Abstract
Trichoderma species belong to a class of free-living fungi beneficial to plants that are common in the rhizosphere. We investigated the role of auxin in regulating the growth and development of Arabidopsis (Arabidopsis thaliana) seedlings in response to inoculation with Trichoderma virens and Trichoderma atroviride by developing a plant-fungus interaction system. Wild-type Arabidopsis seedlings inoculated with either T. virens or T. atroviride showed characteristic auxin-related phenotypes, including increased biomass production and stimulated lateral root development. Mutations in genes involved in auxin transport or signaling, AUX1, BIG, EIR1, and AXR1, were found to reduce the growth-promoting and root developmental effects of T. virens inoculation. When grown under axenic conditions, T. virens produced the auxin-related compounds indole-3-acetic acid, indole-3-acetaldehyde, and indole-3-ethanol. A comparative analysis of all three indolic compounds provided detailed information about the structure-activity relationship based on their efficacy at modulating root system architecture, activation of auxin-regulated gene expression, and rescue of the root hair-defective phenotype of the rhd6 auxin response Arabidopsis mutant. Our results highlight the important role of auxin signaling for plant growth promotion by T. virens.
Plant growth is affected by a plethora of environmental factors, including light, temperature, nutrients, and microorganisms. The region around the root, the rhizosphere, is relatively rich in nutrients, because as much as 40% of plant photosynthesis products can be lost from the roots (Bais et al., 2006). Consequently, the rhizosphere supports large microbial populations capable of exerting beneficial, neutral, or detrimental effects on plant growth.
Trichoderma species are free-living fungi that are common in soil and root ecosystems. They have been widely studied for their capacity to produce antibiotics, parasitize other fungi, and compete with deleterious plant microorganisms (Harman et al., 2004a). Until recently, these traits were considered to be the basis for how Trichoderma exert beneficial effects on plant growth and development. However, it is becoming increasingly clear that certain strains also have substantial direct influence on plant development and crop productivity (Harman, 2006). Trichoderma enhancement of plant growth has been known for many years and can occur in axenic systems or in soils (Chang et al., 1986; Yedidia et al., 2001; Adams et al., 2007).
In maize (Zea mays) plants, Trichoderma inoculation affected root system architecture, which was related to increased yield of plants. Reported effects include enhanced root biomass production and increased root hair development (Bjorkman et al., 1998; Harman et al., 2004b). The root system is important for plant fitness because it provides anchorage, contributes to water use efficiency, and facilitates the acquisition of mineral nutrients from the soil (López-Bucio et al., 2005a). Many lines of evidence strongly support a role for auxin in the regulation of root system architecture. Application of natural and synthetic auxins increases lateral root and root hair development, whereas auxin transport inhibitors reduce root branching (Reed et al., 1998; Casimiro et al., 2001). The auxin-resistant mutants axr1 and axr2 produce fewer lateral roots than wild-type plants (Estelle and Somerville, 1987). Conversely, increased formation of lateral roots has been observed in Arabidopsis (Arabidopsis thaliana) mutants with elevated auxin content, including the rooty mutant and its alleles alf1 and superroot1 (Boerjan et al., 1995; Celenza et al., 1995; King et al., 1995). Additional mutants with auxin-related phenotypes include aux1, doc1, and eir1. The aux1-7 mutant is defective at the AUX1 locus, which encodes an auxin influx facilitator participating in both acropetal and basipetal auxin transport at the root tip (Swarup et al., 2001). doc1 is a mutant allele of BIG, which encodes a protein important for the correct location of certain auxin transport proteins (Gil et al., 2001), whereas EIR1 encodes the auxin transporter AtPIN2 (Luschnig et al., 1998). It has been determined that auxin deprivation keeps pericycle cells in G1 phase and readdition promotes the G1-S transition of the cell cycle, thus promoting lateral root initiation (Himanen et al., 2002). Despite auxin being a major player in root growth regulation, little is known about its role in plant growth promotion by fungi.
To elucidate the signaling mechanisms by which Trichoderma species promote plant growth and development, we evaluated the Arabidopsis response to inoculation with two Trichoderma species, Trichoderma atroviride (formerly known as Trichoderma harzianum) and Trichoderma virens. The two fungal species were found to promote Arabidopsis seedling growth under axenic conditions. Plant growth promotion elicited by these fungi correlated with prolific formation of lateral roots. A role for auxin signaling in mediating the observed developmental alterations by T. virens inoculation in plants was inferred from tests using the auxin-responsive marker constructs DR5:uidA, BA3:uidA, and HS∷AXR3NT-GUS and the analysis of aux1-7, doc1, eir1, and axr1 auxin-related mutants of Arabidopsis. We further show that T. virens is able to produce the indolic compounds indole-3-acetic acid (IAA), indole-3-acetaldehyde (IAAld), and indole-3-ethanol (IEt), which may play roles in mediating plant growth promotion by this fungus.
RESULTS
T. atroviride and T. virens Promote Growth and Development of Arabidopsis Seedlings
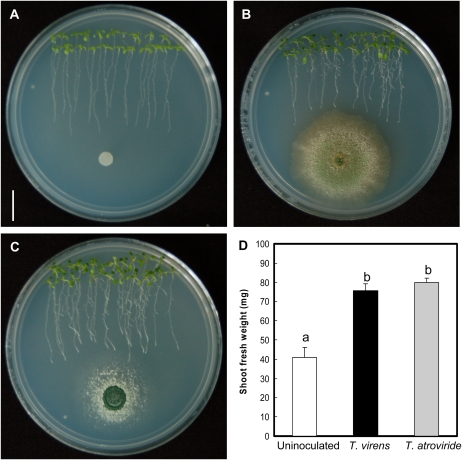
To study the plant growth-promoting activity of T. atroviride and T. virens, we used Arabidopsis as a model. Arabidopsis (ecotype Columbia [Col-0]) seedlings were germinated and grown for a 4-d period on petri plates containing agar-solidified 0.2× Murashige and Skoog (MS) medium. At day 4 after germination, the seedlings were treated with distilled sterilized water (control treatment) or with 106 spores of each fungal species dissolved in water. Fungal spores were placed at a 5-cm distance from the primary root tip to test the possibility that diffusible fungal compounds could affect plant growth and development. After 5 d of growth in the presence of T. atroviride or T. virens, increases in shoot and root growth were observed (Fig. 1, A–C). Interestingly, fungal inoculation stimulated lateral root formation (Fig. 1, A–C) and increased shoot biomass production (Fig. 1D), indicating a beneficial effect of inoculation on plant growth and development.
Figure 1.
Effects of T. virens and T. atroviride inoculation on the growth of Arabidopsis seedlings. A, Photograph of 9-d-old Arabidopsis (Col-0) seedlings grown on the surface of agar plates containing 0.2× MS medium. Seedlings were treated with sterilized water at day 4 and photographed 5 d later. Bar = 1 cm. B, Representative photograph of Arabidopsis seedlings that were inoculated with T. virens at a distance of 5 cm from the root tip at 4 d after germination and grown for a further 5-d period. C, Photograph of Arabidopsis seedlings inoculated with T. atroviride at a distance of 5 cm from the root tip at 4 d after germination and grown for a further 5-d period. D, Effects of fungal inoculation on shoot biomass production. Photographs show representative individuals of four plates per treatment. Data from D show means ± sd from three groups of 10 seedlings that were recovered from the medium, excised at the root/shoot junction, and weighed on an analytical scale. Different letters represent means statistically different at the 0.05 level. The experiment was repeated three times with similar results. [See online article for color version of this figure.]
