Abstract
Previously, we showed that in barley (Hordeum vulgare) leaves with heat-inactivated oxygen-evolving complexes, photosystem II (PSII) has access to a large pool of alternative electron donors. Based on in vitro data, we proposed that this donor was ascorbate, yet this hypothesis has not been substantiated in vivo. In this paper, with the aid of chlorophyll a fluorescence induced by short (5-ms) light pulses and 820-nm absorbance transient measurements on wild-type and ascorbate-deficient (vtc2-1) mutant leaves of Arabidopsis (Arabidopsis thaliana), we show that in heat-treated leaves the rate of electron donation to PSII as well as the 3-(3,4-dichlorophenyl)-1,1-dimethylurea-sensitive electron transport toward photosystem I depend on the ascorbate content of the leaves: upon ascorbate treatment, the donation half-time in the wild type and the mutant decreased from 25 to 22 ms and from 55 to 32 ms, respectively. Thermoluminescence measurements show that TyrZ+ is involved in the electron transfer from ascorbate to PSII. These data and the similar ascorbate dependencies of the heat-treated and the tris(hydroxymethyl)aminomethane-treated thylakoid membranes, with maximal donation half-times of about 16 ms, show that ascorbate is capable of supporting a sustained electron transport activity in leaves containing inactivated oxygen-evolving complexes. This alternative electron transport appears to be ubiquitous in the plant kingdom and is present in the green alga Chlamydomonas reinhardtii, and its rate depends on the physiological state of the plants and on environmental conditions. Our data suggest that ascorbate, as an alternative PSII electron donor, plays a physiological role in heat-stressed plants.
The oxygen-evolving complex (OEC) is one of the most vulnerable complexes of the photosynthetic electron transport chain. It is particularly sensitive to heat stress, and it is probably the first target of donor-side photoinhibition as well (Murata et al., 2007; Tyystjärvi, 2008). The inactivation of the OEC by heat stress includes the removal of the 18-kD and other extrinsic proteins as well as the release of Ca and Mn ions from their binding sites (Nash et al., 1985; Enami et al., 1994; Yamane et al., 1998; Barra et al., 2005). In the case of donor-side photoinhibition, primary damage by light occurs at the OEC and secondary damages occur at the reaction center of PSII (Hakala et al., 2005, Ohnishi et al., 2005). When OEC is inactivated, the supply of electrons from water to the oxidized primary donor, P680+, is interrupted and the residual electron transport activity of PSII is rapidly lost (Blubaugh et al., 1991). The accumulation of P680+ results in the loss of the capacity for primary charge separation and the degradation of the D1 protein of PSII (Klimov et al., 1990; Blubaugh et al., 1991; Jegerschöld and Styring, 1996).
Previously, we showed that when the oxygen evolution in barley (Hordeum vulgare) leaves was inhibited by a short heat pulse (50°C, 40 s in a water bath), PSII was supplied by electrons from a large pool of alternative donors (Tóth et al., 2007a). With the short heat pulse, all OECs were inactivated, while PSII reaction centers retained their activity, and other secondary effects, such as desiccation, adaptation, and partial recovery during the treatment, were also avoided (Tóth et al., 2005, 2007a). The half-time of electron donation (t1/2) to PSII was approximately 30 ms in intact barley leaves, and their presence resulted in a 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU)-sensitive electron flow from PSII to PSI. Based on in vitro data, obtained on thylakoids with inactivated OECs (Katoh and San Pietro, 1967; Yamashita and Butler, 1968; Mano et al., 2004), we suggested that this alternative electron donor of PSII was ascorbate (Asc; Tóth et al., 2007a).
Asc is important in scavenging reactive oxygen species (for review, see Asada, 2006), in redox signaling, and in growth control of plant cells involving cell division and the synthesis of the cell wall (De Tullio et al., 1999; Potters et al., 2002; Shao et al., 2008). It is ubiquitously present in chloroplasts and plays multiple roles. In C3 plants, the Asc content of chloroplasts is approximately 25 to 50 mm (Eskling and Åkerlund, 1998; Smirnoff, 2000). Asc limitation leads to a decrease in nonphotochemical quenching (Müller-Moulé et al., 2002), because Asc is required for the xanthophyll cycle as a cofactor for violaxanthin deepoxidase, which is localized in the thylakoid lumen (Hager and Holocher, 1994). The Asc content in the lumen is approximately 4 mm (Foyer and Lelandais, 1996).
In vitro studies have shown that Asc can replace water, the terminal donor of PSII, when the OEC is inactivated. When added to thylakoids isolated from heat-treated Euglena gracilis cells, Asc has been shown to support the DCMU-sensitive photoreduction of NADP+; whereas heat-treated thylakoids exhibited no or very low Hill activity, 14 mm Asc restored the electron transport to about 70% of its original activity (Katoh and San Pietro, 1967). It has also been shown to donate electrons to PSII in Tris-washed thylakoids (Yamashita and Butler, 1968) and to alleviate photoinhibition, the strong oxidizing capacity of PSII being utilized for the formation of monodehydroascorbate (Mano et al., 1997). Asc has also been shown to donate electrons to PSII in UV-B-irradiated thylakoids (Mano et al., 2004). These data show that Asc might act as an alternative, “emergency” donor to PSII. It has thus been proposed that the lumenal Asc plays a significant role in vivo not only by maintaining the violaxanthin deepoxidase activity but also by supporting the electron transport in reaction center complexes with inactive OECs (Mano et al., 2004). Therefore, we hypothesized that the large pool of alternative electron donors is identical to the pool of Asc molecules in the lumen (Tóth et al., 2007a).
In order to examine the hypothesis that Asc can serve as an alternative electron donor in vivo in whole leaves containing inactivated OECs, we monitored the activity of the two photosystems with fast chlorophyll (Chl) a fluorescence and 820-nm absorbance transient measurements in wild-type Arabidopsis (Arabidopsis thaliana) and its Asc-deficient mutant, vtc2-1 (Conklin et al., 2000), with and without externally supplied Asc. We also compared thylakoid membranes isolated from heat-treated leaves with Tris-washed samples and investigated the pathway of electron donation from Asc to PSII. Our data provide evidence for the existence of an Asc-dependent electron transport via TyrZ, a mechanism that appears to be present in plants and green algae and that might protect PSII reaction centers from photooxidation, especially under moderate heat stress.
RESULTS
Dependence of the Alternative Electron Transport through PSII on the Asc Content of Leaves
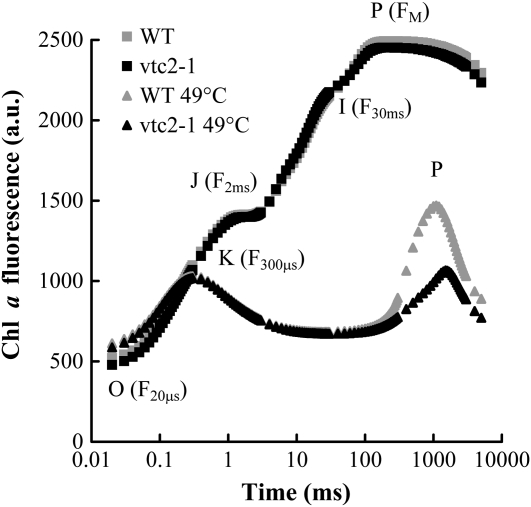
In order to clarify the putative role of Asc as an alternative electron donor, first we compared wild-type and Asc-deficient mutant Arabidopsis plants (vtc mutants; Conklin et al., 2000; Giacomelli et al., 2006; Dowdle et al., 2007; Laing et al., 2007; Linster et al., 2008). The Asc contents in the wild-type and the vtc2-1 mutant leaves were approximately 3.4 and 0.5 μmol g−1 fresh weight, respectively (i.e. the Asc content of the mutant was 15% of that of the wild type) under our growth conditions at moderate light intensity (approximately 200 μmol photons m−2 s−1). The Asc-deficient mutants were about 30% smaller than the wild type plants, as also reported by Müller-Moulé et al. (2004). A decrease in nonphotochemical quenching was observed in this mutant, but the electron transport rate and oxygen evolution were essentially the same (Müller-Moulé et al., 2002, 2004). In agreement with these findings, as shown in Figure 1, the fast Chl a fluorescence (OJIP) curves of wild-type Arabidopsis (ecotype Columbia [Col-0]) and the Asc-deficient mutant were similar and the Fv/Fm (maximum photochemical efficiency of PSII in the dark-adapted state) values of the untreated wild-type and mutant plants were essentially the same (about 0.82). The OJIP transient, induced by strong illumination (usually at 3,000 μmol photons m−2 s−1) and detected at a time resolution of 10 μs, is a very sensitive indicator of the photosynthetic electron transport processes (for review, see Govindjee, 2004; Lazár, 2006). The OJ phase (0–3 ms) is called the photochemical phase because its kinetics depends strongly on the light intensity (Neubauer and Schreiber, 1987; Strasser et al., 1995). The JI phase (approximately 3–30 ms) parallels the reduction of the PQ pool (Schreiber et al., 1989; Tóth et al., 2007b), and the IP phase (approximately 30–300 ms) is correlated with the reduction of ferredoxin in the presence of inactive ferredoxin:NADP+ oxidoreductase, as shown by dibromothymoquinone and methylviologen treatments on intact leaves (Schansker et al., 2005).
