Abstract
Treating wounds in Nicotiana attenuata leaves with Manduca sexta oral secretions (W+OS) mimics most changes elicited by M. sexta herbivory, but an unbiased analysis of the effect of the different OS constituents on volatile emissions is lacking. We used two-dimensional gas chromatography/time-of-flight (GCxGC-ToF) mass spectrometry combined with multivariate statistics to parse volatiles into regulatory patterns. Volatiles released by wounding alone and by the alkalinity of OS were assessed by applying a buffer known to mimic the pH-mediated changes of OS elicitation (pectin methyl esterase activation and methanol release). The activities of fatty acid amino acid conjugates, well-known elicitors of antiherbivore defenses, and of 2-hydroxyoctadecatrienoic acid, a newly discovered signal in OS, were determined. Approximately 400 analytes were detected after deconvolution and alignment of GCxGC data; 35 volatiles were significantly regulated upon W+OS. Two-thirds of these were specifically regulated by OS, being either amplified (most terpenoids and certain hexenylesters) or strongly repressed (many short-chain alcohols and some aromatic and hexenylester derivatives). Fatty acid amino acid conjugates played a central role in this pattern of regulation, since they induced the emission of half of OS-elicited volatiles and inhibited the production of almost all OS-repressed volatiles; 2-hydroxyoctadecatrienoic acid influenced emission of trans-α-bergamotene, while other unknown OS constituents amplified hexenylester production. We conclude that the complex bouquet of herbivory-elicited volatiles results from the complex modulations of the wound response by diverse cues found in OS. This work also underscores the value of ultra-high-resolution GCxGC-ToF analysis combined with the nontargeted mining of the resulting data.
Plants employ a complex arsenal of defense mechanisms to protect themselves from insect herbivores. In addition to constitutive defenses such as trichomes or thick secondary walls, plants also use induced defenses, which are deployed specifically when herbivores start to chew on a leaf (Karban and Baldwin, 1997). These conditional responses allow plants to forgo the costs of defense production (Zavala et al., 2004) and optimize their ability to compete with neighboring plants or to tolerate the damage of resistant herbivores (Stowe et al., 2000; Schwachtje et al., 2006). Induced defenses function either directly via the production of amino acid-catabolizing enzymes, antidigestive proteinase inhibitors, or toxic and repelling chemicals that render plant tissues less suitable as food for herbivores (Duffey and Stout, 1996) or indirectly through traits that increase the apparency of infested plants to the natural enemies of their herbivores (Karban and Baldwin, 1997; Pare and Tumlinson, 1999).
An intensively studied example of an inducible indirect defense is the production and emission of volatile organic compounds (VOCs) by plants attacked by insects (for review, see Turlings and Wäckers, 2004). The first complete evidence that plants use odors to attract natural enemies of their predators was obtained from studies on plant-mite interactions (Sabelis and van de Baan, 1983; Dicke et al., 1988; Dicke et al., 1990a, 1990b). These first studies and many since, performed under optimized laboratory conditions (Dicke, 1994; Mattiacci et al., 1995 Alborn et al., 1997; Turlings et al., 1990, 1991a, 1991b, 1998) or under field conditions (Shimoda et al., 1997; De Moraes et al., 1998; Bernasconi Ockroy et al., 2001; Kessler and Baldwin, 2001), have demonstrated that parasitoids and predators make effective use of herbivory-induced plant VOCs (HIPV) to locate their prey. Certain of these volatiles have notably been shown to decrease oviposition rates and increase egg predation on the emitting plant (Feeny et al., 1989; Kessler and Baldwin, 2001; Colazza et al., 2004; Johne et al., 2006). Moreover, some of these plant odors can also serve as direct repellents against insect herbivores (Quiroz et al., 1997; Bernasconi et al., 1998; Landolt et al., 1999). In addition, the volatiles can be perceived by neighboring plants and increase or potentiate their defensive abilities (Arimura et al., 2000a, 2000b; Baldwin et al., 2006; Kessler et al., 2006; Kost and Heil, 2006). On the other hand, volatile signaling is also used by some plant species to overcome the vascular constraints on systemic signaling to elicit defense responses in adjacent leaves with little or no vascular connection with the attacked leaves (Frost et al., 2007).
Despite their common feature of being volatile at ambient temperatures, these major players of plant signaling are chemically highly diverse (Holopainen, 2004). HIPV bursts have been partially characterized in several plant species (for overview, see van Poecke and Dicke, 2004; Van Dam and Poppy, 2008), including, for instance, maize (Zea mays), cotton (Gossypium hirsutum), lima bean (Phaseolus lunatus), and native tobacco (Nicotiana attenuata). The major constituents of these VOC bouquets consist of products of the shikimic acid pathway, terpenes and fatty acid (FA) derivatives. The best characterized metabolites from this latter subpopulation are the green leaf volatiles (GLVs), originating from the degradation of C18 FAs (linolenic and linoleic acids) when hydroperoxidated by a lipoxygenase and cleaved into C12 and C6 units by a hydroperoxide lyase (Halitschke et al., 2004; Matsui, 2006).
Fatty acid amino acid conjugates (FACs), produced in the insect gut by conjugation of host-derived FAs to amino acids (Spiteller et al., 2000) and contained in the oral secretions (OS) of many caterpillar species, were the first nonenzymatic elicitors of HIPV characterized (Alborn et al., 1997; Halitschke et al., 2001). More generally, recent “omic” approaches have demonstrated that FACs are responsible for eliciting a large portion of the hundreds of genes regulated during the plant-herbivore interaction (Halitschke et al., 2003; Roda et al., 2004) as well as the reconfiguration of the proteome (Giri et al., 2006). However, such untargeted analyses have not been conducted for HIPV, and until now the regulation of only a few components of plants' volatile blends (e.g. trans-α-bergamotene in N. attenuata [Halitschke et al., 2001] and hexenylacetate or trans-β-farnesene in maize [Alborn et al., 1997]) has been associated with FAC signaling.