T. atroviride and T. virens Alter Root System Architecture in Arabidopsis
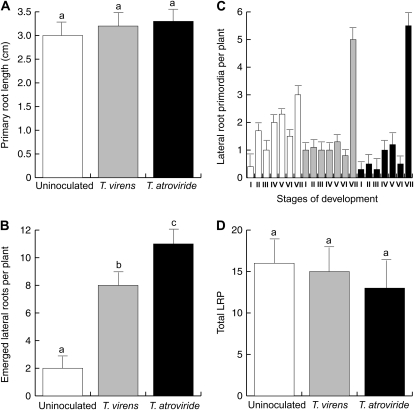
To more closely analyze the effects of Trichoderma on plant development, primary root length and number of emerged lateral roots were determined in 9-d-old Arabidopsis seedlings grown on petri plates containing agar-solidified 0.2× MS medium after 5 d of fungal inoculation. No significant effects of inoculation with T. atroviride or T. virens were observed for primary root growth (Fig. 2A). However, a 4- to 6-fold increase in lateral root number was observed in seedlings inoculated with each fungus (Fig. 2B). The effect of Trichoderma at increasing the number of lateral roots could be due to the stimulation of lateral root growth or to the de novo formation of lateral root primordia (LRP) by activation of pericycle cells. To distinguish between these two possibilities, LRP were quantified at day 5 after fungal inoculation. Seedling roots were first cleared to enable LRP at early stages of development to be visualized and counted. Each LRP was classified according to its stage of development as reported by Malamy and Benfey (1997). We found that the stage distribution of LRP was affected by inoculation with T. atroviride or T. virens. In particular, stage VII LRP, which belongs to developing LRP with fully active meristems, was significantly increased in T. atroviride- and T. virens-inoculated seedlings (Fig. 2C). The total number of LRP per plant was similar between uninoculated and Trichoderma-inoculated seedlings (Fig. 2D). These data suggest that Trichoderma can promote root branching in Arabidopsis by inducing lateral root growth rather than by increasing de novo formation of LRP.
Figure 2.
Effects of Trichoderma inoculation on Arabidopsis root system architecture. Arabidopsis Col-0 seedlings were germinated and grown for 4 d on the surface of agar plates containing 0.2× MS medium. Half of the plates were inoculated with T. virens or T. atroviride at a distance of 5 cm from the primary root tip and grown for an additional 5-d period. A, Primary root length. B, Lateral root number per plant. C, Stage number of lateral root primordia per plant. D, Total lateral root primordia per plant. Values shown are means ± sd (n = 30). Different letters represent means statistically different at the 0.05 level. The experiment was repeated three times with similar results.
T. virens Alters Auxin-Inducible Gene Expression in Arabidopsis
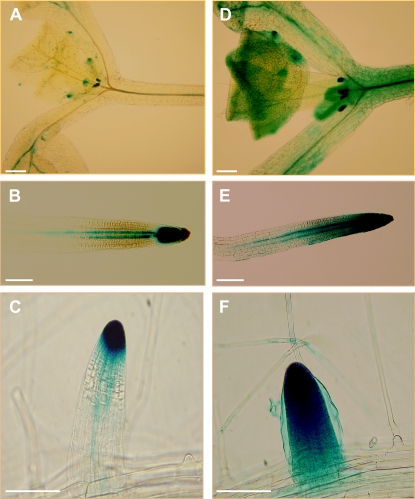
The observed effect of Trichoderma in promoting lateral root development is similar to that described for auxins in plants (Casimiro et al., 2001). We next tested whether T. virens could alter auxin-regulated gene expression in Arabidopsis by inoculating DR5:uidA transgenic seedlings with this fungus. The DR5:uidA line has been used to study auxin-regulated gene expression in Arabidopsis (Ulmasov et al., 1997). DR5:uidA seedlings were germinated and grown for 4 d on petri plates containing agar-solidified 0.2× MS medium and then inoculated with T. virens at 5 cm from the primary root tip. After an additional 5-d growth period, DR5:uidA seedlings were stained for GUS activity and further cleared to visualize changes in GUS expression. Although no significant effect of fungal inoculation was observed for primary root growth for wild-type (Fig. 2A) and DR5:uidA (data not shown) seedlings, an increase in GUS expression could be detected in shoots (Fig. 3, A and D), primary root tips (Fig. 3, B and E), and developing lateral roots (Fig. 3, C and F) from T. virens-inoculated seedlings when compared with uninoculated seedlings. These data suggest that T. virens inoculation increases auxin-regulated gene expression.
Figure 3.
Effects of T. virens inoculation on auxin-regulated gene expression. Twelve-hour GUS staining of DR5:uidA primary roots of Arabidopsis seedlings grown for 4 d on agar-solidified 0.2× MS medium. A to C, Uninoculated seedlings. D to F, T. virens-inoculated seedlings. Photographs show representative individuals of at least 20 stained seedlings. The experiment was repeated twice with similar results. Bars = 100 μm. [See online article for color version of this figure.]
Effects of T. virens Inoculation on Growth and Lateral Root Development of Auxin-Related Arabidopsis Mutants
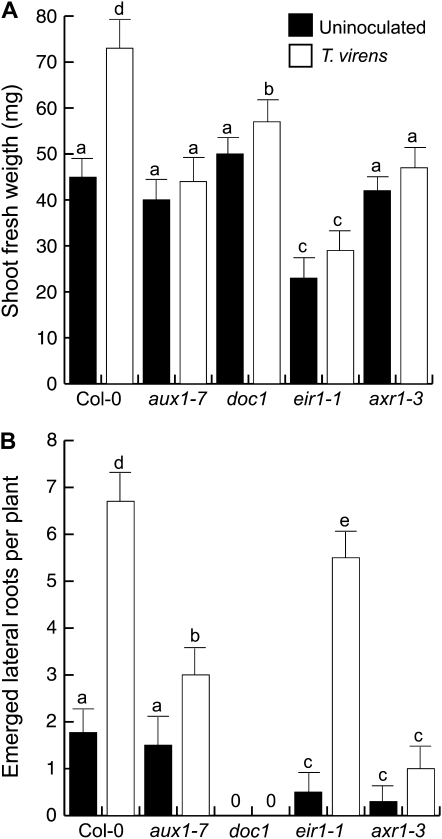
Next, we evaluated the effects of T. virens inoculation on growth of Arabidopsis wild-type seedlings and mutants defective in auxin transport (aux1-7, doc1, and eir1) or auxin response (axr1-3). Five days after plants were inoculated, T. virens increased by 62% shoot fresh weight in wild-type seedlings when compared with uninoculated seedlings. In contrast, all four mutant lines, aux1-7, doc1, eir1, and axr1-3, showed decreased or null responses in growth promotion by the fungus (Fig. 4A). We also quantified lateral root number in the wild type and all above mentioned mutants. It was found that T. virens inoculation induced up to a 4-fold increase in lateral root number when compared with uninoculated plants. Interestingly, a reduction in lateral root formation when compared with inoculated wild-type plants was observed for aux1-7 and axr1-3 inoculated seedlings, and no lateral root induction was registered for uninoculated or inoculated doc1 seedlings (Fig. 4B). These results indicate that both normal auxin transport and response are important for promoting the effects of T. virens on plant growth and lateral root development.
Figure 4.
Effects of T. virens inoculation on biomass production and lateral root development in wild-type Arabidopsis (Col-0) and auxin-related mutants. Plant material was harvested 5 d after fungal inoculation. Shoots were excised at the root/shoot junction, and the fresh weight was determined on an analytical balance. A, Shoot fresh weight. B, Lateral root number per plant. Values shown represent means of four groups of 10 seedlings ± sd. Lateral roots were quantified for 30 seedlings. Different letters are used to indicate means that differ significantly (P < 0.05). The experiment was repeated three times with similar results.