Figure 1.
Fast Chl a fluorescence transients (OJIP curves) of untreated and heat-treated (49°C, 40 s) wild-type (WT) and Asc-deficient vtc2-1 Arabidopsis mutant plants measured at 3,500 μmol m−2 s−1 photon flux density. The approximate positions of the different steps of the OJIP transient and of the K peak are indicated in parentheses. The curves are averages of eight to 10 measurements. The excitation light was produced by three 650-nm LEDs, and the Chl a fluorescence was measured at wavelengths above 700 nm. a.u., Arbitrary units.
If the oxygen evolution is completely inhibited, the J and I steps disappear and the K peak develops (Srivastava et al., 1997), as can be seen in Figure 1 in both the wild-type and mutant leaves treated with a heat pulse (49°C, 40 s). The kinetics of the K peak strongly depends on the measuring light intensity, and when the measurements are carried out at a standard, approximately 3,000 μmol quanta m−2 s−1 photon flux density, the maximum is located between 300 and 400 μs (Srivastava et al., 1997; Tóth et al., 2007a). It has been estimated that the excitation rate of PSII for pea (Pisum sativum) leaves is once every 50 μs at 12,000 μmol quanta m−2 s−1 photon flux density (Schreiber and Neubauer, 1990; Lazár, 1999). This suggests that the rise of the K peak represents approximately one charge separation, with TyrZ being the electron donor. Following the K peak, the fluorescence intensity decreases to a level approaching F0 in a few milliseconds, due to the reoxidation of QA− by the secondary quinone acceptor, QB. In leaves, another peak appears in the 0.2- to 2-s time range (Fig. 1), representing a second phase of QA reduction. In our previous study, we have shown that this latter phase depends on the presence of alternative electron donors (Tóth et al., 2007a). In heat-treated (49°C, 40 s) Asc-deficient leaves, this peak, emerging in the 0.2- to 2-s time range, was considerably smaller than in the wild type, and its rise was slower (Fig. 1). This indicates that the pool of alternative donors is smaller in the mutant than in the wild type.
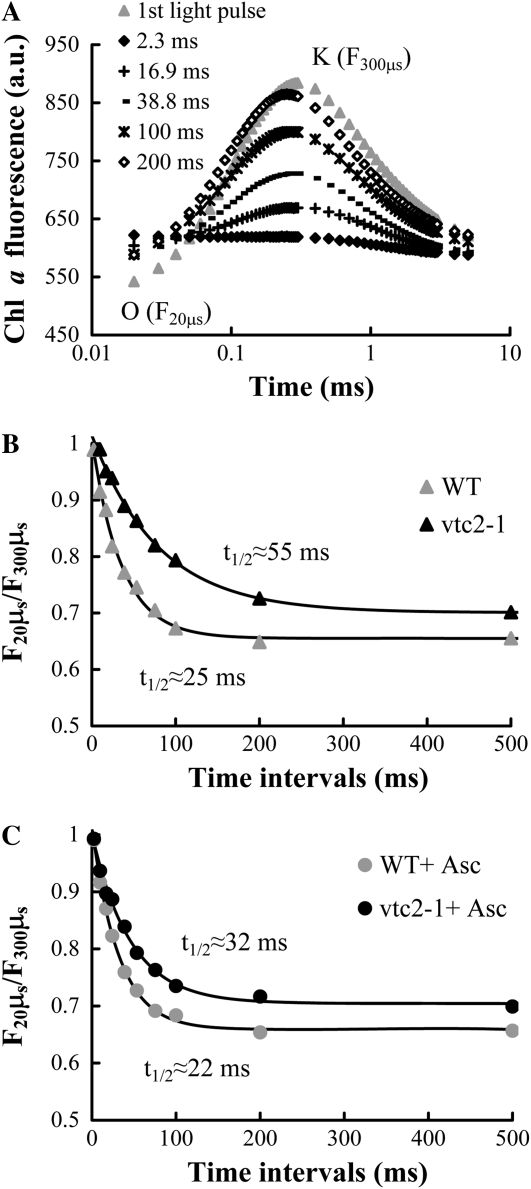
The t1/2 to PSII with inactive OEC can be determined, as described earlier (Tóth et al., 2007a), by using short (5-ms) light pulses and by varying the dark interval for the rereduction of TyrZ+. During the 5-ms light pulse, one charge separation and the reoxidation of QA− by QB can take place; therefore, recombination between QA− and TyrZ+ in the dark, with t1/2 approximately 120 ms (Dekker et al., 1984), is unlikely to occur. Figure 2A shows that after a 2.3-ms dark interval following the first light pulse, there is no variable fluorescence. This is due to the fact that all OECs have been inhibited by the heat pulse: the electron donation from active OEC occurs with t1/2 of about 0.1 to 1 ms (Babcock et al., 1976), and TyrZ+ could not be rereduced by the alternative donor in this short time. With longer dark intervals, the K peak recovers; at 16.9 ms, the peak can be clearly discerned, and at 200 ms after the first flash, its amplitude reaches its maximal value, approaching the amplitude of the dark control (Fig. 2A). The t1/2 of the regeneration of the K peak can be used as the half-time of the rereduction of TyrZ+ by the alternative electron donor (the electron transfer steps between TyrZ and QA and between QA and QB are much faster; Govindjee, 2004). The regeneration of the K peak, calculated as F20μs/F300μs, followed exponential kinetics, and the t1/2 was approximately 25 ms in wild-type Arabidopsis leaves; this value compares well with the 30-ms half-time obtained earlier in barley (Tóth et al., 2007a). In the Asc-deficient mutant, the electron donation was much slower, t1/2 ℵ 55 ms (Fig. 2B), which is in very good agreement with our hypothesis that Asc is the alternative electron donor of PSII.
Figure 2.
Dependence of the recovery of the K peak of the fast Chl a fluorescence transient in heat-treated wild-type and Asc-deficient Arabidopsis plants (vtc2-1 mutant) on the dark interval between the light pulses. A, Fast Chl a fluorescence transients measured on heat-treated (49°C, 40 s) wild-type (WT) leaves during 5-ms light pulses that were spaced 2.3 to 200 ms apart, as indicated. The fluorescence curves are averages of eight to 10 measurements. a.u., Arbitrary units. B and C, Regeneration of the K peak (calculated as F20μs/F300μs) as a function of dark intervals between the light pulses in heat-treated wild-type and vtc2-1 plants in the absence (B) and the presence (C) of externally supplied Asc (incubation of leaves in 20 mm Asc for 2 h in low light). The points are mean values from four to eight individual measurements that were fitted with exponentials for the determination of the half-recovery time. The error of the fitting was 5% to 6%.