Notably, FACs are not always active: in lima bean and cowpea (Vigna unguiculata), for instance, FAC treatment of wounds does not elicit VOCs (Spiteller et al., 2001). Rather, Mithöfer et al. (2005) demonstrated that a continuous wounding treatment that resembled insect feeding was sufficient for HIPV emission. In cowpea, inceptin, an elicitor produced from the postingestive digestion of host plant ATP synthase, triggers changes in phytohormone and VOC production similar to those detected during herbivory (Schmelz et al., 2006; Carroll et al., 2008). In N. attenuata, the large methanol burst, which is many times larger than the GLV burst elicited by Manduca sexta herbivory, originates from the demethylation of pectin cell walls and in turn is elicited by the alkalinity of OS (von Dahl et al., 2006). β-Glucosidase hydrolytic enzymes from Pieris brassicae OS also trigger HIPV emissions when applied to cabbage (Brassica capitata) plants (Mattiacci et al., 1995). 2-Hydroxyoctadecatrienoic acid (2-HOT) is a newly identified component of M. sexta OS (E. Gaquerel, A. Steppuhn, and I.T. Baldwin, unpublished data). This oxylipin is produced from linolenic acid through the action of the α-dioxygenase (α-DOX; Hamberg et al., 2002, 2003). In N. attenuata, the production of 2-HOT during herbivory and its accumulation in OS allow the plant to monitor the progression of the insect's attack and to sustain its production of jasmonic acid, the central hormone that coordinates antiherbivore defenses. The striking postingestive stability and high activity of N. attenuata's α-DOX1 proteins in the alkaline and proteolytic milieu of the M. sexta midgut mediates 2-HOT's particular signaling role (E. Gaquerel, A. Steppuhn, and I.T. Baldwin, unpublished data).
Given the rapid advances in characterizing the active elicitors of M. sexta OS, a full unbiased analysis of N. attenuata's HIPV blend, an analysis that has not been conducted for any plant, is overdue. To examine the extent to which FACs, 2-HOT, and alkalinity contribute to the OS-elicited HIPV bouquet, we used comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry (GCxGC-ToFMS) in combination with multivariate statistics. This nontargeted approach was applied to a suite of elicitation treatments in which the amount of mechanical wounding was held constant.
RESULTS
A Stepwise Preprocessing of Volatile Blends
OS collected from M. sexta caterpillars fed on wild-type N. attenuata plants was purified by ion-exchange chromatography (IEX) to remove, among others, FACs and 2-HOT (IOS; Fig. 1A). This latter extract, as well as solutions of synthetic FACs and 2-HOT at concentrations that mimicked those found in OS (Halitschke et al., 2001; E. Gaquerel, A. Steppuhn, and I.T. Baldwin, unpublished data), were prepared as summarized in Figure 1B. We analyzed VOCs emitted locally during three consecutive 12-h periods (Fig. 1, B and C) after treating wounded (W) leaves with these solutions and compared these blends with those elicited by the complete OS or an alkaline buffer (B). We started VOC collections 1 h after treatment, when the effect of the mechanical disturbance had attenuated, to emphasize the exact contributions of each of the solutions tested.
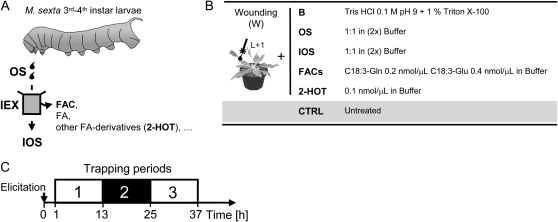
Figure 1.
Elicitation and volatile collection procedures. A, M. sexta's OS were collected from third to fourth instar larvae reared on wild-type N. attenuata plants. OS purified by IEX (IOS), which are free of FACs, 2-HOT, and other FA metabolites, were prepared as described previously (Halitschke et al., 2001) using Amberlite IRA-400 resin. OS and IOS extracts were flushed with argon and stored at −20°C until use. B, The first fully expanded leaf (L+1) of rosette-stage N. attenuata plants (randomized; n = 6 biological replicates per treatment) was wounded (W) with a fabric pattern wheel, and 20 μL of one of the different eliciting solutions to test was directly applied to the leaf surface. Volatile emissions strictly induced by the mechanical wounding and/or the alkalinity of M. sexta's OS were assessed by applying a 0.1 m Tris, pH 9, buffer solution (B) containing 0.1% (v/v) Triton X-100 to wounded leaves. This nonionic surfactant was added to the B solution to evaluate its potential eliciting effect, as it was used for the preparation of the FAC solution. Synthetic N-linolenoyl-l-Gln (C18:3-Gln) and N-linolenoyl-l-Glu (C18:3-Glu), the two major FACs, and 2-HOT were diluted in the B solution at concentrations similar to those found in M. sexta's OS. Control plants (CTRL) were left untreated. C, Leaves were enclosed, 1 h after elicitation, in food-quality plastic containers secured with miniature clips. Plant volatiles were collected, using self-packed Super Q traps connected to a manifold vacuum pump, as described by Halitschke et al. (2000), during three consecutive periods of 12 h, the second one occurring during the glasshouse dark time. Background contaminants present in ambient air were also tested using empty trapping containers.
Unbiased comparative analyses require exceptionally high resolution given the high structural diversity of HIPV constituents (Holopainen, 2004) and the range of concentrations at which they occur, which in this study was provided by GCxGC-ToFMS (Fig. 2A). Once the 108 samples were analyzed, the raw chromatograms were deconvoluted to find analyte peaks using the LECO ChromaToF software version 2.21 (Fig. 2B). Routinely, approximately 600 peaks were detected. The peak lists were then checked prior to further processing for known artifact peaks and contaminants (e.g. column bleeding and plasticizers) identified from the analysis of ambient air samples trapped in the glasshouse.
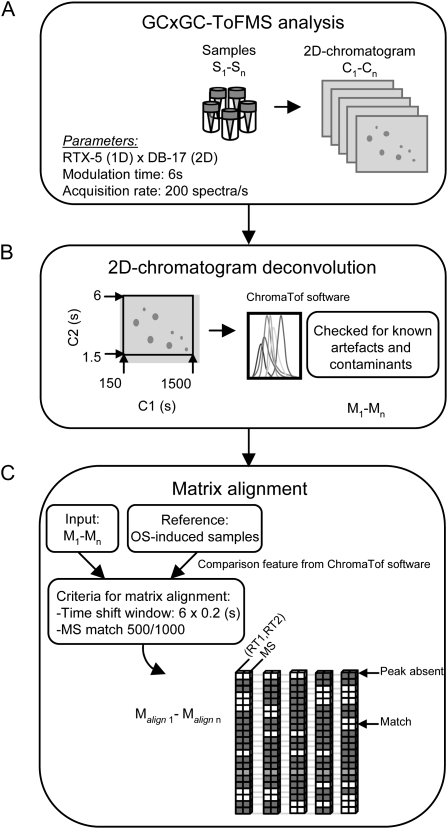
Figure 2.