T. virens Produces IAA, IAAld, and IEt
The induced expression of DR5:uidA by T. virens and the decreased response of auxin-related Arabidopsis mutants to fungal inoculation opens the possibility that the fungus could produce IAA or other auxin-like compounds. We conducted experiments aimed at identifying IAA or IAA-related substances by growing T. virens on liquid cultures and determining indolic compounds from the supernatant by gas chromatography-mass spectrometry (GC-MS) analysis. We determined the actual (no Trp addition) and potential (100 mg L−1 Trp) production of indolic compounds produced by T. virens from either derivatized or underivatized samples from the growth medium. When derivatized samples were analyzed by GC-MS, we identified IAA (Fig. 5), which increases up to 17-fold in concentration in T. virens growth medium supplied with Trp (Table I). When underivatized samples from T. virens growth medium without Trp were analyzed for indolic compounds, the presence of IEt (retention time = 9.97 min) and IAAld (retention time = 8.83 min) was found (Fig. 6). The production of IEt was enhanced upon Trp addition, while a small yet significant increase in IAAld production was also detected in Trp-supplied cultures (Table I). IAA could not be further detected from underivatized samples.
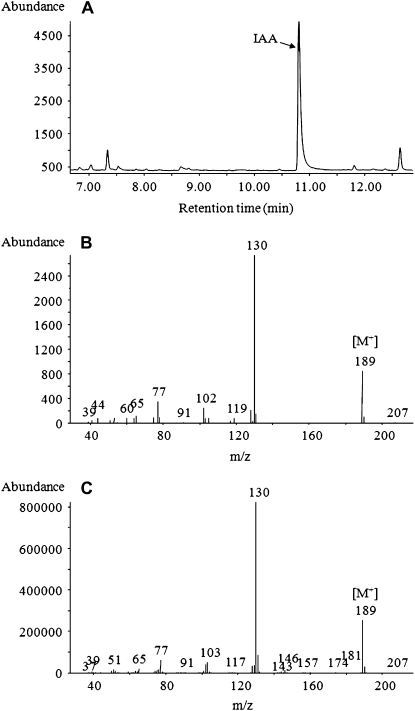
Figure 5.
Determination of IAA from derivatized samples from T. virens growth medium by GC-MS. A, Total ion chromatogram of IAA from acidic ethyl acetate extract obtained from 1 L of culture medium of T. virens. B and C, The 70-eV electron-impact full-scan mass spectra from m/z 50 to 500 of IAA methyl ester identified in the extract (B) and the methylated IAA standard (C). Determinations were done from at least five independent samples.
Table I.
Quantification of auxin-like compounds from T. virens
T. virens was inoculated in 1 L of nutrient solution with or without 100 mg of l-Trp, and determinations were performed by GC-MS after 3 d of growth. Data shown are means ± se for samples from three independent cultures (n = 3). Different letters represent means statistically different at the 0.05 level.
| Compound | Retention Time | Concentration
|
|
|---|---|---|---|
| (−) l-Trp | (+) l-Trp | ||
| min | μg L−1 | ||
| IAAld | 8.83 | 59.4 ± 4.47a | 70.15 ± 3.78b |
| IEt | 9.97 | 72.33 ± 1.41a | 141.88 ± 4.85b |
| IAA | 10.81 | 13.48 ± 0.97a | 233.64 ± 3.06b |
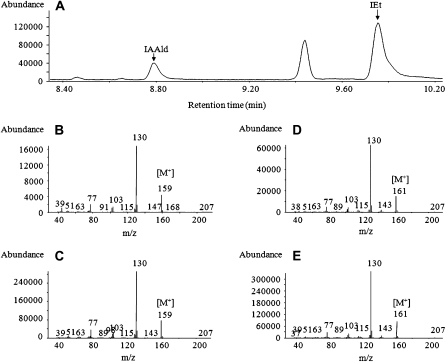
Figure 6.
Determination of indolic compounds from underivatized samples from T. virens growth medium by GC-MS. A, Total ion chromatogram of IAAld and IEt from neutral ethyl acetate extract obtained from 1 L of culture medium of T. virens. B to E, The 70-eV electron-impact full-scan mass spectra from m/z 50 to 500 of IAAld identified in the extract (B), the standard IAAld (C), IEt identified in the extract (D), and the standard IEt (E). Determinations were done from at least five independent samples.
IAAld Activates Auxin-Inducible Gene Expression
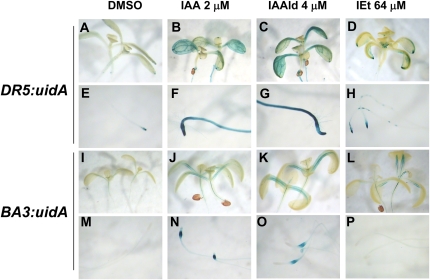
To determine if IAAld and IEt act in an auxin-related signaling pathway, we conducted analyses of the expression of the auxin-inducible DR5:uidA and BA3:uidA gene markers. Figure 7 shows histochemical staining for transgenic DR5:uidA and BA3:uidA seedlings that were grown for 6 d under IAA, IAAld, or IEt treatment. As reported previously (Ulmasov et al., 1997), in untreated control plants, DR5:uidA is absent from cotyledons and leaves and expressed primarily in the root tip region (Fig. 7, A and E). DR5:uidA seedlings grown at a concentration of 2 μm IAA showed GUS activity in the cotyledons and the primary root (Fig. 7, B and F). The pattern of GUS expression in DR5:uidA seedlings treated with 4 μm IAAld remained similar to that observed for IAA-treated plants (Fig. 7, C and G). In contrast, up to a 64 μm concentration of IEt showed a modest increase in expression of this marker (Fig. 7, D and H), indicating different auxin-related activity for the compounds. Untreated BA3:uidA plants did not show detectable levels of GUS activity (Fig. 7, I and M), whereas when treated with 2 μm IAA, they showed GUS expression mainly in petioles of cotyledons (Fig. 7J) and in the root elongation zone (Fig. 7N). GUS expression in plants treated with IAAld was clearly observed in the same regions as in IAA-treated seedlings (Fig. 7, K and O). IEt failed to activate BA3:uidA expression (Fig. 7, L and P). These results show that IAAld, IEt, and IAA treatments can differentially activate the expression of auxin-inducible gene markers.
Figure 7.
Effects of indolic compounds produced by T. virens on auxin-regulated gene expression. A to H, Twelve-hour GUS staining of DR5:uidA Arabidopsis seedlings grown for 6 d on agar plates containing 0.2× MS medium (A and E) and on medium supplied with 2 μm IAA (B and F), 4 μm IAAld (C and G), or 64 μm IEt (D and H). Notice the increase in GUS expression in shoots and roots in the treatments with IAAld. I to P, Twelve-hour GUS staining of BA3:uidA Arabidopsis seedlings grown for 6 d on agar plates containing 0.2× MS medium (I and M) and on medium supplied with 2 μm IAA (J and N), 4 μm IAAld (K and O), or 64 μm IEt (L and P). Notice the increase in GUS expression in the root elongation region in the treatments with IAA or IAAld. Photographs are representative individuals of at least 20 stained seedlings. The experiment was repeated twice with similar results. DMSO, Dimethyl sulfoxide. [See online article for color version of this figure.]