In order to make certain that the observed differences in intact leaves are not caused by an inherent difference between the leaves other than the Asc content, we incubated intact leaves in Asc solution before the heat pulse. This treatment significantly accelerated the electron donation rates in the vtc2-1 mutant: the t1/2 decreased from 55 to 32 ms. In the case of wild-type plants, the t1/2 decreased to a much smaller extent, from 25 to 22 ms (Fig. 2C). These data show that the rate of electron donation depends substantially on the Asc content of the leaves.
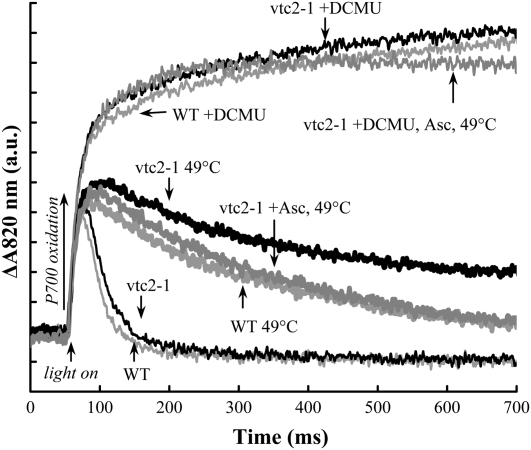
In order to confirm that also under these experimental conditions regeneration of the K peak originated from electron donation to PSII, rather than from recombination, we monitored the electron transport toward PSI. This was performed by measuring the light-induced absorbance changes at 820 nm. This transient is an indicator of the changes in the redox states of P700 and plastocyanin (PC), with PC being the minor component (Klughammer and Schreiber, 1991). In dark-adapted untreated leaves, the 820-nm absorbance increased for about 20 ms after the onset of the actinic illumination (Fig. 3), indicating oxidation of P700 and PC (Schansker et al., 2003). This was followed by rereduction of P700+ and PC+ in about 200 ms, with no significant differences between the untreated wild-type and vtc2-1 leaves.
Figure 3.
Light-induced 820-nm absorbance transients in wild-type (WT) and Asc-deficient Arabidopsis plants (vtc2-1 mutant). Heat treatment (49°C, 40 s), DCMU treatment (200 μm), and the addition of Asc (20 mm) were carried out as described in “Materials and Methods.” The kinetics were measured on dark-adapted samples during continuous illumination with blue light of 1,800 μmol m−2 s−1 photon flux density. The traces are averages of four to six measurements. a.u., Arbitrary units.
In the case of wild-type Arabidopsis leaves subjected to a heat pulse that fully inactivated the OECs, the 820-nm absorbance increase was followed by rereduction of P700+ and PC+ (Fig. 3). These data agree well with our earlier observations on heat-treated barley leaves (Tóth et al., 2007a). In heat-treated vtc2-1 leaves, the rereduction of P700+ and PC+ was very limited and slow. This phase could be accelerated by preincubating the mutant leaves in 20 mm Asc before the heat pulse. With externally supplied Asc, the rereduction rate was very close to the rates obtained in the wild type. These findings are in agreement with the data obtained on the regeneration of the K peak (Fig. 2). As reported earlier for barley, the rereduction phase of P700+ and PC+ was sensitive to DCMU both in the control and in the heat-treated leaves (Fig. 3). Incubation of the mutant leaves in Asc solution led to some rereduction of P700+ and/or PC+, which was manifested in a 5% to 10% decrease in the amplitude at 1 s compared with the samples without externally supplied Asc, but the DCMU sensitivity was retained. Hence, these data confirm that in leaves with inactive OECs, the alternative donors support electron transport through PSII in continuous light that depends on the Asc content of the leaves; at the same time, Asc acts only as a weak donor to PSI.
Asc-Dependent Electron Transfer in Thylakoids Isolated from Heat-Treated Leaves and in Tris-Washed Membranes
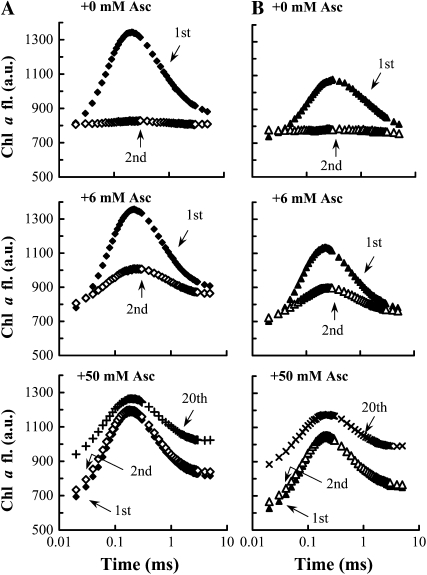
The dependence of the alternative electron donation to PSII on Asc can be further substantiated by fast Chl a fluorescence measurements on thylakoid membranes isolated from heat-treated leaves. These measurements, and data on Tris-treated thylakoid membranes, are also suitable for determining the Km for Asc that is associated with the electron donation to PSII. Polarographic measurements performed in the presence of phenyl-p-benzoquinone as electron acceptor showed that thylakoid membranes isolated from heat-treated barley leaves (48°C, 40 s) and Tris-washed thylakoids contained no active OECs (data not shown). As shown in Figure 4, in the absence of Asc, only the first light pulse was able to induce variable fluorescence, the K peak, which originated from a charge separation and the oxidation of TyrZ (see first section of “Results” above). The second light pulse spaced 200 ms after the first one induced no sizeable peak, as expected, since during thylakoid isolation the Asc in the lumen is strongly diluted or lost (Ivanov and Edwards, 2000; Müller-Moulé et al., 2002). Upon the addition of Asc, the regeneration of the K peak could be achieved in a manner that depended on its concentration. When 6 mm Asc was added, variable fluorescence could be detected on the second light pulse, whereas the addition of 50 mm Asc allowed almost complete regeneration of the K peak on the second and consecutive light pulses (Fig. 4), similar to intact barley leaves, in which half-depletion of the alternative electron donor pool occurred after several hundred light pulses and it regenerated in the dark (Tóth et al., 2007a). It can also be seen that the data obtained with thylakoids isolated from heat-treated leaves (Fig. 4A) were very similar to those obtained with Tris-washed samples (Fig. 4B). This shows that heat treatments and Tris washing lead to similar inactivation of the OEC and yield PSII complexes with similar inherent abilities to oxidize Asc upon illumination.
Figure 4.
Regeneration of the K peak of the fast Chl a fluorescence (fl.) transient after multiple 5-ms light pulses in thylakoid membranes isolated from heat-treated leaves (A) and in Tris-washed thylakoids (B) in the presence of 0, 6, and 50 mm Asc, as indicated. The dark interval between the consecutive light pulses was 200 ms. The Chl content was adjusted to 20 μg mL−1. The traces are averages of four to six measurements. a.u., Arbitrary units.
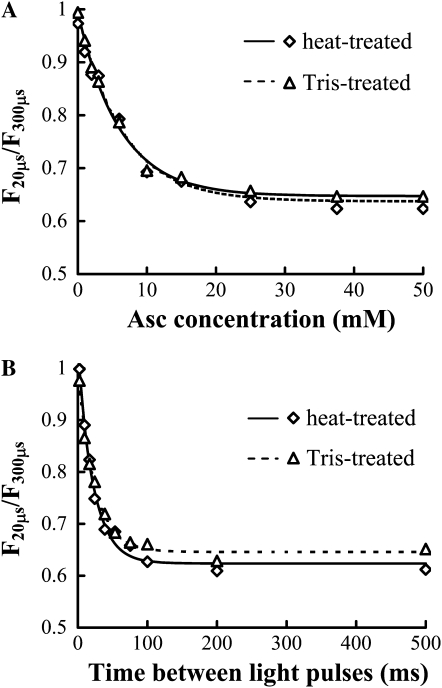
The regeneration of the K peak on a second light pulse with a 200-ms dark interval was plotted versus the Asc concentration of the sample (Fig. 5A). Half-saturation was reached between 4.2 and 4.5 mm Asc in both heat-treated and Tris-washed samples. This value, the apparent Km value for Asc in Tris-treated thylakoids and in thylakoids isolated from heat-treated leaves, compares well with 2.5 mm determined by Mano et al. (2004).
