SIM-BIM model: towards more realistic prediction of isoprene emissions from adult Quercus petraea forest trees. Atmos Environ 37 1665–1671 [Google Scholar]