IAAld Enhances Aux/IAA Protein Degradation
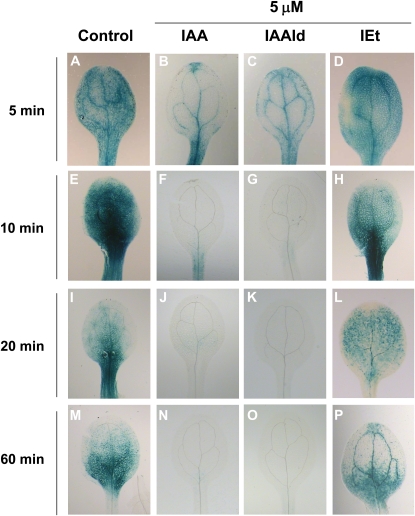
Auxin promotes the degradation of Aux/IAA repressor proteins via the ubiquitin-proteasome pathway and thereby induces primary auxin-responsive gene expression (Gray et al., 2001). To address the effect of IAA, IAAld, and IEt on auxin-mediated degradation of Aux/IAA proteins, we examined the effects of these compounds on Aux/IAA stability using the Arabidopsis HS∷AXR3NT-GUS line, in which a translational fusion between domains I and II of AXR3 and the GUS reporter protein is expressed under the control of a heat shock promoter (Gray et al., 2001). Seedlings expressing the HS∷AXR3NT-GUS construct were heat shocked at 37°C for 2 h and further treated with 5 μm IAA, IAAld, or IEt for 5, 10, 20, and 60 min. Treatment with IAA or IAAld showed enhanced degradation of the fusion protein in a similar way, but IEt failed to induce degradation of the fusion protein even after 60 min of treatment (Fig. 8, A–P). Our data indicate that IAAld likely acts in an auxin-mediated signaling pathway, either by direct binding to an auxin receptor or by its conversion to IAA, which rapidly destabilizes the AXR3 protein.
Figure 8.
Analysis of Aux/IAA stability with HS∷AXR3NT-GUS fusions. Wild-type seedlings expressing the HS∷AXR3NT-GUS constructs were heat shocked at 37°C for 2 h. After heat induction, the seedlings were treated with IAA, IAAld, or IEt for different time periods at the indicated concentrations and stained overnight for GUS activity. Notice the degradation of the fusion protein by either IAA or IAAld. A to P, Representative photographs of cotyledons (n = 10 stained seedlings). Similar results were obtained in two independent experiments. [See online article for color version of this figure.]
IAAld and IEt Differentially Regulate Arabidopsis Root System Architecture
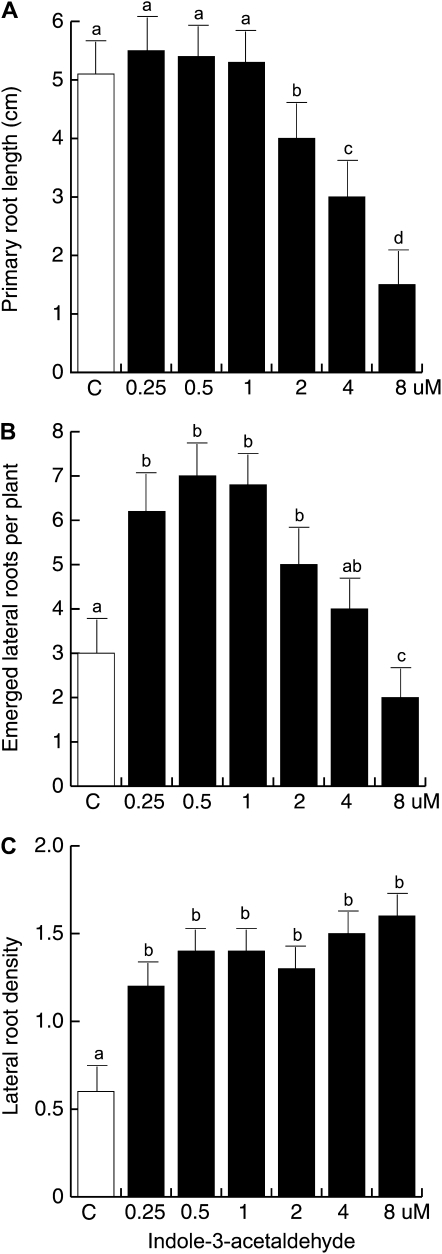
To determine more closely the effects of IAAld and IEt on the architecture of the Arabidopsis root system, wild-type Arabidopsis seedlings were germinated and grown on vertically oriented agar plates containing 0.2× MS medium supplied with IAAld or IEt concentrations ranging from 0.25 to 8 μm. Under these conditions, primary root length, number of lateral roots, and lateral root density were quantified. After 10 d of growth, it was observed that concentrations of IAAld greater than 1 μm inhibited primary root growth in a dose-dependent way (Fig. 9A). It was observed that IAAld-treated Arabidopsis seedlings produced a highly branched root system with abundant lateral roots. A roughly 2-fold increase in lateral root number per plant was found at concentrations of IAAld from 0.25 to 2 μm when compared with solvent-treated control seedlings (Fig. 9B). The density of lateral roots was also calculated by dividing the number of lateral roots by the length of the primary root to normalize for the effects of IAAld on primary root length. Lateral root density increased over 2-fold in plants treated with IAAld when compared with untreated seedlings (Fig. 9C). This increase in lateral root density was due to a stimulatory effect of IAAld on both LRP formation and lateral root emergence (Supplemental Fig. S1).
Figure 9.
Effects of IAAld on Arabidopsis root architecture. Wild-type Col-0 seedlings were grown for 10 d under increasing IAAld concentrations on vertically oriented agar plates. Data are given for the length of the primary root (A), lateral root number (B), and lateral root density (C). Values shown represent means of 30 seedlings ± sd. Different letters represent means statistically different at the 0.05 level. The experiment was repeated three times with similar results.
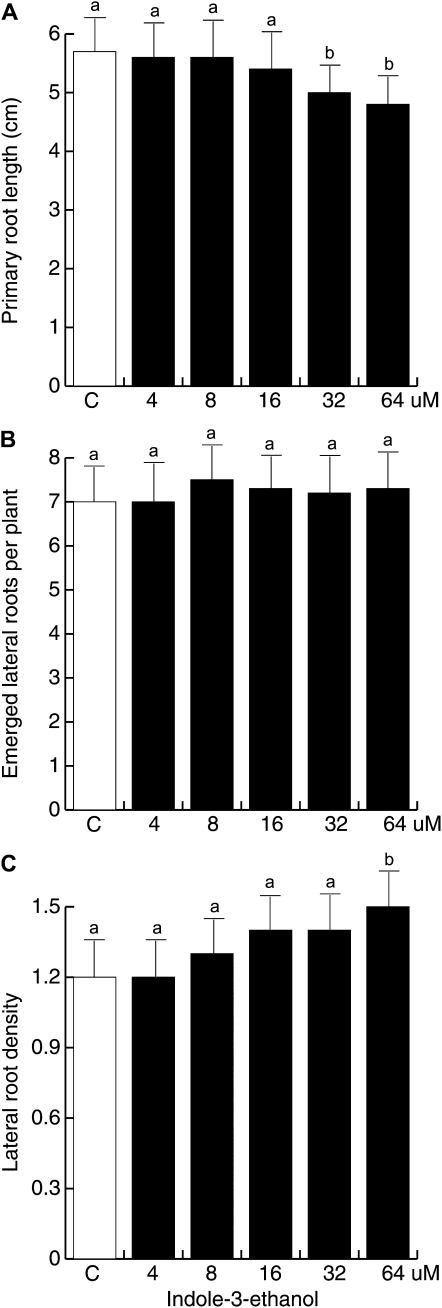
Interestingly, after 12 d of growth, IEt showed modest activity at inhibiting primary root growth (Fig. 10A) and failed to increase lateral root formation even when supplied at concentrations up to 64 μm (Fig. 10B). Lateral root density significantly increased only at 64 μm IEt concentration in the medium (Fig. 10C), indicating that this compound acts at high concentrations to activate pericycle cells. These results show that IAAld and IEt have different activity in Arabidopsis root system architecture modulation and that the effects of fungal inoculation on root development are likely due to a combined effect of all three indolic compounds, IAA, IAAld, and IEt, produced by the fungus.