Figure 5.
Regeneration of the K peak (calculated as F20μs/F300μs) of Tris-washed thylakoids and thylakoid membranes isolated from heat-treated (48°C, 40 s) barley leaves as a function of Asc concentration (A) and the dark interval between the light pulses (B). In A, the dark interval between the light pulses was 200 ms. In B, the Asc concentration was 50 mm. The Chl content was adjusted to 20 μg mL−1. The data points are mean values from four to six measurements. The Km value (A) and the half-recovery time (B) were determined by fitting the data points with exponentials. The error of the fitting was 5% to 6%.
The regeneration of the K peak as a function of the dark interval between the light pulses was studied at saturating Asc concentration (50 mm; Fig. 5B). There was no significant difference between heat-treated and Tris-washed samples. It is also interesting that the maximal t1/2 (15.5 ms) in thylakoids in the presence of 50 mm Asc was comparable to the half-time measured with the same heat treatment on intact barley leaves (t1/2 = 23.6 ms; Table I).
Table I.
t1/2 from alternative donors to PSII in different species and in barley of different ages and physiological conditions
The oxygen evolution was completely inactivated by a heat pulse (for details, see “Materials and Methods”). The half-times were determined as in Figure 2, by two 5-ms light pulses with varying dark intervals. The data points are mean values from eight to 12 measurements for each time interval, and the error of the fitting is also indicated.
| Sample | t1/2 |
|---|---|
| ms | |
| Synechocystis PCC 6803 | 25.5 ± 2.5 |
| C. reinhardtii | 17.5 ± 0.6 |
| M. polymorpha | 37.5 ± 3.5 |
| N. exaltata | 20.4 ± 1.4 |
| J. chinensis | 21.3 ± 1.4 |
| P. sativum | 27.6 ± 1.5 |
| A.thaliana Col-0 | 23.9 ± 2.5 |
| A. elongatum ‘Szarvasi-1’ | 15.5 ± 0.9 |
| H. vulgare ‘Scarlett’ | |
| 1-week-old plants | 23.6 ± 0.7 |
| 1-month-old plants | 16.7 ± 1.0 |
| 0.5 h of dark adaptation (1-week-old plants) | 21.5 ± 0.7 |
| 24 h of dark adaptation (1-week-old plants) | 37.1 ± 1.8 |
| 250 mm NaCl (1-week-old plants) | 17.7 ± 0.6 |
| 500 mm NaCl (1-week-old plants) | 25.3 ± 1.5 |
| 1 m NaCl (1-week-old plants) | 31.6 ± 2.1 |
These results show that the regeneration of the K peak strictly depends on the Asc concentration. When there is no Asc added, the K peak does not regenerate, confirming that the electrons responsible for its regeneration do not arise from remaining OEC activity. Furthermore, the comparison of Tris-washed thylakoids and thylakoids isolated from heat-treated leaves also shows that the heat pulse does not have any effect on the regeneration of the K peak other than the inactivation of OECs.
Involvement of TyrZ in the Electron Donation from Asc to PSII
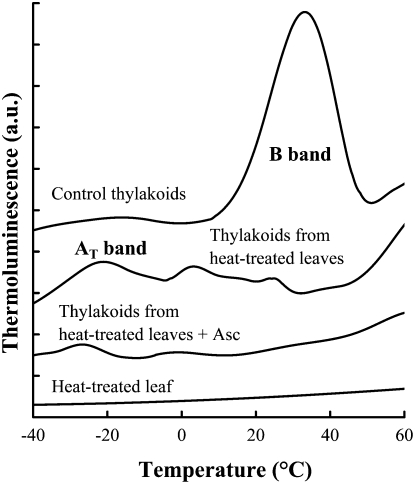
Information on the pathway of electron donation from Asc to PSII was obtained using thermoluminescence (TL) measurements. In thylakoid membranes in which the oxygen evolution has been inactivated, the B band, which is produced by recombination reactions between the S2/S3 states of the OEC and QB− (for review, see Vass, 2003), disappears with a concomitant appearance of the AT band around −20°C. The AT band is produced by recombinations between TyrZ+ and QA− (Ducruet and Vass, 2009).
Upon the addition of Asc to heat-treated thylakoid membranes, the AT band significantly decreased (Fig. 6), indicating that TyrZ+ was reduced by Asc. When the TL measurements were carried out on heat-treated leaves that naturally contain Asc, the AT band did not appear at all (Fig. 6). These data indicate that Asc provided electrons to PSII via TyrZ+. Earlier EPR data obtained with artificial electron donors are in agreement with the involvement of TyrZ in the reaction pathway between Asc and PSII (Yerkes and Babcock, 1980).
Figure 6.
TL glow curves of heat-treated (48°C, 40 s) leaves, control thylakoid membranes, and thylakoids isolated from heat-treated barley leaves in the absence and presence of 50 mm Asc. The curves are averages of three measurements. a.u., Arbitrary units.
Detection of Alternative Electron Transport in Different Organisms, under Different Physiological Conditions, and in Moderately Heat-Stressed Leaves
We investigated the occurrence of alternative electron donors in different photosynthetic organisms, including the cyanobacterium Synechocystis PCC 6803, the green alga Chlamydomonas reinhardtii, the moss Marchantia polymorpha, the fern Nephrolepis exaltata, the coniferous plant Juniperus chinensis, as well as in pea and Agropyron elongatum (‘Szarvasi-1’; a new variety of tall wheatgrass that is produced in Hungary for its biomass). Following a heat treatment, the alternative electron donors could be detected in all investigated species and the t1/2, determined by two 5-ms light pulses with varying dark intervals, was found in a relatively narrow range, between 15 and 37 ms (Table I). We also observed that the t1/2 varied with the age of the plants. In 1-week-old barley seedlings, the t1/2 was 23.6 ms, whereas in 1-month-old plants, it decreased to 16.7 ms. In plants exposed to moderate salt stress (250 mm NaCl treatment) before the heat treatment, the electron donation became faster than in the control (18 and 23 ms, respectively). In contrast, stronger salt stress (watering the plants with 1 m NaCl) decelerated the alternative electron donation (t1/2 = 31.6 ms; Table I). The length of the dark adaptation also influenced the rate: if the plants were dark adapted for 24 h, electron donation became much slower, which might be due to the depletion of Asc in the dark (Kiyota et al., 2006). These data show that the alternative electron donor(s) function(s) in evolutionarily distant species and that the t1/2 depends on the physiological state of the plant.
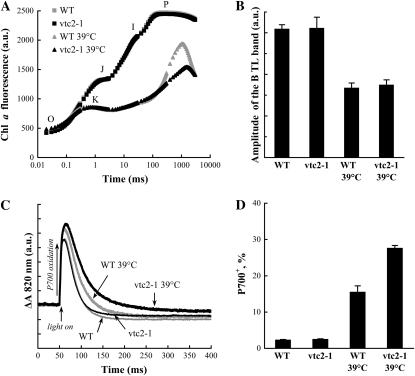
The functioning of the alternative electron donors was investigated in moderately stressed leaves as well. After a heat treatment of the leaves at 39°C for 15 min, approximately 50% of the OECs were inactivated in both the wild type and mutant plants, as shown by the equal, approximately 50% decrease in the amplitude of the B bands of the TL curves (Fig. 7B). As already shown in Figure 1, in another set of experiments, the fast Chl a fluorescence transients were almost identical in untreated wild-type and vtc2-1 leaves (Fig. 7A). Also, in heat-stressed leaves, the fluorescence transients were very similar up to about 100 ms, which confirms that the two genotypes were equally inhibited by the heat stress. However, there was a large difference between the fluorescence amplitudes at around 1 s, which shows that the extent of QA reduction was higher in the wild type than in the Asc-deficient mutant. Since the extent of inhibition in oxygen evolution was the same in the two genotypes, this difference must be attributed to the differences in the Asc content of the leaves. In perfect agreement with the above Chl a fluorescence induction data, the rereduction of P700+ was decelerated in the 39°C-treated mutant leaves, whereas, again, there was no difference between the untreated leaves (Fig. 7C).