Preprocessing of GCxGC-ToFMS data. A, Two-dimensional chromatograms (C1 × C2) obtained after analysis of the volatile extracts by GCxGC-ToFMS were processed in two steps. B, Raw data were first deconvoluted to find analyte peaks using the LECO ChromaToF software version 2.21, and peak lists were then checked prior to further processing for known artifact peaks and contaminants. C, Peak matrices from each of three trapping periods were then aligned separately using the comparison feature embedded in the LECO ChromaToF software. For each data point, the largest peak table obtained from the deconvolution of samples collected from leaves wounded and treated with M. sexta's W+OS was used as a reference matrix. Peaks providing matches with metabolites from the reference list (i.e. W+OS-induced plant volatiles) were retained for statistical analysis. Processing parameters and peak list alignment procedures are explained in more detail in the text.
Inconsistencies in the size of the peak tables rendered it difficult to place the information into a suitable matrix format in which rows represented an individual peak, columns represented an individual sample, and the values are peak intensities obtained by integration of deconvoluted peaks. As discussed previously by Shellie et al. (2005), the comparison feature embedded in the LECO ChromaToF software helped in aligning the different peak lists. This required the comparison of each individual chromatogram with a unique reference matrix in order to obtain output tables with the same numbers of peaks. To that end, for each of the three volatile-trapping periods, we selected the largest peak table obtained from the deconvolution of OS-induced samples as a reference matrix; this matrix contained the largest range of HIPV released during each of the sampling intervals. Samples from each of the three trapping periods were processed separately. A mass spectrum similarity threshold of 500/1,000 was used, as it provided sufficient selectivity for peak matching. Retention time (RT) window parameters for peak comparison were set as one modulation period for column 1 (C1) and 0.2 s for column 2 (C2); an evaluation of RT precision with these parameters revealed the parameters to be within the range of typical peak widths of nontailing peaks (Supplemental Table S2). Only the peaks providing matches with metabolites from the reference list (i.e. constituents of the OS-elicited HIPV profiles) were retained. These procedures shortened matrices to approximately 400 peaks, which were subsequently normalized by the tetralin internal standard @ RT (846; 3.76).
W+OS Triggers Pronounced Changes in Volatile Emission
In order to evaluate the performance and value of data deconvolution and pretreatment for statistical analysis, we compared chromatographic peak areas in W+OS and control (CTRL) samples. The intensity and statistical significance of the changes elicited after W+OS were examined using Volcano plots, a procedure commonly used to assess the quality of microarray data sets (Cui and Churchill, 2003). To that end, ANOVAs (W+OS versus CTRL) were performed on normalized log2-transformed intensities to screen for differentially regulated analytes. Volcano plots were obtained by plotting the negative log10-transformed P value issued from these ANOVA tests against the log2 ratio of mean intensities (W+OS/CTRL). We selected a P value of 0.05 for statistical significance threshold and a 1.5-fold change ratio as cutoff threshold to discriminate between significantly up- and down-regulated peaks.
A total of 61 peaks were significantly more intense in chromatograms obtained from leaves induced by W+OS than from untreated leaves during the first trapping period. Concomitantly, no plant volatiles were significantly decreased (Fig. 3A). The number of significantly regulated peaks decreased to 23 and finally to 17 in the two next data points, which underscores that W+OS treatment elicited rapidly waxing and waning changes in VOC releases. Prior to being “blasted” against libraries, lists of significantly regulated analytes were checked for false positives potentially created during peak detection and alignment. We estimated the false discovery rate to be 7% of the total of differentially regulated peaks. These errors, which were mainly produced during the alignment of peak matrices, were manually corrected, and contaminants remaining from the preprocessing were removed. Finally, significantly emitted plant-derived compounds were identified from comparison with standards, based on their mass spectral and retention index homologies shared with entries from the NIST and homemade VOC libraries or reports from the literature, or they were putatively assigned to VOC classes (Supplemental Table S1).
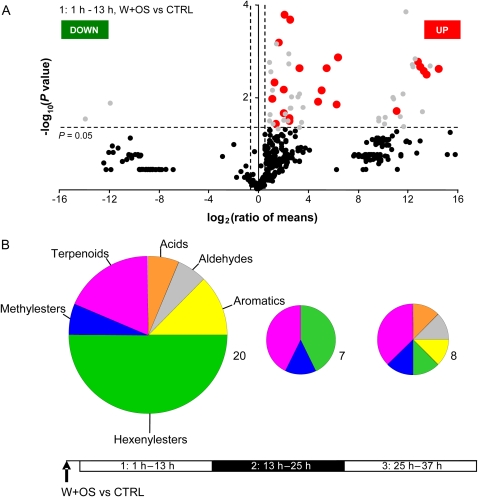
Figure 3.
W+OS triggers pronounced changes in volatile emission. A, Volcano plot analysis of the orientation, intensity, and statistical significance of the changes in chromatographic peak areas between volatile samples collected 1 to 13 h after N. attenuata plants were wounded with a fabric pattern wheel and treated with M. sexta's W+OS and those collected from untreated plants (CTRL). The log2 ratio of mean intensities (W+OS/CTRL; n = 6 biological replicates per treatment) is shown on the x axis, and the negative log10-transformed P value, from ANOVA on normalized log-transformed peak intensities, is shown on the y axis. Each dot represents an analyte from the common set of 463 normalized peaks between samples. The vertical dashed lines represent 1.5-fold change ratios, and the horizontal dashed line represents the statistical significance level at P = 0.05. Peaks with large fold change values lie outside the vertical threshold lines. Up- and down-regulated peaks are localized in the top left and top right parts of the plot, respectively. Gray dots represent significantly regulated contaminants or false positives created by the peak alignment procedure. Plant volatiles found to be up-regulated when W+OS elicited were compared with CTRL samples and are highlighted in red. No plant volatiles were found to be significantly down-regulated. B, Classes of plant volatiles significantly regulated after plants were elicited by W+OS. Nineteen volatiles were emitted at significantly higher rates by plants elicited by W+OS than by untreated plants at 1 to 13 h after elicitation (Supplemental Table S1). Seven volatiles were up-regulated at 13 to 25 h and 25 to 37 h after elicitation; none was down-regulated. Volatiles detected were classified as acids (e.g. pentanoic acid, 3-methyl-), aldehydes (e.g. octanal), aromatics (e.g. hexenylesters [e.g. cis-3-hexenylacetate]), methylesters (e.g. pentanoic, 3-methyl-, methylester), and terpenoids (e.g. β-pinene).