Figure 10.
Effects of IEt on Arabidopsis root architecture. Wild-type Col-0 seedlings were grown for 12 d under increasing IEt concentrations on vertically oriented agar plates. Data are given for the length of the primary root (A), lateral root number (B), and lateral root density (C). Values shown represent means of 30 seedlings ± sd. Different letters represent means statistically different at the 0.05 level. The experiment was repeated three times with similar results.
IAAld Rescues the Root Hair-Defective Phenotype of the Auxin-Related rhd6 Arabidopsis Mutant
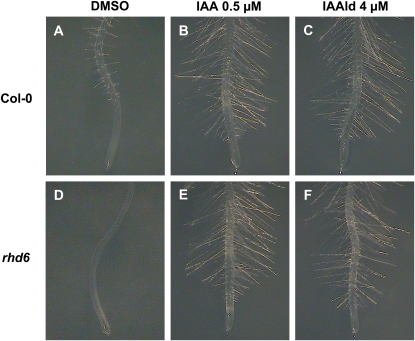
Arabidopsis root hairs are a good system in which to study cell differentiation and morphogenesis in plants. The study of their development is also of great interest because of their putative function in water and nutrient uptake. Several auxin-related mutations have been found to alter root hair development (Parker et al., 2000). Of particular interest is the rhd6 mutant, which is defective in root hair initiation and has been shown previously to be rescued by auxin (Masucci and Schiefelbein, 1994). We used the rhd6 mutant as a tool to probe the mechanism of IAAld action. We compared the root hair response of Arabidopsis wild-type seedlings and rhd6 mutants with IAA and IAAld treatments at day 5 after germination. As shown in Figure 10, treatments with 0.5 μm IAA or 4 μm IAAld stimulated root hair elongation and increased root hair formation at the primary root tip region in Arabidopsis wild-type seedlings (Fig. 11, A–C). rhd6 mutant seedlings grown in medium without auxin were completely devoid of root hairs (Fig. 11D). Interestingly, both IAA and IAAld were found to rescue the rhd6 root hair-defective phenotype (Fig. 11, E and F). The root hairs produced in each of these experiments exhibited normal growth and morphology. These results imply that the application of IAAld can suppress the root hair formation defects of rhd6.
Figure 11.
IAAld rescues the rhd6 mutant phenotype. A, Wild-type Col-0 Arabidopsis root with normal root hair formation. B and C, Root hair formation in response to IAA (B) or IAAld (C) treatment. D, A typical rhd6 Arabidopsis mutant root showing a reduction in root hair formation. E and F, Formation of root hairs in rhd6 roots in response to IAA (E) or IAAld (F) treatment. The experiment was repeated three times with similar results. DMSO, Dimethyl sulfoxide. [See online article for color version of this figure.]
IAA and IAAld Alter Arabidopsis Biomass Production in a Dose-Dependent Way
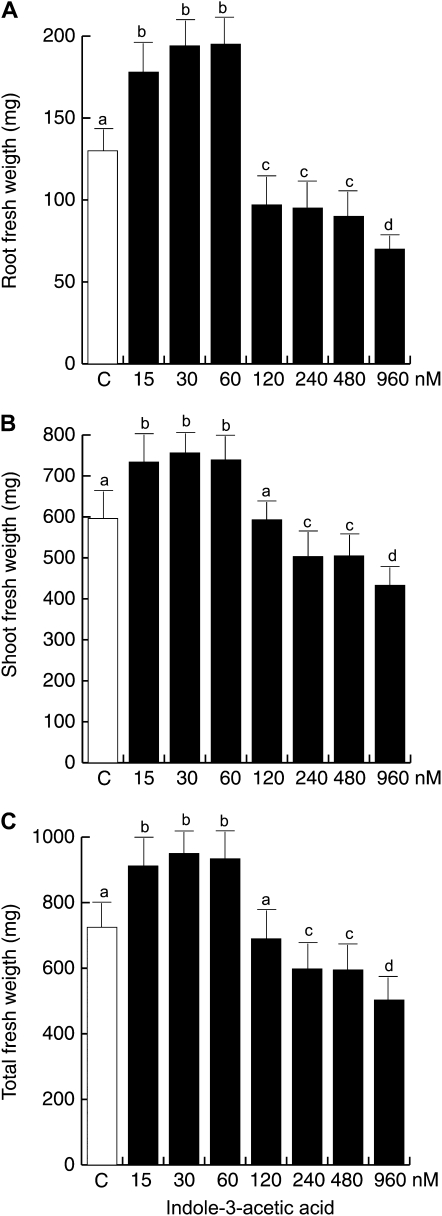
The fact that T. virens-enhanced shoot biomass production was dependent on auxin transport/signaling prompted us to determine whether exogenous auxin application could increase the growth of Arabidopsis seedlings. We quantified root, shoot, and total fresh weight of plants grown under varied concentrations of IAA or IAAld. Treatments of 15 to 60 nm IAA significantly increased root, shoot, and total fresh weight when compared with control plants, while concentrations of 120 to 960 nm did not affect or decreased biomass production (Fig. 12). Similar dose-dependent effects on growth were observed for IAAld-treated plants, albeit at greater concentrations than IAA (Supplemental Fig. S2).
Figure 12.
Effects of IAA on Arabidopsis biomass production. Wild-type Col-0 seedlings were grown for 14 d under increasing IAA concentrations on vertically oriented agar plates. Data are given for the mean root fresh weight (A), shoot fresh weight (B), and total fresh weight (C). Plants were excised at the root/shoot junction, and fresh weights were determined on an analytical scale for four groups of 25 plants. Different letters represent means statistically different at the 0.05 level. The experiment was repeated twice with similar results.
To further define whether the effects of IAAld are mediated by auxin transport/signaling, we performed experiments to investigate the resistance of auxin-related mutants to exogenous application of IAAld. A commonly used developmental marker for auxin responses is primary root growth. Therefore, we grew wild-type plants and the auxin-related mutants aux1-7, doc1, eir1-1, and axr1-3 in medium with or without 8 μm IAAld, a concentration that inhibits root growth. Our results show that aux1-7, eir1-1, and axr1-3 are indeed very resistant to IAAld and sustained primary root growth in an IAAld concentration that drastically inhibits growth in wild-type plants (Supplemental Fig. S3). Thus, we conclude that both auxin transport and response are important for root developmental responses to IAAld.
DISCUSSION
T. virens Promotes Arabidopsis Growth and Development through an Auxin-Dependent Mechanism
Trichoderma species are naturally occurring soil fungi that colonize roots and stimulate plant growth. Such fungi have been applied to a wide range of plant species for the purpose of growth enhancement, with a positive effect on plant weight, crop yields, and disease control. Their agricultural use could be expanded if the mechanisms of growth enhancement were known. A number of mechanisms for plant growth promotion by Trichoderma have been proposed (Harman et al., 2004a). Among these, fungal interaction with auxin signaling has not been examined, despite auxin being a central plant growth-regulating substance.