Figure 7.
The effect of moderate heat stress (39°C, 15 min) on the electron transport of wild-type and vtc2-1 Arabidopsis leaves. A, Fast Chl a fluorescence transients (OJIP curves) of untreated and heat-treated leaves measured at 3,500 μmol m−2 s−1 photon flux density. The curves are averages of eight to 10 measurements. WT, Wild type; a.u., arbitrary units. B, Amplitudes of the B TL band of untreated and heat-treated wild-type and mutant plants; mean and se values from 10 to 12 measurements are shown. C, Light-induced 820-nm absorbance transients on control and heat-treated wild-type and vtc2-1 leaves. The kinetics were measured with a Dual-PAM-100 instrument on dark-adapted samples during continuous illumination with red light of 1,950 μmol m−2 s−1 photon flux density for 1 s. The traces are averages of six to seven transients. D, Amounts of P700+ in continuous red light of 95 μmol m−2 s−1 photon flux density, relative to the maximum oxidizable amounts, induced by saturating pulses of 10,000 μmol m−2 s−1 photon flux density, in untreated and heat-stressed wild-type and vtc2-1 leaves. Average traces and se values were calculated from six to seven 820-nm absorbance transient measurements.
In order to establish the role of Asc in the linear electron transport, we determined the amount of oxidized P700+ in leaves illuminated with red light (95 μmol photons m−2 s−1) in the two genotypes before and after moderate heat stress. This was performed following the protocol of Schreiber and Klughammer (2008), which provides information on the magnitude of the maximum photooxidizable P700 as well as on the relative concentration of P700+ in continuous light. As shown in Figure 7D, in untreated leaves, P700 was almost fully reduced. In contrast, after the heat treatment, P700+ accumulated both in the wild type and in the mutant, but significantly more P700+ was found in the Asc-deficient mutant than in the control, despite the identical inhibition of their OECs. These data show that Asc acts as an alternative PSII electron donor also in moderately heat-stressed plants.
DISCUSSION
In this study, we have provided experimental evidence that Asc is a naturally occurring alternative electron donor of PSII. We have shown that the t1/2 to PSII depends on the Asc content of the leaves: in wild-type Arabidopsis plants, it is approximately 25 ms, whereas in the Asc-deficient vtc2-1 mutant, it is much slower, about 55 ms, which, however, can be accelerated with externally supplied Asc to a level approaching the wild-type level (Fig. 2).
Our method for determining the t1/2 to PSII is based on the regeneration kinetics of the K peak of the fast fluorescence transient (Fig. 2). In heat-treated leaves, the rate of regeneration of this peak depends on the Asc content of the sample. The regeneration is slower in the Asc-deficient mutant than in the wild type, and it does not occur in isolated thylakoid membranes but can be restored by externally supplied Asc (Figs. 2 and 4). These data show that the electrons originate from an external pool, the Asc, and not from cofactors of PSII (e.g. cytochrome b559, TyrD, ChlZ, or β-carotene). The role of recombination and cyclic electron transport around PSII is also ruled out by the detection of the Asc-dependent electron transport to PSI, which is sensitive to DCMU (Fig. 3). TL measurements on leaves and thylakoids (Fig. 6) suggest that TyrZ is involved in the reaction pathway between Asc and PSII. (The possible involvement of residual Mn atoms, mediating between Asc and TyrZ, cannot be ruled out, although this is unlikely; see below.)
The Asc concentration has been estimated to be approximately 4 mm in the thylakoid lumen (Foyer and Lelandais, 1996) and 25 to 50 mm in chloroplasts (Eskling and Åkerlund, 1998; Smirnoff, 2000). In vitro, the Asc-dependent electron flux has been determined to be 40 and 50 μmol NADPH (mg Chl)−1 h−1 at 10 and 50 mm, respectively, approximately half of the electron transport rate detected in thylakoids with active OECs (Mano et al., 1997). Nevertheless, the relatively high Km values, of about 2.5 and 4.5 mm, determined by Mano et al. (2004) and in this work (Fig. 5A), respectively, suggest that the rates can be limited by the substrate concentration. This explains the observation that a substantial (85%) reduction of the Asc concentration in the mutant cells, and thus presumably also in the thylakoid lumen of vtc2-1, significantly decelerates the electron donation to PSII.
For a sustained electron transport from Asc to PSII, an efficient Asc regeneration system is required. It has been shown that upon donating electrons to PSII, Asc is oxidized to monodehydroascorbate radical in the lumen (Mano et al., 1997), as during the xanthophyll cycle, where Asc is required as a cofactor for violaxanthin deepoxidase (Hager and Holocher, 1994). The monodehydroascorbate radicals are disproportionated to Asc and dehydroascorbate, which diffuses to the stroma, where it is reduced to Asc by glutathione in a reaction catalyzed by dehydroascorbate reductase (Trümper et al., 1994). Asc is then transferred to the lumen by a postulated transporter or by diffusion (Foyer and Lelandais, 1996; Mano et al., 2004).
Based on in vitro studies, it has been suggested that Asc can donate electrons also to PSI, when electrons from PSII are blocked by DCMU, and support substantial electron flow at high Asc concentrations (50 mm; Mano et al., 1997). Ivanov et al. (2001) suggested that Asc participates in the cyclic electron transport around PSI in thylakoid membranes of bundle sheath and mesophyll cells of maize (Zea mays). In this and in our previous study (Tóth et al., 2007a), we found no evidence for significant electron donation rates to PSI by alternative electron donors. The oxidation kinetics of P700 and PC in the presence of DCMU are very similar in the wild type and in the Asc-deficient mutant, both showing complete oxidation of P700 and PC in continuous light, although their steady-state oxidation level is slightly decreased by externally supplied Asc (Fig. 3). Hence, we conclude that under our conditions, in heat-treated leaves, the affinity of Asc to PSII is much higher than to PSI, a conclusion reached earlier by Mano et al. (2004) for NH2OH-treated thylakoid membranes.
The alternative electron donation to PSII appears to be ubiquitous in the plant kingdom and is present in C. reinhardtii (Table I). It also appears to operate in Synechocystis PCC 6803, even though cyanobacteria contain no or only low amounts of Asc (Tel-Or et al., 1986). Therefore, the currently available data could not rule out the possibility that besides Asc, other thylakoid lumenal compounds also can donate electrons to PSII. Cyanobacterial cells have been shown to contain 2 to 5 mm glutathione (Tel-Or et al., 1985), which might serve as PSII donor. In vitro, hydrogen peroxide has been shown to donate electrons to PSII, but at high (millimolar) concentrations (Pan and Izawa, 1979; Mano et al., 1987), which, however, are unlikely to occur in the lumen. Alternative electron donation to PSII was also observed in the presence of 20 mm Cys (Katoh and San Pietro, 1967), but it is also very unlikely that the lumen would contain such a large amount of free Cys. It has been suggested by de Ronde et al. (2004) that Pro is an alternative electron donor of PSII; their idea was based on strong Pro accumulation and the faster initial rise of the OJIP transient in leaves exposed to drought stress. To test if this compound can act as an alternative electron donor of PSII, we added Pro to isolated thylakoids with inactivated OECs. However, we did not observe any variable fluorescence upon a second light pulse 200 ms after the first one, even at concentrations as high as 200 mm (data not shown).
It should be noted that the affinity of PSII to Asc might be very different in different species. It is equally possible that the applied heat treatments lead to different conformations on the donor side of PSII (i.e. release of two or more Mn atoms and of the extrinsic proteins). These factors, as discussed below, can influence the accessibility of Asc to PSII.