We called the subset of volatiles highlighted by this type of comparison the W+OS response. The W+OS response collected at 1 to 13 h after treatment included the up-regulation of the 20 volatiles (Fig. 3B; Supplemental Table S1, column A). Hexenylesters were the most significantly up-regulated (e.g. ANOVA, W+OS versus CTRL, cis-3-hexenylpropionate, P = 0.001; cis-3-hexenylisobutyrate, P = 0.002; hexylisobutyrate, P = 0.002) and represented half of the total up-regulated metabolites. Terpenoid (e.g. β-pinene, P = 0.002) and aromatic (e.g. unknown @ RT [822; 2.81], P = 0.007) derivatives constituted the second largest groups. Fewer metabolites (by a factor of more than two) were induced at 13 to 25 h after elicitation. Most of them, like cis-3-hexenylpropionate (P = 0.001) and cis-3-hexenyl,trans-2-butenoic ester (P = 0.011), were already significantly up-regulated during the first trapping period (Supplemental Table S1, column A). But only the emission of cis-3-hexenylisobutyrate was significantly sustained throughout the 36 h of trapping (13–25 h, P = 0.030; 25–37 h, P = 0.009). The W+OS response collected at 25 to 37 h after treatment (eight significantly up-regulated metabolites) was dominated by terpenoid derivatives such as the sesquiterpene trans-α-bergamotene (P = 0.002) and other sesquiterpenes that remain to be identified (unknown @ RT [714; 2.71], P = 0.041; unknown @ RT [1,176; 3.27], P = 0.012).
OS Reconfigures the Plant Wound Response Partly through the Action of FACs
To explore the extent to which the introduction of OS to wounds specifically regulated volatile emission, we compared the total changes elicited after W+OS with those detected after W+B (Fig. 4A). We called the ensemble of volatiles found differentially regulated in such comparisons the OS response (Supplemental Table S1, column C). More precisely, up-regulated volatiles were interpreted as being amplified by OS application, while down-regulated volatiles were considered repressed by this treatment. Similarly, volatile substances regulated by the wound and/or the alkalinity of OS were identified by statistically comparing W+B and CTRL blends (Supplemental Table S1, column B).
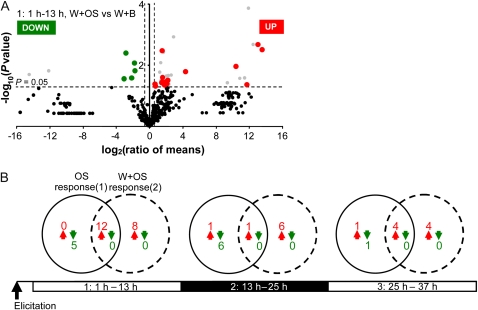
Figure 4.
Application of OS reconfigures the plant wound response. A, Volcano plot analysis of the orientation, intensity, and statistical significance of the changes in chromatographic peak areas between volatile samples collected at 1 to 13 h after plants were wounded with a fabric pattern wheel and treated with M. sexta's W+OS and those collected from wounded plants treated with a buffer at the same pH as M. sexta's OS (W+B). The log2 ratio of mean intensities (W+OS/W+B; n = 6 biological replicates per treatment) is shown on the x axis, and the negative log10-transformed P value, from ANOVAs on normalized log-transformed peak intensities, is shown on the y axis. Each dot represents an analyte from the common set of 463 normalized peaks between samples. The vertical dashed lines represent 1.5-fold change ratios, and the horizontal dashed line represents the statistical significance level at P = 0.05. Peaks with large fold change values lie outside vertical threshold lines. Up- and down-regulated peaks are localized in the top left and top right parts of the plot, respectively. Gray dots represent significantly regulated contaminants or false positives created by the peak alignment procedure. Plant volatiles found to be up-regulated and down-regulated in W+OS- and W+B-elicited samples are highlighted in red and green. B, Two types of responses were considered from samples collected at 1 to 13 h, 13 to 25 h, and 25 to 37 h after elicitation: the W+OS response was detected by comparing W+OS to control (CTRL; 1; dashed circle; see also Fig. 3), and the OS response was detected by comparing W+OS versus W+B (2; black circle). Venn diagrams show the number of overlapping and nonoverlapping volatiles induced (red arrows) or repressed (green arrows) between those two sets of comparisons (Supplemental Table S1). Numbers specific to the W+OS response (1) represent W-induced/repressed volatiles for which no significant effect of the application of OS was observed. Numbers in the common area represent volatiles for which the application of OS amplifies the W regulation. Numbers specific to the OS response (2) represent W-repressed volatiles being induced by OS or W-induced volatiles being repressed by OS application.
During the first period of the experiment, the emission of 12 volatiles (58% of the W+OS response; Fig. 4B; Supplemental Table S1, column A): one acid (pentanoic acid, 3-methyl-, ANOVA W+OS versus W+B, P = 0.043), one aldehyde (octanal, P = 0.012), one aromatic (unknown @ RT [954; 4.13], P = 0.012), five hexenylesters (cis-3-hexenylpropionate, P = 0.005; cis-3-hexenyl,trans-2-butenoic ester, P = 0.023; cis-3-hexenyl-3-methylbutyrate, P = 0.012; cis-3-hexenyltigilate, P = 0.038; and cis-3-hexenyl-methylpentanoate, P = 0.033), and four terpenoids (β-pinene, P = 0.026; unknowns @ RT [714; 2.71], P = 0.045; @ RT [720; 2.34], P = 0.034; and @ RT [1,176; 3.27], P = 0.039) was amplified by OS factors. By contrast, we observed that the emission of certain wound-inducible hexenylesters, such as cis-3-hexenylacetate (ANOVA W+B versus CTRL, P = 0.034), cis-3-hexenylbutyrate (P = 0.042), and cis-3-hexenyl-2-methylbutyrate (P = 0.048), was not significantly altered by the OS treatment. In samples collected at sampling times 2 and 3, the OS response accounted for 14% (one of seven: unknown @ RT [1,176; 3.27], P = 0.034) and 50% (four of eight: cis-3-hexenylisobutyrate, P = 0.012; unknown @ RT [714; 2.71], P = 0.032; unknown @ RT [1,176; 3.27], P = 0.003; and trans-α-bergamotene, P = 0.005) of the up-regulated volatiles from the W+OS response. We interpreted this as evidence that a large proportion of the emission of wound-inducible volatiles was amplified by factors contained in M. sexta's OS. On the other hand, we also observed that OS factors specifically repressed the emission of certain wound-induced volatiles. This latter trend was clearly apparent for alcohol derivatives (2-pentanol,4-methyl, ANOVA W+OS versus W+B, P = 0.008; 2-pentanol,3-methyl, P = 0.017; cis-3-hexenol, P = 0.004; and 1-hexanol, P = 0.027) and one unidentified terpenoid @ RT (1,476; 2.88) (P = 0.027) collected between 1 and 13 h after elicitation (Supplemental Table S1, columns B and C). Certain volatiles collected at later times also exhibited such a trend (e.g. terpineol, P = 0.027 between 13 and 25 h; limonene, P = 0.001 between 25 and 37 h).