It was noticeable that inoculation with Trichoderma affected lateral root development in Arabidopsis wild-type plants in a way that suggests that the effects are mediated by auxin (Figs. 1 and 2). IAA is a molecule that is synthesized by plants and a few microbes (Woodward and Bartel, 2005). In plants IAA plays a key role in root and shoot development. The hormone moves from one part of the plant to another by specific transporter systems that involve auxin importer (AUX1) and efflux (PIN1-7) proteins. IAA is a key regulator of lateral root development and root hair development (Casimiro et al., 2001). Expression studies of the auxin-inducible marker DR5:uidA suggested that T. virens inoculation increases the auxin response in Arabidopsis seedlings (Fig. 3). To further elucidate some of the aspects of auxin transport/perception involved in the Arabidopsis response to T. virens, we analyzed the growth and development of Arabidopsis mutants with defects in the auxin signal transduction pathway. We found that the auxin transport mutants (aux1-7, eir1, and doc1) have a reduced response to the fungus in terms of growth promotion (Fig. 4A) and lateral root development (Fig. 4B). In particular, the doc1 mutant, which shows defects in lateral root initiation that can be complemented by nutrient deficiency (López-Bucio et al., 2005b), showed null induction of lateral roots when inoculated with T. virens (Fig. 4B). These results indicate that normal auxin transport is important for plant responses to T. virens. The finding that the auxin-resistant axr1-3 mutant also shows a reduced response to inoculation suggests that the corresponding wild-type gene is required in Arabidopsis for increased growth and lateral root formation when inoculated with the fungus (Fig. 4). AXR1 encodes a protein related to the ubiquitin-activating enzyme E1 (Leyser et al., 1993). These results indicate that plant growth promotion by T. virens operates through the classical auxin response pathway.
T. virens Produces IAA, IAAld, and IEt
In this study, we determined the presence of IAA (Fig. 5) and of two substances structurally related to IAA, namely IAAld and IEt, in T. virens growth medium (Fig. 6). When Trp was added to the growth medium of T. virens, an increased production of all three metabolites was evident (Table I). Although it is widely accepted that plants use several pathways to synthesize IAA, none of the pathways are yet defined to the level of knowing each relevant gene, enzyme, and intermediate. Several Trp-dependent pathways have been proposed: the indole-3-pyruvic acid (IPA) pathway, the indole-3-acetamide pathway, the tryptamine pathway, and the indole-3-acetaldoxime pathway (Woodward and Bartel, 2005). The IPA pathway (Trp→IPA→IAAld→IAA) is important in some IAA-synthesizing microorganisms (Koga, 1995), and recently it was demonstrated that it operates in plants as well (Stepanova et al., 2008; Tao et al., 2008). The final enzyme in the proposed IPA pathway is an IAAld-specific aldehyde oxidase (AAO1) that has increased activity in the IAA-overproducing superroot1 mutant (Seo et al., 1998). The identification of Arabidopsis AAO1 suggests that plant- and microbe-produced IAAld can be used to produce IAA in plants. Several lines of evidence support the view that the rate of auxin biosynthesis is subject to regulation, with several IAA precursors acting as storage compounds. IAAld can be converted to IEt by an indole acetaldehyde reductase enzyme. This enzyme has been characterized in cucumber (Cucumis sativus) seedlings, where it plays an important role in auxin biosynthesis (Brown and Purves, 1980). Both IAAld and IEt occur naturally in plants (Purves and Brown, 1978; Magnus et al., 1982), which suggests that these compounds can act as flexible storage pools for IAA. Although IEt does show a modest auxin-like activity in activating the auxin-regulated gene markers DR5:uidA and BA3:uidA (Fig. 7), conversion of IEt to IAA has been already demonstrated in cucumber seedling shoots (Rayle and Purves, 1967).
Relatively little information is available on IAA biosynthesis in fungi. Production of IAA through the IPA pathway was identified in the fungus Colletotrichum acutum (Chung et al., 2003). HPLC analysis and chromogenic stains after a fluorescence thin-layer chromatography separation unambiguously identified IAA, IEt, IAAld, and IPA from cultures supplemented with Trp. Interestingly, increasing Trp concentrations drastically increased the levels of IEt but not IAA (Chung et al., 2003). It has been suggested that in this case IEt may be the end product of Trp metabolism rather than a side product of the IPA pathway. In contrast, our results show that IAA levels dramatically increase in Trp-supplied cultures of T. virens (Table I); it is tempting to speculate that Trp supply to T. virens cultures increases IAA accumulation as a direct product of its metabolism.
IAAld Shows Auxin-Like Activity in Arabidopsis
To maximize the capability of an organ to expand or elongate, or to establish a particular developmental program such as lateral root formation, plants have evolved mechanisms tightly coupled to the perception of biotic and abiotic stimuli. Many of the plant responses to environmental factors are mediated by phytohormones, such as auxin.
IAA has been found to be the typical auxin in plants, mainly evaluated by cell elongation tests in hypocotyls and primary root growth responses (Woodward and Bartel, 2005). However, the chemical space, which encompasses the term “auxin,” is actually not easily achieved, since many compounds were found to exhibit an auxin-like activity in several different bioassays (Ferro et al., 2007). Our comparative analysis of auxin activity for IAA, IAAld, and IEt (Fig. 7) identified IAAld, an IAA precursor in the IPA pathway, as an active auxin. Three additional lines of evidence indicate that IAAld acts as an auxin: (1) the effect of the compound on Aux/IAA stability using the Arabidopsis HS∷AXR3NT-GUS line; (2) the regulation of root system architecture by its exogenous application to the seedlings; and (3) the rescue of the root hair-defective phenotype of the rhd6 mutant of Arabidopsis when exogenously supplied to the growth medium. Treatment with IAA or IAAld showed enhanced degradation of the fusion protein HS∷AXR3NT-GUS in a similar way, but IEt failed to induce degradation of the fusion protein even after 60 min of treatment (Fig. 8). These data indicate that IAAld likely acts in an auxin-mediated signaling pathway. Interestingly, exogenously supplied IEt was found to inhibit primary root growth and to increase lateral root density at a 64 μm concentration (Fig. 10), a much higher concentration than that required for IAA or IAAld to affect the same developmental traits. Compelling evidence that IAAld shows auxin-like activity came from the analysis of the root hair response in wild-type and rhd6 mutant seedlings to this compound. The reported association between auxin and the rhd6 mutation indicated that the RHD6 gene product could be used as a tool to probe the mechanism of action of auxin-like compounds (Masucci and Schiefelbein, 1994). Treatment with IAAld was found to rescue the root hair phenotype of the rhd6 mutant in a similar way to that of IAA (Fig. 11). Inoculation with T. virens or application of T. virens extracts also induced normal formation of root hairs in the rhd6 mutant (data not shown), suggesting that developmental effects of fungal inoculation in Arabidopsis likely occur by the production of an auxin, presumably IAA or IAAld.
Role of Auxin Signals in Trichoderma-Plant Interactions
The importance of auxins for plant development has been long recognized, and redundancy for IAA biosynthesis is widespread in plants and among plant-associated microorganisms. Accumulation of auxins or increased responses to auxins might lead to diverse outcomes on the plant side, varying from pathogenesis to growth promotion. T. virens and T. atroviride were found to stimulate the growth of Arabidopsis plants in vitro (Fig. 1), suggesting that these fungi likely act as plant growth-promoting microorganisms. It was previously reported that Trichoderma was able to colonize the entire root system of maize plants and to persist for the entire lifespan of this crop (Harman et al., 2004b). Fungal colonization stimulated plant growth by factors including increased root size and rooting depth, which aid in nutrient uptake (Yedidia et al., 2001; Harman et al., 2004a).
To further investigate whether IAA and IAAld produced by T. virens could have a positive effect on Arabidopsis growth, we quantified biomass production in plants treated with varied concentrations of these compounds. Both compounds showed a dose-dependent effect on growth by increasing biomass production in small amounts but repressing growth at higher concentrations (Fig. 12; Supplemental Fig. S2). Thus, the effect of inoculation with Trichoderma strains in plants under natural conditions may depend on the type and concentration of auxins being produced by the fungi.
Little is known about the molecular determinants involved in the interaction of T. virens with plants. We hypothesize that auxin production by this fungus promotes the interaction with roots by circumvention of basal plant defense mechanisms, as recently reported by Navarro et al. (2006), who showed that repression of auxin signaling restricts Pseudomonas syringae growth, implicating auxin in disease susceptibility and RNA-mediated suppression of auxin signaling in resistance. The fungus can also produce auxins as part of its colonization strategy, as published information indicates that fungus-produced IAA induces adhesion and filamentation of Saccharomyces cerevisiae (Prusty et al., 2004).