Our finding that the rate of alternative electron donation, with inactivated OECs, depends on environmental conditions and the age of plants (Table I) appears to indicate a dependence on the physiological state of plants. This is conceivable because the Asc content is known to vary, for example, with the growth light intensity (Grace and Logan, 1996; Eskling and Åkerlund, 1998). It must be noted, however, that differences in the heat sensitivity of the OEC might also cause variations in the donation rates, as indicated by the observation that the regeneration of the K peak in barley leaves depends on the temperature of the heat pulse. Between 48°C and 52°C, the t1/2 was 24 to 25 ms, whereas at 54°C, 56°C, and 58°C, it decreased to 19, 14, and 11 ms, respectively, suggesting a steric hindrance below 54°C. It is known that while at moderately high temperatures (around 50°C), two Mn atoms are released and the unbinding of the additional Mn atoms occurs only at higher temperatures or with longer treatments, which might also bring about more severe damage to the extrinsic proteins (Nash et al., 1985; Barra et al., 2005). From these data, one can infer that the remaining Mn atoms hinder rather than facilitate the electron donation from Asc to TyrZ.
Evidently, under physiological conditions, inactivation of all OECs, as induced by high-temperature heat pulses (48°C–50°C, 40 s), is unlikely to occur. It must be emphasized that our purpose with the use of these heat pulses was merely to provide clear evidence for the role of Asc as PSII electron donor. This can most clearly be shown when all OECs are fully inactivated while keeping the activity of the reaction centers (Tóth et al., 2007a). The elimination of all OECs was also instrumental in the determination of the t1/2 from Asc.
In the moderate temperature range (e.g. between 10°C and 25°C), the inactivation of the OECs is a stochastic event. As long as its extent is low, detection of the electron donation from Asc might not be possible. Similarly, the existence of the Asc-dependent electron transport is difficult to identify if, together with the OEC, or as a consecutive event, the photochemical reaction center is also damaged. Whereas in moderately UV-B-stressed isolated thylakoid membranes electron donation from Asc could clearly be observed, higher doses of UV-B light extensively inactivated the reaction centers and the photooxidation of Asc disappeared (Mano et al., 2004). In unreported experiments, we found that in UV-B-treated leaves, electron donation by Asc was obscured by the effects of UV-B light on the reaction centers.
There are environmental conditions in which oxygen evolution is substantially inhibited. It has been shown in potato (Solanum tuberosum) leaves that the disassembly of OECs occurs at relatively low temperatures, with a half-inactivation temperature being 39°C, whereas the inactivation of the reaction centers occurs at somewhat higher temperatures (Havaux, 1993). Our experiments on leaves exposed to moderate heat stress have shown that the Asc-dependent electron transport can clearly be identified also when only a fraction of OECs are inactivated (Fig. 7). These data clearly demonstrate the role of Asc, as alternative PSII electron donor, under physiologically relevant conditions.
The most likely physiological significance of this alternative electron donation, as suggested previously by Mano et al. (1997), is to protect the PSII reaction centers from photooxidation. The extent and effectiveness of this protection probably depend on the extent of damage, the available Asc concentration, its turnover rate, the light intensity, and possible other factors, the understanding of which is outside the scope of this paper. Nevertheless, we note that when heat-treated (49°C, 40 s) Arabidopsis plants are transferred to light, the decrease of primary charge separation, estimated from fast Chl a fluorescence transients, was more pronounced in the vtc2-1 mutants (data not shown). Furthermore, if leaves of Asc-deficient plants were incubated in 1 mm diphenylcarbazide (an artificial electron donor of PSII that has no scavenging effect for reactive oxygen species), the inactivation of PSII reaction centers was in large part alleviated in the vtc2-1 plants. These data suggest that alternative donors can indeed protect PSII reaction centers in vivo.
In conclusion, our data provide experimental evidence that Asc donates electrons to PSII reaction centers that possess no active OEC and maintains a DCMU-sensitive linear electron transport in leaves. This mechanism, which appears to be ubiquitous in organisms with oxygenic photosynthesis, might represent a defense mechanism, which appears to be particularly important in plants exposed to heat stress.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) Col-0 wild-type plants and Asc-deficient vtc2 mutants were grown in a greenhouse under short-day conditions, from September to January, at approximately 200 μmol m−2 s−1 photon flux density. The night temperature was 18°C to 20°C, and the day temperature was 22°C to 25°C.
Barley (Hordeum vulgare ‘Scarlett’), Juniperus chinensis, pea (Pisum sativum ‘Rajnai törpe’), and Agropyron elongatum ‘Szarvasi-1’ plants were grown in a greenhouse in the summer season. The temperature was 20°C to 27°C during the day and 20°C to 23°C at night, and no supplemental light was provided. Barley seedlings were used when they were 7 d old except in a set of experiments in which 1-month-old plants were used. Weak, moderate, and relatively strong salt stresses (the Fv/Fm values were unchanged, 0.81, for all treatments) on barley plants were applied by watering 4-d-old barley seedlings once with 250 mm, 500 mm, or 1 m NaCl, respectively, and the plants were measured when they were 8 d old. Marchantia polymorpha and Nephrolepis exaltata were grown under similar conditions but at moderate light intensity.
Cells of Synechocystis PCC 6803 and Chlamydomonas reinhardtii were grown photoautotrophically in BG11 (pH 7.5) and TAP (pH 7.0) media, respectively. Cells were grown at 30°C under continuous illumination at 30 μmol m−2 s−1 photon flux density. Cultures were aerated on a gyratory shaker operating at 100 rpm.
Heat Treatments
Complete inactivation of oxygen evolution was achieved by a heat pulse: immersing whole leaves in a water bath for a very short time (40 s) at elevated temperature (49°C for Arabidopsis). This treatment induces no visible symptoms or secondary effects (Tóth et al., 2005). The heat-treated plants or leaves were kept in the dark, and the measurements were carried out at room temperature at 30 to 60 min after the heat treatment.
M. polymorpha, N. exaltata, J. chinensis, pea, A. elongatum, and barley were treated at 48°C, 52°C, 54°C, 48°C, 50°C, and 48°C, respectively, where complete inactivation of oxygen evolution occurred. In a set of experiments, 48°C to 58°C treatments were applied to 7-d-old barley seedlings and 1-month-old barley plants were treated at 50°C. In all cases, oxygen evolution became zero as measured on thylakoids isolated from heat-treated leaves using phenyl-p-benzoquinone as an electron acceptor (Tóth et al., 2005). The B band arising from recombination reactions between QB− and the S2 or S3 state of the OEC was also completely eliminated.
Moderate heat stress was induced by treating detached Arabidopsis leaves at 39°C for 15 min in a water bath in the dark, and then leaves were cooled down in room-temperature water (Havaux, 1993).
Asc Content Determination and Asc Feeding to Leaves
Asc and dehydroascorbate contents of wild-type Arabidopsis and vtc2 mutants were determined by a spectroscopic method using the absorption at 265 nm of Asc (Takahama and Oniki, 1992). Three lines of the vtc2 mutant were available (vtc2-1, vtc2-2, and vtc2-3), of which the vtc2-1 mutant had the lowest and the least variable Asc content (data not shown) and was used for the experiments.
Feeding of Asc was performed by incubating detached Arabidopsis leaves in 20 mm Na-Asc. Leaves were placed in petri dishes and covered with one layer of filter paper for 2 h at a photon flux density of approximately 15 μmol m−2 s−1. This was followed by a heat pulse of 49°C, and the measurements were carried out after 30 min of dark adaptation. Asc+DCMU treatments were carried out in a similar way; the DCMU concentration was 200 μm, and the solution contained 1% ethanol to dissolve DCMU.
Thylakoid Isolation and Tris Washing
Thylakoids for fluorescence measurements were isolated according to the method of Robinson and Yocum (1980), except that the pH of the buffers was 7.5. Samples were isolated from 7-d-old barley seedlings that were heat treated at 48°C for 40 s after 30 min of incubation in the dark. Tris washing was carried out on thylakoids isolated from untreated leaves by 0.8 m Tris-HCl (pH 8.0 at 4°C) for 30 min under dim light (Yamashita and Butler, 1969).