To determine the extent to which FACs, 2-HOT, or other OS components not removed by IEX could account for the OS response, we examined peak matrices for FAC-responsive (Supplemental Table S1, column E), 2-HOT-responsive (column F), and IOS-responsive (column D) volatiles. Following the same logic, we defined these responses as the subsets of volatiles being differentially emitted in W+FAC-, W+2-HOT-, or W+IOS-treated samples when compared with W+B-treated samples. During the first trapping period, the FAC response accounted for 75% (three of four) of the amplified terpenoid emissions detected after OS was supplied to wounds (Supplemental Table S1, column E): unknown @ RT (714; 2.71), ANOVA, W+FACs versus W+B, P = 0.037; unknown @ RT (720; 2.34), P = 0.044; and unknown @ RT (1,176; 3.27), P = 0.032. Surprisingly, none of the OS-induced hexenylesters was significantly up-regulated after FAC application. Similar distinctions in the regulation of these two classes of volatiles were observed for the two next sampling times. Remarkably, FAC treatments inhibited the production of 2-pentanol,4-methyl (P = 0.013), 2-pentanol,3-methyl (P = 0.021), cis-3-hexenol (P = 0.016), and 1-hexanol (P = 0.020) in the same range of statistical significance as had been observed after application of OS. FAC signaling also accounted for the decreases, compared with wounded leaves, in the release of benzothiazole (P = 0.047) and terpineol (P = 0.004) at 13 to 25 h after treatment. As expected, removing FACs from OS by IEX abolished most of the FAC-mediated amplification and inhibitory patterns detected above (Supplemental Table S1, column D). Only two volatile substances, cis-3-hexenylbutyrate (P = 0.047) and trans-α-bergamotene (P = 0.039 and 0.007), were significant amplified by the 2-HOT treatment compared with the W+B response (Supplemental Table S1, column F).
The OS and FAC Responses Are Most Similar
The above analysis was conducted by comparing one volatile at a time. Although this univariate procedure reveals interesting patterns, it does not provide insights into the relationships among classes of treatments. Numerous multivariate procedures exist, and of these, hierarchical clustering analyses (HCA) and principal component analyses (PCA) are the most commonly used procedures in transcriptomic, proteomic, and metabolomic analyses of plant samples. Prior to performing such analyses, a filtering procedure is usually required to emphasize information linked to group separation. Thus, as recommended by Boccard et al. (2007), multiple univariate ANOVAs (P < 0.05, n = 6 groups of treatments) were performed in an effort to eliminate, in a supervised way, analytes shared by all samples and therefore containing a common pattern of noninformative signals. Using this filter, only approximately 15% of the initial information was retained for the multivariate analyses. Again, these filtered lists of variables were checked for contaminants and false positives produced during peak matrix alignment.
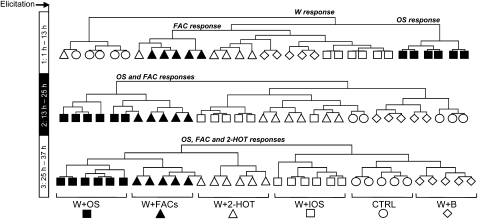
To determine if these prefiltered variables were sufficient to discriminate classes of treatments, we performed HCA. Filtered data were autoscaled, and HCA was performed using the Euclidean distance as a clustering metric and the complete linkage aggregation method. For each of the three data points, preselected variables with equivalent abilities to classify samples were used to group treatments during HCA. Control samples collected 1 to 13 h after treatment were, as expected, clearly separated by the first branch of the dendogram (W response, Fig. 5). The second branch subdivided profiles collected from W+OS-elicited samples from the other samples (OS response). The first branch of the dendogram obtained from the clustering of samples collected 13 to 25 h after the start of the experiment separated OS- and FAC-treated samples from those untreated or elicited with FAC-free solutions (OS and FAC responses). For the last sampling time, 2-HOT-treated samples clustered with OS and FAC-elicited samples (OS and FAC and 2-HOT responses).
Figure 5.
Hierarchical clustering analyses of sample specimens. N. attenuata volatiles were collected at 1 to 13 h, 13 to 25 h, and 25 to 37 h after elicitation. For each data point, an ANOVA-based filter (P < 0.05) was applied on normalized peak intensities in an effort to eliminate redundant signals from GCxGC-ToFMS matrices (see text). This shortened the peak lists by approximately 70% to 80%. The resulting data sets were checked manually for contaminants and false positives from the peak alignment procedure, autoscaled, and classified by hierarchical clustering analysis with The Institute for Genomic Research MultiExperiment Viewer software using the Euclidean distance as a grouping parameter and the complete linkage aggregation method. Symbols indicate different groups of treatments: OS (black squares), IOS (white squares), FACs (black triangles), 2-HOT (white triangles), and buffer (B; white diamonds) solutions were applied onto leaves wounded with a fabric pattern wheel. Control plants (CTRL; white circles) were left untreated. One outlier sample (midvein disrupted during handling) was deleted from the W+FACs, CTRL, and W+B classes of treatments.
PCA Identified Multiple Regulatory Patterns of Volatile Production
PCA was used as an unsupervised method to produce interpretable projections of samples in a reduced dimensionality (score plots) and to highlight biomarkers responsible for group separation (loading plots). Prior to performing these analyses, different types of transformations were applied to correct for a nonconstant signal variance. Since for analytical chemical measurements the total uncertainty is often proportional to the expected value of the signal, a logarithmic transformation is often appropriate. Therefore, we log2 transformed and mean-center normalized the values. The two first principal components (PCs) extracted accounted for approximately 60% of the total variance existing in the three sample populations (Supplemental Fig. S1). In addition, as expected from the HCA analyses, CTRL and W+OS samples were the treatments best separated by these two PCs. Examining other PCs that accounted for lower amounts of variance, we were unable to obtain the additional power needed to discriminate among treatments. To address this problem, we performed two new series of PCA per data point after dividing the data sets into two subparts.