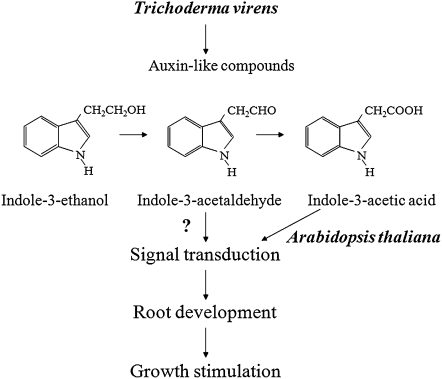
Although we cannot exclude the possibility that IAAld could be converted to IAA and in this way exert its biological action, the concerted action of all three indolic compounds identified may account for the plant growth-promoting properties of T. virens (Fig. 13). In the plant partner, alteration in lateral root formation may provide a greater root surface area for fungal colonization. In turn, increased absorptive surface by branched roots may increase water and nutrient uptake capacity of plants. It is tempting to speculate that production of auxins by Trichoderma may benefit plant hosts by initiating or reinforcing symbiotic behaviors with fungal partners in the rhizosphere.
Figure 13.
Arabidopsis growth responses to T. virens and their regulation. T. virens induces lateral root proliferation and enhances biomass accumulation by production of IAA and IAAld. IAAld can be converted to IAA by plant enzymes or can directly regulate auxin-inducible gene expression, possibly by interacting with auxin receptors. IEt did not show clear auxin-like activity, but it can act as a storage form for other active indolic compounds such as IAAld or IAA.
The data presented in this work suggest an important role for auxin signaling in plant growth regulation by T. virens. Our results show great promise for the use of Trichoderma species as inoculants for plant improvement under controlled and field conditions.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana Col-0), the Arabidopsis transgenic lines HS∷AXR3NT-GUS (Gray et al., 2001), DR5:uidA (Ulmasov et al., 1997), and BA3:uidA (Oono et al., 1998), and the mutant lines eir1-1 (Roman et al., 1995), doc1 (Li et al., 1994), axr1-3 (Lincoln et al., 1990), aux1-7 (Picket et al., 1990), and rhd6 (Masucci and Schiefelbein, 1994) were used for the different experiments. Seeds were surface sterilized with 95% (v/v) ethanol for 5 min and 20% (v/v) bleach for 7 min. After five washes in distilled water, seeds were germinated and grown on agar plates containing 0.2× MS medium. The MS medium (Murashige and Skoog Basal Salts Mixture, catalog no. M5524) was purchased from Sigma. Phytagar (commercial grade) was purchased from Gibco-BRL. Plates were placed vertically at an angle of 65° to allow root growth along the agar surface and to allow unimpeded aerial growth of the hypocotyls. Plants were placed in a plant growth chamber (Percival AR-95L) with a photoperiod of 16 h of light/8 h darkness, light intensity of 300 μmol m2 s−1, and temperature of 22°C.
Fungal Growth and Indolic Compound Determinations
The following strains were used in this work: Trichoderma virens Gv. 29-8 and Trichoderma atroviride (formerly Trichoderma harzianum) IMI 206040. The strains of Trichoderma were grown and maintained on potato dextrose agar medium (Difco).
For the production of indolic compounds, an active inoculum of 1 × 106 spores of T. virens was added to 1 L of potato dextrose broth (Difco) and grown for 3 d at 28°C with shaking at 200 rpm. To evaluate the effect of Trp supply on indolic compounds, the medium was supplemented with l-Trp (Merck) at a concentration of 100 mg L−1. For IAAld and IEt determinations, the fungal culture was filtered and the supernatant was adjusted to pH 7 using 2 n NaOH. Indolic compounds in supernatant solutions were extracted three times with 1 L of ethyl acetate. The extracts were combined and evaporated to dryness under a stream of nitrogen and then diluted in 1 mL of ethyl acetate.
For IAA determination, the fungal culture was filtered and the supernatant was adjusted to pH 3 using 1 n HCl. IAA from supernatant solutions was extracted three times with 1 L of ethyl acetate, and the extracts were combined, evaporated to dryness under a stream of nitrogen, and diluted in 1 mL of ethyl acetate. IAA was methyl esterified with 600 μL of acetyl chloride in 2 mL of dry methanol, sonicated for 15 min, and heated at 75°C for 1 h. The IAA methyl ester was evaporated under a stream of nitrogen and redissolved in 1 mL of ethyl acetate. The sample was diluted 1:10 (v/v) without l-Trp in the medium and 1:100 (v/v) with l-Trp before GC-MS analysis.
The indolic compounds were analyzed in an Agilent 6850 Series II gas chromatograph equipped with an Agilent MS detector model 5973 and a 30-m × 0.2-μm × 0.25-mm, 5% phenyl methyl silicone capillary column (HP-5 MS). Operating conditions used 1 mL min−1 helium as carrier gas, detector temperature of 300°C, and injector temperature of 250°C. The volume of the injected sample was 1 μL. The column was held for 3 min at 80°C and programmed at 6°C min−1 to a final temperature of 230°C for 5 min. Indolic compounds were identified by comparison with a mass spectra library (National Institute of Standards and Technology/Environmental Protection Agency/National Institutes of Health; Chem Station; Hewlett-Packard). The identities of the indolic compounds were further confirmed by comparison of the retention time in the fungal extract with samples of the pure IAAld, IEt, and IAA standards (Sigma). A selected ion monitoring analysis was used to verify the presence of these indolic compounds in the samples. The molecular ions were monitored after electron impact ionization (70 eV). For IAAld, mass-to-charge ratios (m/z) were m/z 144, m/z 116, and m/z 89; for IEt, they were m/z 161, m/z 130, m/z 103, and m/z 77; and for IAA methyl ester, they were m/z 189, m/z 130, m/z 103, and m/z 77. To estimate the amount of compounds produced by T. virens, we constructed individual calibration curves for all three standards using concentrations from 40 to 400 μg for IAAld, 30 to 300 μg for IEt, and 0.5 to 5 μg for IAA.
Inoculation Experiments
T. virens and T. atroviride were evaluated in vitro for their plant growth-promoting ability using the Arabidopsis Col-0 ecotype. Fungal spore densities of 1 × 106 spores were inoculated by placing the spores at the opposite ends of agar plates containing 4-d-old germinated Arabidopsis seedlings (10 seedlings per plate). Plates were sealed with Parafilm and arranged in a completely randomized design. The seedlings were cultured for different time periods in a Percival AR95L growth chamber. Plants were sectioned at the root/shoot interface to quantify shoot weight. The fresh weight was measured on an analytical scale immediately after plant harvest, stem and root lengths were measured with a ruler, and lateral roots were counted and measured with a dissection microscope.
Determination of Developmental Stages of LRP
LRP were quantified at day 5 after fungal inoculation. Seedling roots were first cleared to enable LRP at early stages of development to be visualized and counted. Each LRP was classified according to its stage of development as reported by Malamy and Benfey (1997). The developmental stages are as follows. Stage I, LRP initiation. In the longitudinal plane, approximately 8 to 10 “short” pericycle cells are formed. Stage II, the formed LRP is divided into two layers by a periclinal division. Stage III, the outer layer of the primordium divides periclinally, generating a three-layer primordium. Stage IV, LRP with four cell layers. Stage V, the LRP is midway through the parent cortex. Stage VI, the LRP has passed through the parent cortex layer and has penetrated the epidermis. It begins to resemble the mature root tip. Stage VII, the LRP appears to be just about to emerge from the parent root.