For TL and oxygen evolution measurements, heat-treated and untreated leaves were homogenized in 40 mm HEPES (pH 7.5) containing 0.4 m Suc, 1% (w/v) bovine serum albumin, 5 mm MgCl2, 15 mm NaCl, and 2 mm Na2-EDTA. The homogenate was filtered and centrifuged at 3,000g for 5 min, and then the pellet was resuspended in the same buffer and centrifuged at 3,000g for 5 min. Then the membrane pellet was resuspended in 40 mm HEPES buffer (pH 7.5) containing 0.4 m Suc, 5 mm MgCl2, and 15 mm NaCl. The Chl content was adjusted (Porra et al., 1989) to 200 μg mL−1. Thylakoids were kept on ice in darkness and were used within 3 h after isolation.
Fast Chl a Fluorescence (OJIP) Measurements
Leaves were kept in darkness after the heat treatments and measured within 30 to 60 min. Fluorescence measurements were carried out at room temperature with a special version of the Handy-PEA instrument (Hansatech Instruments) that allows reducing the length of the measurement to 300 μs. Leaf samples were illuminated with continuous red light (3,500 μmol photons m−2 s−1, 650-nm peak wavelength; the spectral half-width was 22 nm; the light emitted by the LEDs is cut off at 700 nm by a near-infrared short-pass filter). The light was provided by an array of three LEDs focused on a circle of 5 mm diameter of the sample surface. The first reliably measured point of the fluorescence transient is at 20 μs, which was taken as F0. The length of the measurements was 5 s or 5 ms. In the case of the double 5-ms pulses, the dark intervals between the light pulses were 2.3, 9.6, 16.9, 31.5, 38.8, 53.4, 75.3, 100, 200, or 500 ms. In the case of fluorescence measurements on isolated thylakoid membranes, 20 μL of suspension (20 μg Chl mL−1) was placed on a Whatman glass microfiber filter (GF/C) inserted in a leaf clip.
Measurement of the Oxidation-Reduction Kinetics of P700
The light-induced redox changes of P700 were monitored by measuring absorbance changes at 820 nm using a PAM 101 Chl fluorometer (Heinz Walz) equipped with an ED 800 T emitter-detector system. Continuous blue actinic light (approximately 1,800 μmol photons m−2 s−1 for 2 s) was provided by a halogen lamp (KL 1500; Schott) connected to an electronic shutter. Absorbance changes at 820 nm were recorded in continuous light on a millisecond time scale. In a set of experiments, a Dual-PAM-100 instrument was used to measure the 820-nm absorbance changes and the steady-state oxidation level of P700 during continuous illumination, relative to the maximum photooxidizable amount (Klughammer and Schreiber, 1994; Schreiber and Klughammer, 2008).
TL Measurements
TL was measured using a custom-made TL apparatus described by Wiessner and Demeter (1988). For TL measurements, thylakoid suspension (0.5 mL, 200 μg Chl [a+b] mL−1) was placed on the sample holder. Dark-adapted samples were illuminated at −20°C by a saturating single-turnover flash and cooled to −40°C. In the case of TL measurements on leaves, thoroughly dark-adapted samples were cooled to 0°C and illuminated by a single-turnover flash. Glow curves were recorded while heating the sample to 70°C in darkness with a constant heating rate of 20°C min−1.
Acknowledgments
We thank Prof. Patricia Conklin (State University of New York College at Cortland) for providing us with the vtc2 mutants, Prof. Jun'ichi Mano (Yamaguchi University) and Dr. Gert Schansker (University of Geneva) for helpful discussions, and Dr. Éva Hideg (Biological Research Center Szeged) for advice on the method of determination of the Asc content of leaves. We also thank Dr. László Kovács (Biological Research Center Szeged) for his help with the oxygen evolution and P700 measurements, Ms. Mary Prathiba Joseph (Biological Research Center Szeged) for help with growing Arabidopsis plants, and Dr. Rudolf Tóth-Boconádi (Biological Research Center Szeged) for his help in the UV-B experiments.
This work was supported by the Hungarian Research Foundation (grant nos. PD72718 and K63252 to S.Z.T. and G.G., respectively), by the Bolyai János Research Foundation of the Hungarian Academy of Sciences (research scholarship to S.Z.T.), and by the Department of Science and Technology, Government of India (BOYSCAST fellowship to J.T.P.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Szilvia Z. Tóth (sztoth@brc.hu).
Open Access articles can be viewed online without a subscription.
References
- Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock GT, Blankenship RE, Sauer K (1976) Reaction kinetics for positive charge accumulation on the water side of chloroplast photosystem II. FEBS Lett 61 286–289 [DOI] [PubMed] [Google Scholar]
- Barra M, Haumann M, Dau H (2005) Specific loss of the extrinsic 18 kDa protein from photosystem II upon heating to 47°C causes inactivation of oxygen evolution likely due to Ca release from the Mn-complex. Photosynth Res 84 231–237 [DOI] [PubMed] [Google Scholar]
- Blubaugh DJ, Atamian M, Babcock GT, Golbeck JH, Cheniae GM (1991) Photoinhibition of hydroxylamine-extracted photosystem II membranes: identification of the sites of photodamage. Biochemistry 30 7586–7597 [DOI] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL (2000) Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker JP, van Gorkom HJ, Brok M, Ouwehand L (1984) Optical characterization of photosystem II electron donors. Biochim Biophys Acta 764 301–309 [Google Scholar]
- de Ronde JA, Cress WA, Krüger GHJ, Strasser RJ, van Staden J (2004) Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J Plant Physiol 161 1211–1224 [DOI] [PubMed] [Google Scholar]
- De Tullio MC, Paciolla C, Vecchia FD, Rascio N, D'Emerico S, De Gara L, Liso R, Arrigoni O (1999) Changes in onion root development induced by the inhibition of peptidyl-prolyl hydroxylase and influence of the ascorbate system on cell division and elongation. Planta 209 424–434 [DOI] [PubMed] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N (2007) Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52 673–689 [DOI] [PubMed] [Google Scholar]
- Ducruet JM, Vass I (2009) Thermoluminescence: experimental. Photosynth Res (in press) [DOI] [PubMed]
- Enami I, Kitamura M, Tomo T, Isokawa Y, Ohta H, Katoh S (1994) Is the primary cause of thermal inactivation of oxygen evolution in spinach PSII membranes release of the extrinsic 33 kDa protein or of Mn? Biochim Biophys Acta 1186 52–58 [Google Scholar]
- Eskling M, Åkerlund HE (1998) Changes in the quantities of violaxanthin de-epoxidase, xanthophylls and ascorbate in spinach upon shift from low to high light. Photosynth Res 57 41–50 [Google Scholar]
- Foyer CH, Lelandais M (1996) A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cells. J Plant Physiol 148 391–398 [Google Scholar]
- Giacomelli L, Rudella A, van Wijk KJ (2006) High light response of the thylakoid proteome in Arabidopsis wild type and the ascorbate-deficient mutant vtc2-2: a comparative proteomics study. Plant Physiol 141 685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee (2004) Chlorophyll a fluorescence: a bit of basics and history. In GC Papageorgiou, Govindjee, eds, Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration, Vol 19. Springer, Dordrecht, The Netherlands, pp 1–42
- Grace SC, Logan BA (1996) Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol 112 1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A, Holocher K (1994) Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta 192 581–589 [Google Scholar]
- Hakala M, Tuominen I, Keränen M, Tyystjärvi T, Tyystjärvi E (2005) Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of photosystem II. Biochim Biophys Acta 1706 68–80 [DOI] [PubMed] [Google Scholar]
- Havaux M (1993) Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci 94 19–33 [Google Scholar]
- Ivanov B, Edwards G (2000) Influence of ascorbate and the Mehler peroxidase reaction on non-photochemical quenching of chlorophyll fluorescence in maize chloroplasts. Planta 210 765–774 [DOI] [PubMed] [Google Scholar]
- Ivanov BN, Sacksteder CA, Kramer DM, Edwards GE (2001) Light-induced ascorbate-dependent electron transport and membrane energization in chloroplasts of bundle sheath cells of the C4 plant maize. Arch Biochem Biophys 385 145–153 [DOI] [PubMed] [Google Scholar]