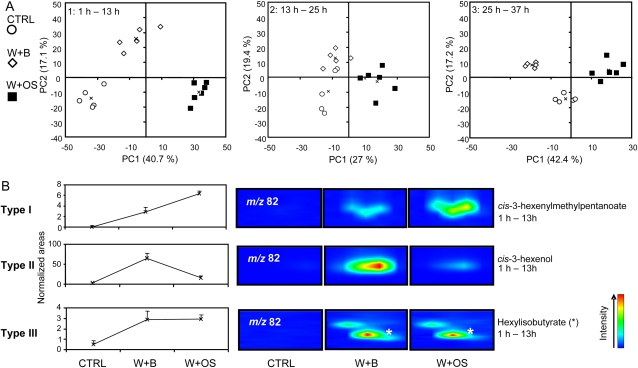
The first category of PCA aimed at classifying CTRL, W+B, and W+OS samples (Fig. 6). The two first PCs extracted using those samples as inputs captured approximately 45% to 60% of the total variance (Fig. 6A) and segregated samples according to two biological responses. The first one represented the W response and the second was associated with the OS response, as described previously. In accordance with HCA results, group separation was less efficient for volatile profiles collected in the dark phase of the experiment (13–25 h). To simplify the interpretation, metabolites contributing to these projections were categorized as follows (Figs. 6B and 8 [below]; Table I). OS-responsive volatiles, high-ranking loadings on PC1, were divided into those up-regulated (type I, W induced and OS amplified; 28 volatiles) and those down-regulated (type II, W induced and OS repressed; 11 volatiles). We classified as type III, high-ranking loadings on PC2, metabolites being W inducible but not OS responsive (seven volatiles). Globally, the clustering of the volatiles identified with this procedure was largely commensurate with that detected from the univariate statistical comparisons.
Figure 6.
PCA of W- and OS-responsive volatiles. A, Filtered data sets used for HCA (Fig. 5) were mean centered and subjected to PCA using an Excel add-in developed by the Bristol Chemometrics group. Projection plots were obtained from the coordinates calculated for the two first PCs extracted. The percentage of the total variance explained by each PC is reported in parentheses. Symbols indicate different groups of treatments: OS (black squares) and buffer (B; white diamonds) solutions were applied onto leaves wounded with a fabric pattern wheel (W). Control plants (CTRL; white circles) were left untreated. Centers of each group of treatment were calculated and are indicated on projections by crosses. B, For each data point, analytes contributing to the PCA projection were examined and screened for plant volatiles (Table I). To simplify the analysis, we categorized volatile accumulation patterns into three main types: type I contains W-induced and OS-amplified volatiles (e.g. cis-3-hexenylmethylpentanoate between 1 and 13 h); type II contains those being W induced and OS repressed (e.g. cis-3-hexenol between 1 and 13 h); and type III contains those detected as W induced but not OS responsive (e.g. hexylisobutyrate between 1 and 13 h). Normalized intensities and magnified areas of representative two-dimensional chromatograms obtained from W+OS, W+B, and CTRL samples illustrate for one member each of the three types of regulation.
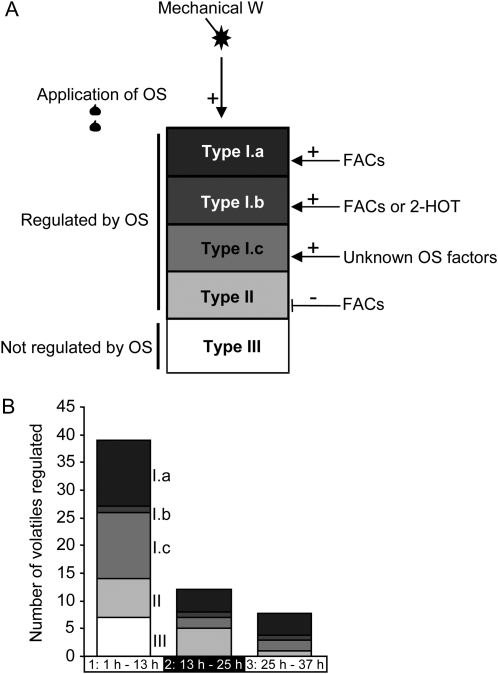
Figure 8.
Patterns of regulation of volatile emissions observed during W+OS elicitation. A, When wounded with a fabric pattern wheel (W), N. attenuata leaves emit large amounts of volatiles (types I.a–I.c, II, and III). This volatile signature is reconfigured by the application of M. sexta's OS during W+OS elicitations. Types I.a to I.c and type II contain leaf volatiles that were induced after W and significantly induced or repressed after application of M. sexta's OS. Amplification of the wound response was mediated by the action of FACs (type I.a; dark square), the action of FACs or 2-HOT (type I.b; dark gray square), or the action of unknown factors (type I.c; gray square). Type II (light gray square) represents volatiles induced after W that were repressed after application of M. sexta's OS through the action of FACs. Type III (white square) represents W-induced volatiles for which no significant effect of the application of OS was observed. B, Number of volatiles significantly regulated following types I to III (see Table I) at 1 to 13 h, 13 to 25 h, and 25 to 37 h after elicitation.
Table I.
Summary of differentially emitted volatiles and their corresponding regulatory patterns
N. attenuata leaf volatiles were collected at 1 to 13 h, 13 to 25 h, and 25 to 37 h after elicitation, and those differentially emitted (ANOVA, P < 0.05, n = 6) between the groups of treatments were analyzed by PCA (Figs. 6 and 7). Types I.a to I.c and type II contain leaf volatiles that were induced after W and induced or repressed after application of M. sexta OS. Amplification of the wound (W) response was mediated by the action of FACs (type I.a), the action of FACs or 2-HOT (type I.b), or the action of unknown factors (type I.c). Type II represents volatiles induced after W that were repressed after the application of M. sexta OS mainly through the action of FACs. Type III represents W-induced volatiles for which no significant effect of the application of OS was observed. Volatiles are listed by chemical classes with their RTs on the first and second dimensions. Numbers indicate the type of identification (Id.): 1, comparison with standards; 2, putative identification. Retention indices were calculated according to Kováts (1958) and compared with literature (Lit.) reports: a (Ruther, 2000), b (Adams, 2004), c (Engel et al., 2002), d (Triqui and Reineccius, 1995), e (Guth and Grosch, 1991), and f (Jirovetz et al., 2002).