Histochemical Analysis
For histochemical analysis of GUS activity, Arabidopsis seedlings were incubated overnight at 37°C in a GUS reaction buffer (0.5 mg mL−1 5-bromo-4-chloro-3-indolyl-β-d-glucuronide in 100 mm sodium phosphate, pH 7). The stained seedlings were cleared using the method of Malamy and Benfey (1997). For each marker line and for each treatment, at least 10 transgenic plants were analyzed. A representative plant was chosen and photographed using Nomarski optics on a Leica DMR microscope.
Aux/IAA Protein Degradation Assay
Six-day-old HS∷AXR3NT-GUS Arabidopsis transgenic seedlings were incubated on liquid 0.2× MS medium for 2 h at 37°C, followed by transfer of the seedlings into liquid 0.2× MS medium supplied with the different indolic compounds for 5, 10, 20, or 60 min at 22°C. The seedlings were washed with fresh 0.2× MS medium and, 12 to 14 h later, histochemically stained for GUS activity.
Data Analysis
Arabidopsis root systems were viewed with an AFX-II-A stereomicroscope (Nikon). All lateral roots emerging from the primary root and observed under the 3× objective were taken into account for lateral root number data. For all experiments, the overall data were statistically analyzed in the SPSS 10 program (SPSS). Univariate and multivariate analyses with Tukey's posthoc test were used for testing differences in growth and root developmental responses in wild-type and mutant plants. In the figures, different letters are used to indicate means that differ significantly (P < 0.05).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effects of IAAld on Arabidopsis lateral root development.
Supplemental Figure S2. Effects of IAAld on Arabidopsis biomass production.
Supplemental Figure S3. Effects of IAAld on primary root growth in wild-type (Col-0) plants and auxin-related Arabidopsis mutants.
Supplementary Material
Acknowledgments
We thank Athanasios Teologis, Tom Guilfoyle, Claire Grierson, Joanne Chory, Mark A. Estelle, and John W. Schiefelbein for kindly providing us with seeds of transgenic and mutant lines.
This work was supported by the Consejo Nacional de Ciencia y Tecnología (grant nos. 43978 and 60999) and the Consejo de la Investigación Científica (grant no. CIC 2.26).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: José López-Bucio (jbucio@zeus.umich.mx).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Adams P, De-Leij FA, Lynch JM (2007) Trichoderma harzianum Rifai 1295-22 mediates growth promotion of Crack willow (Salix fragilis) saplings in both clean and metal-contaminated soil. Microb Ecol 54 306–313 [DOI] [PubMed] [Google Scholar]
- Bais HP, Weir TL, Perry L, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57 233–266 [DOI] [PubMed] [Google Scholar]
- Bjorkman T, Blanchard LM, Harman GE (1998) Growth enhancement of shrunken-2 sweet corn when colonized with Trichoderma harzianum 1295-22: effect of environmental stress. J Am Soc Hortic Sci 123 35–40 [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, Beeckman T, DeWitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagú M, Inzé D (1995) superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7 1405–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HM, Purves WK (1980) Indoleacetaldehyde reductase of Cucumis sativus L. Plant Physiol 65 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, Bennett M (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL, Grisafi PL, Fink GR (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9 2131–2142 [DOI] [PubMed] [Google Scholar]
- Chang YC, Baker R, Klefield O, Chet I (1986) Increased growth of plants in the presence of the biological control agent Trichoderma harzianum. Plant Dis 70 145–148 [Google Scholar]
- Chung KR, Shilts T, Esturk U, Timmer LW, Ueng P (2003) Indole derivatives produced by the fungus Colletotrichum acutum causing lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol Lett 226 23–30 [DOI] [PubMed] [Google Scholar]
- Estelle M, Somerville C (1987) Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet 206 200–206 [Google Scholar]
- Ferro N, Bultnick P, Gallegos A, Jacobsen HJ, Carbo-Dorca R, Reinard T (2007) Unrevealed structural requirements for auxin-like molecules by theoretical and experimental evidences. Phytochemistry 68 237–250 [DOI] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putrill J, Palme K, Estelle M, Chory J (2001) BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 15 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414 271–276 [DOI] [PubMed] [Google Scholar]
- Harman GE (2006) Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96 190–194 [DOI] [PubMed] [Google Scholar]
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004. a) Trichoderma species: opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2 43–56 [DOI] [PubMed] [Google Scholar]
- Harman GE, Petzoldt R, Comis A, Chen J (2004. b) Interaction between Trichoderma harzianum strain T-22 and maize inbred line Mo17 and effects of these interactions on disease caused by Phytium ultimum and Colletotrichum graminicola. Phytopathology 94 147–153 [DOI] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vaneste S, de Almedida-Engler J, Inzé D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JJ, Stimart DP, Fisher RH, Bleecker AB (1995) A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell 7 2023–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga J (1995) Structure and function of indolepyruvate decarboxylase, a key enzyme in indole-3-acetic acid biosynthesis. Biochim Biophys Acta 1249 1–13 [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M (1993) Arabidopsis auxin resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature 364 161–164 [DOI] [PubMed] [Google Scholar]
- Li HM, Altschmied L, Chory J (1994) Arabidopsis mutants define downstream branches in the phototransduction pathway. Genes Dev 8 339–349 [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutant of Arabidopsis. Plant Cell 2 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Pérez-Torres A, Ramírez-Pimentel JG, Sánchez-Calderón L, Herrera-Estrella L (2005. a) Root architecture. In C Turnbull, ed, Plant Architecture and Its Manipulation. Blackwell Annual Review Series. Blackwell Scientific, Oxford, pp 181–206
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Pérez-Torres A, Rampey RA, Bartel B, Herrera-Estrella L (2005. b) An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis: identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol 137 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport is required for gravitropism in Arabidopsis thaliana. Genes Dev 12 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus V, Simaga S, Iskrig S, Kveder S (1982) Metabolism of tryptophan, indole-3-acetic acid, and related compounds in parasitic plants from the genus Orobanche. Plant Physiol 69 853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124 33–44 [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW (1994) The rhd6 mutation of Arabidopsis thaliana alters root hair initiation through an auxin and ethylene associated process. Plant Physiol 106 1335–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JDG (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312 436–439 [DOI] [PubMed] [Google Scholar]
- Oono Y, Chen QG, Overvoorde PJ, Kohler C, Theologis A (1998) age mutants of Arabidopsis exhibit altered auxin-regulated gene expression. Plant Cell 10 1649–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JS, Cavell A, Dolan L, Roberts K, Grierson C (2000) Genetic interactions during root hair morphogenesis in Arabidopsis. Plant Cell 12 1961–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picket FB, Wilson AK, Estelle M (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94 1462–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusty R, Grisafi P, Fink GR (2004) The plant hormone indoleacetic acid induces invasive growth in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 101 4153–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves WK, Brown HM (1978) Indoleacetaldehyde in cucumber seedlings. Plant Physiol 61 104–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle DL, Purves WK (1967) Conversion of indole-3-ethanol to indole-3-acetic acid in cucumber seedling shoots. Plant Physiol 42 1091–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday G (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Akaba S, Oritani T, Delarue M, Bellini C, Caboche M, Koshiba T (1998) Higher activity of an aldehyde oxidase in the auxin-overproducing superroot mutant of Arabidopsis thaliana. Plant Physiol 116 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson J, Yun J, Bevavente LM, Xie D, Dolezal K, Schlereth A, Jürgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133 177–191 [DOI] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev 15 2648–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle T (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedidia I, Shrivasta AK, Kapulnik Y, Chet I (2001) Effect of Trichoderma harzianum on microelement concentration and increased growth of cucumber plants. Plant Soil 235 235–242 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.