- Jegerschöld C, Styring S (1996) Spectroscopic characterization of intermediate steps involved in donor-side-induced photoinhibition of photosystem II. Biochemistry 35 7794–7801 [DOI] [PubMed] [Google Scholar]
- Katoh S, San Pietro A (1967) Ascorbate-supported NADP photoreduction by heated Euglena chloroplasts. Arch Biochem Biophys 122 144–152 [DOI] [PubMed] [Google Scholar]
- Kiyota M, Numayama N, Goto K (2006) Circadian rhythms of the L-ascorbic acid level in Euglena and spinach. J Photochem Photobiol B 84 197–203 [DOI] [PubMed] [Google Scholar]
- Klimov VV, Shafiev MA, Allakhverdiev SI (1990) Photoinactivation of the reactivation capacity of photosystem II in pea subchloroplast particles after a complete removal of manganese. Photosynth Res 23 59–65 [DOI] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U (1991) Analysis of light-induced absorbance changes in the near-infrared spectral region. I. Characterization of various components in isolated chloroplasts. Z Naturforsch 46c 233–244 [Google Scholar]
- Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192 261–268 [Google Scholar]
- Laing WA, Wright MA, Cooney J, Bulley SM (2007) The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc Natl Acad Sci USA 104 9534–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazár D (1999) Chlorophyll a fluorescence induction. Biochim Biophys Acta 1412 1–28 [DOI] [PubMed] [Google Scholar]
- Lazár D (2006) The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct Plant Biol 33 9–30 [DOI] [PubMed] [Google Scholar]
- Linster CL, Adler LN, Webb K, Christensen KC, Brenner C, Clarke SG (2008) A second GDP-L-galactose phosphorylase in Arabidopsis en route to vitamin C: covalent intermediate and substrate requirements for the conserved reaction. J Biol Chem 27 18483–18492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano J, Hideg É, Asada K (2004) Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem II and photosystem I. Arch Biochem Biophys 429 71–80 [DOI] [PubMed] [Google Scholar]
- Mano J, Takahashi M, Asada K (1987) Oxygen evolution from hydrogen peroxide in photosystem II: flash-induced catalytic activity of water-oxidizing photosystem II membranes. Biochemistry 26 2495–2501 [Google Scholar]
- Mano J, Ushimaru T, Asada K (1997) Ascorbate in thylakoid lumen as an endogenous electron donor to photosystem II: protection of thylakoids from photoinhibition and regeneration of ascorbate in stroma by dehydroascorbate reductase. Photosynth Res 53 197–204 [Google Scholar]
- Müller-Moulé P, Conklin PL, Niyogi KK (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128 970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé P, Golan T, Niyogi KK (2004) Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol 134 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767 414–421 [DOI] [PubMed] [Google Scholar]
- Nash D, Miyao M, Murata N (1985) Heat inactivation of oxygen evolution in photosystem II particles and its acceleration by chloride depletion and exogenous manganese. Biochim Biophys Acta 807 127–133 [Google Scholar]
- Neubauer C, Schreiber U (1987) The polyphasic rise of chlorophyll fluorescence upon onset of strong continuous illumination. I. Saturation characteristics and partial control by the photosystem II acceptor side. Z Naturforsch 42c 1246–1254 [Google Scholar]
- Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 44 8494–8499 [DOI] [PubMed] [Google Scholar]
- Pan RL, Izawa S (1979) Photosystem II energy coupling in chloroplasts with H2O2 as electron donor. Biochim Biophys Acta 547 311–319 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedeman PE (1989) Determination of accurate extinction coefficients and simultaneous equations for essaying chlorophylls-a and -b with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975 384–394 [Google Scholar]
- Potters G, De Gara L, Asard H, Horemans N (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40 537–548 [Google Scholar]
- Robinson HH, Yocum CF (1980) Cyclic photophosphorylation reactions catalyzed by ferredoxin, methyl viologen and anthraquinone sulfonate: use of photochemical reactions to optimize redox poising. Biochim Biophys Acta 590 97–106 [DOI] [PubMed] [Google Scholar]
- Schansker G, Srivastava A, Govindjee, Strasser RJ (2003) Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct Plant Biol 30 785–796 [DOI] [PubMed] [Google Scholar]
- Schansker G, Tóth SZ, Strasser RJ (2005) Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim Biophys Acta 1706 250–261 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Klughammer C (2008) Non-photochemical fluorescence quenching and quantum yields in PS I and PS II: analysis of heat-induced limitations using Maxi-Imaging-PAM and Dual-PAM-100. PAM Application Notes 1 15–18 [Google Scholar]
- Schreiber U, Neubauer C (1990) O2-dependent electron flow, membrane energization and the mechanism of non-photochemical quenching of chlorophyll fluorescence. Photosynth Res 25 279–293 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Neubauer C, Klughammer C (1989) Devices and methods for room-temperature fluorescence analysis. Philos Trans R Soc Lond B 323 241–251 [Google Scholar]
- Shao HB, Chu LY, Lu ZH, Kang CM (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4 8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci 355 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Guissé B, Greppin H, Strasser RJ (1997) Regulation of antenna structure and electron transport in photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim Biophys Acta 1320 95–106 [Google Scholar]
- Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61 32–42 [Google Scholar]
- Takahama U, Oniki T (1992) Regulation of peroxidase-dependent oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol 33 379–387 [Google Scholar]
- Tel-Or E, Huflejt M, Packer L (1985) The role of glutathione and ascorbate in hydroperoxide removal in cyanobacteria. Biochem Biophys Res Commun 132 533–539 [DOI] [PubMed] [Google Scholar]
- Tel-Or E, Huflejt ME, Packer L (1986) Hydroperoxide metabolism in cyanobacteria. Arch Biochem Biophys 246 396–402 [DOI] [PubMed] [Google Scholar]
- Tóth SZ, Schansker G, Garab G, Strasser RJ (2007. a) Photosynthetic electron transport activity in heat-treated barley leaves: the role of internal alternative electron donors to photosystem II. Biochim Biophys Acta 1767 295–305 [DOI] [PubMed] [Google Scholar]
- Tóth SZ, Schansker G, Kissimon J, Kovács L, Garab G, Strasser RJ (2005) Biophysical studies of photosystem II-related recovery processes after a heat pulse in barley seedlings (Hordeum vulgare L.). J Plant Physiol 162 181–194 [DOI] [PubMed] [Google Scholar]
- Tóth SZ, Schansker G, Strasser RJ (2007. b) A non-invasive assay of the plastoquinone pool redox state based on the OJIP-transient. Photosynth Res 93 193–203 [DOI] [PubMed] [Google Scholar]
- Trümper S, Follmann H, Häberlein I (1994) A novel dehydroascorbate reductase from spinach chloroplasts homologous to plant trypsin inhibitor. FEBS Lett 352 159–162 [DOI] [PubMed] [Google Scholar]
- Tyystjärvi E (2008) Photoinhibition of photosystem II and photodamage of the oxygen evolving manganese cluster. Coord Chem Rev 252 361–376 [Google Scholar]
- Vass I (2003) The history of photosynthetic thermoluminescence. Photosynth Res 76 303–318 [DOI] [PubMed] [Google Scholar]
- Wiessner W, Demeter S (1988) Comparative thermoluminescence study of autotrophically and photoheterotrophically cultivated Chlamydobotrys stellata. Photosynth Res 18 345–356 [DOI] [PubMed] [Google Scholar]
- Yamane Y, Kashino Y, Koike H, Satoh K (1998) Effects of high temperatures on the photosynthetic systems in spinach: oxygen-evolving activities, fluorescence characteristics and the denaturation process. Photosynth Res 57 51–59 [Google Scholar]
- Yamashita T, Butler WL (1968) Photoreduction and photophosphorylation with Tris-washed chloroplasts. Plant Physiol 43 1978–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Butler WL (1969) Photooxidation by photosystem II of Tris-washed chloroplasts. Plant Physiol 44 1342–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes CT, Babcock GT (1980) Photosystem II oxidation of charged electron donors: surface charge effects. Biochim Biophys Acta 590 360–372 [DOI] [PubMed] [Google Scholar]