| Class | Compound | Id. | RT(s)
|
Retention Index
|
Regulation (Type)
|
||||
|---|---|---|---|---|---|---|---|---|---|
| RT1 | RT2 | Calculated | Lit. | 1, 1–13 h | 2, 13–25 h | 3, 25–37 h | |||
| Acid | Pentanoic acid, 3-methyl- | 2 | 450 | 2.49 | 963 | – | I.c | I.c | |
| Alcohol | 2-Pentanol,4-methyl | 2 | 216 | 2.02 | 837 | – | II | ||
| 2-Pentanol,3-methyl | 2 | 228 | 2.17 | 845 | – | II | |||
| cis-3-Hexenol | 1 | 246 | 2.57 | 858 | 849a | II | |||
| 1-Hexanol | 1 | 270 | 2.56 | 872 | 876a | II | |||
| Aldehyde | Heptanal | 2 | 336 | 3.01 | 910 | 903c,d | I.a | ||
| Aromatic | Octanal | 2 | 552 | 2.73 | 1,004 | 1,002c,d | I.c | ||
| Unknown | – | 564 | 5.23 | 1,012 | – | I.a | I.a | ||
| Unknown | – | 858 | 4.03 | 1,168 | – | I.c | I.c | ||
| Inden-2-one, octahydro | 2 | 954 | 4.13 | 1,215 | – | I.c | |||
| Benzene, 2,4-diethyl-1-methyl- | 2 | 822 | 2.58 | 1,144 | – | II | II | ||
| Benzothiazole | 2 | 960 | 5.51 | 1,220 | 1,221f | II | II | ||
| Hexenylester | cis-3-Hexenylisobutyrate | 1 | 834 | 2.51 | 1,151 | 1,147b | I.c | I.c | I.c |
| cis-3-Hexenylpropionate | 1 | 750 | 2.73 | 1,104 | 1,100a | I.c | I.c | ||
| cis-3-Hexenyl,trans-2-butenoic ester | 1 | 882 | 2.71 | 1,176 | – | I.c | II | ||
| cis-3-Hexenyl-3-methylbutyrate | 1 | 996 | 2.51 | 1,241 | 1,245b | I.c | |||
| cis-3-Hexenylmethylpentanoate | 1 | 1,170 | 2.51 | 1,348 | – | I.c | |||
| cis-3-Hexenyltigilate | 1 | 1,140 | 2.85 | 1,328 | 1,322a | III | II | ||
| cis-3-Hexenylbutyrate | 1 | 912 | 2.56 | 1,191 | 1,187a | III | |||
| cis-3-Hexenylacetate | 1 | 570 | 2.84 | 1,014 | 1,008a | III | |||
| Hexylisobutyrate | 1 | 840 | 2.32 | 1,154 | 1,152b | III | |||
| trans-2-Hexenylbutyrate | 1 | 924 | 2.65 | 1,197 | 1,198a | III | |||
| cis-3-Hexenyl-2-methylbutyrate | 1 | 984 | 2.51 | 1,234 | 1,231a | III | |||
| Methylester | Pentanoic, 3-methyl-, methyl ester | 2 | 300 | 2.80 | 888 | – | I.c | ||
| Terpenoid | Unidentified sesquiterpene | – | 1,176 | 3.27 | 1,352 | – | I.a | I.a | I.a |
| Unidentified monoterpene | – | 714 | 2.71 | 1,090 | – | I.a | I.a | ||
| Unidentified monoterpene | – | 738 | 2.84 | 1,107 | – | I.a | I.a | ||
| 1-Cyclopentylcyclopentene | 2 | 648 | 2.56 | 1,054 | – | I.a | |||
| Unknown sesquiterpene | – | 720 | 2.34 | 1,088 | – | I.a | |||
| trans-α-Bergamotene | 1 | 1,302 | 2.28 | 1,435 | 1,435b | I.b | I.b | ||
| β-Pinene | 1 | 474 | 2.29 | 396 | 979c | I.c | |||
| Unknown | – | 1,476 | 2.88 | 1,541 | – | II | |||
| Terpineol | 1 | 906 | 3.02 | 1,188 | 1,189b | II | |||
| Limonene | 1 | 600 | 2.46 | 1,030 | 1,031b | II | |||
| Unknown | – | – | 312 | 4.47 | 897 | – | I.a | I.a | |
| – | – | 306 | 4.22 | 895 | – | I.a | |||
| – | – | 612 | 3.51 | 1,036 | – | I.a | |||
| – | – | 756 | 2.45 | 1,110 | – | I.a | |||
| – | – | 1,056 | 3.56 | 1,287 | – | I.a | |||
| – | – | 1,074 | 3.49 | 1,305 | – | I.a | |||
| – | – | 522 | 5.44 | 984 | – | I.b | |||
| – | – | 354 | 3.24 | 348 | – | I.c | |||
| – | – | 594 | 2.66 | 1,026 | – | III | III | ||
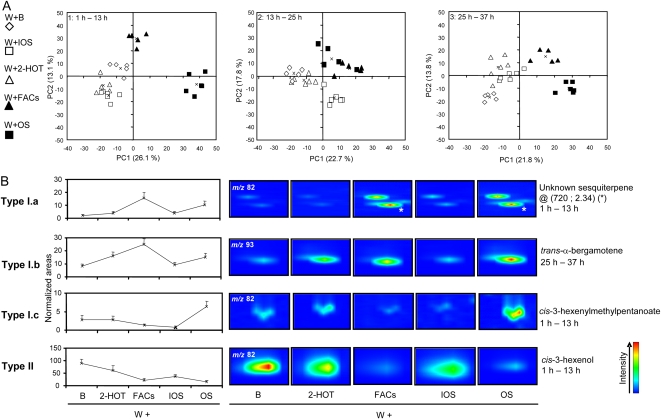
The contributions of B, IOS, 2-HOT, and FACs to the OS response were analyzed in a second series of PCA (Fig. 7). The two first PCs extracted using W+B, W+IOS, W+2-HOT, W+FAC, and W+OS samples as inputs explained approximately 35% to 40% of the total variance (Fig. 6A). Even though none of the OS factors tested could completely restore the OS response, FAC-treated samples were the ones that most closely mimicked OS-elicited samples. As already revealed by HCA, this observation was more pronounced for samples collected at later times during the experiment. In addition, samples treated with IOS, which is free of FACs, were clearly separated from OS-treated samples. An efficient segregation of W+B- and W+2-HOT-treated samples was apparent only 25 to 37 h after elicitation. Following the same logic as presented above, we discovered that three main biological patterns structured those projections (Figs. 7B and 8; Table I). Type I.a volatiles, high-ranked loadings on PC1 and PC2, were amplified by OS through the action of FACs (13 volatiles; W induced and OS and FAC amplified). Terpenoid metabolites were the predominant volatiles regulated in this manner (Table I). Surprisingly, we also detected that the application of 2-HOT amplified the production of trans-α-bergamotene (W+2-HOT versus W+B; ANOVA, 13−25 h, P = 0.039; 25–37 h, P = 0.007; Supplemental Table S1, column F; Fig. 7B; Supplemental Fig. S2), a well-known FAC-regulated sesquiterpene (Halitschke et al., 2001). We called the volatiles with this pattern of regulation type I.b (W induced and OS, FAC, and 2-HOT amplified). Metabolites that were amplified by unknown OS factors were classified as type I.c (12 volatiles). Most of these were identified as hexenylesters and aromatic derivatives (Table I). Volatiles that were repressed by FACs corresponded mainly to those classified in the aforementioned type II (11 volatiles; W induced and OS repressed). Altogether, the number of differentially regulated volatiles largely decreased over the experimental time, while the relative proportion of FAC-regulated substances increased (Fig. 8B).
Figure 7.
PCA of OS-, FAC-, and 2-HOT-responsive volatiles. A, Filtered data sets used for HCA (Fig. 5) were mean centered and subjected to PCA using an Excel add-in developed by the Bristol Chemometrics group. Projection plots were obtained from the coordinates calculated for the two first PCs extracted. The percentage of the total variance explained by each PC is reported in parentheses. Symbols indicate different groups of treatments: OS (black squares), IOS (white squares), FACs (black triangles), 2-HOT (white triangles), and buffer (B; white diamonds) solutions were applied to leaves wounded with a fabric pattern wheel (W). Centers of each group of treatment were calculated and are indicated on projections by crosses. B, For each data point, analytes contributing to the PCA projection were examined and screened for plant volatiles (Table I). To simplify the analysis, we categorized volatile accumulation patterns into two main types and three subtypes: type I volatiles, whose emission was W induced and OS amplified, with type I.a for FAC induced (e.g. unknown sesquiterpene @ RT [720; 2.34] between 1 and 13 h), type I.b for OS, FAC, and 2-HOT induced (e.g. trans-α-bergamotene between 25 and 37 h), and type I.c containing volatiles that were OS induced through the action of unknown factors (e.g. cis-3-hexenylmethylpentanoate); type II (see Fig. 6) represents OS- and FAC-repressed volatiles (e.g. cis-3-hexenol between 1 and 13 h). Normalized intensities and magnified areas of representative two-dimensional chromatograms obtained from W+OS, W+B, and control (CTRL) samples illustrate for one member each of the four types of regulation.
DISCUSSION
We present here a conceptual approach for the untargeted comparative processing via GCxGC-ToFMS of volatile bouquets emitted after insect herbivory. When combined with univariate and multivariate statistics, this approach (1) identifies wound-dependent metabolites and those being further modulated by the application of M. sexta's OS and (2) demonstrates that FACs act as major orchestrators of the OS-elicited response, eliciting and repressing suites of volatiles (Fig. 8).
Nontargeted Functional Analysis of GCxGC-ToFMS Data
The number of biological questions in plant science to which metabolomics have been applied is exponentially growing (for review, see Shulaev et al., 2008). However, a majority of these studies have focused on the regulation of endogenous metabolites rather than on what is sometimes called the plant's exometabolome (e.g. root exudates and floral nectars). As discussed in the recent publication of Van Dam and Poppy (2008), this also applies to metabolites emitted in the gas phase by plants, for which few studies have been carried out using unsupervised approaches.
GCxGC-ToFMS technology has been shown to provide high-quality mass spectra with great sensitivity, largely as a result of the enhanced resolution and zone compression obtained from the orthogonal separation (Shellie et al., 2001). These qualities were exemplified by the 5- to 10-fold increase in the signal-to-noise ratio of a series of commonly emitted volatiles that we compared with the results from one-dimensional analyses performed with the same instrument (data not shown). The approach was found to be reliable and accurate for the automated processing of a large number of samples using parameters based on the recommendations described by Shellie et al. (2005) and preliminary analyses performed with standard compounds (Fig. 2; Supplemental Table S2). For each collection period, each individual chromatogram was compared with the OS-elicited volatile profile reference list. This procedure reduced the size of the output tables presenting the same numbers of peaks and enriched in OS-specific volatiles. These peak matrices, as already documented by several authors (Shellie et al., 2005; Hagan et al., 2007; Kusano et al., 2007; Tu et al., 2007), were suitable for univariate and multivariate statistical analyses (Figs. 3–7), after correction for false positives resulting from the alignment procedure. With the caveat that these aligning and statistical procedures might exclude metabolites up-regulated only by FACs or 2-HOT and not by the OS treatment, they allowed regulatory patterns orchestrating OS induction to be efficiently discriminated (Fig. 8). In that sense, this study constitutes one of the first applications of GCxGC-ToFMS for the nontargeted processing of plant volatile bouquets. Using the approach developed here, we also report the use of this procedure in the analyses of polymorphism in volatile emission among individual plants collected from a native N. attenuata population (M.C. Schumann, N. Heinzel, E. Gaquerel, A. Svatos, and I.T. Baldwin, unpublished data).
FACs Are the Major Determinants of HIPV Emissions
Applying FACs to wounded leaves has been shown to trigger the emission of particular volatiles in amounts similar to those detected during caterpillar feeding (Alborn et al., 1997; Halitschke et al., 2001). Here, we rediscovered through an unbiased approach the dominant role of this class of elicitors in coordinating N. attenuata's volatile emission. Precisely, we detected that the FAC response accounted for approximately half (sample time 1, seven of 17; sample time 2, seven of eight; sample time 3, three of five) of the significant changes in volatile emission elicited by OS treatments. Terpenoids defined the largest proportion of FAC-inducible volatiles (Table I; Supplemental Table S1). In addition to trans-α-bergamotene, whose regulation through FAC signaling has already been described (Halitschke et al., 2001), a suite of new FAC targets was discovered, for instance unknown @ RT (720; 2.34) and unknown @ RT (1,176; 3.27). The identification of these two metabolites will require further work, as will the determination of their potential activity in recruiting predators. In contrast, the diversity of hexenylesters emitted after W+OS treatment appeared to be either strictly associated with the mechanical stress or regulated through the action of unknown OS factors (Table I; Supplemental Table S1).
Even though FACs could not fully account for all of the qualitative changes in volatile emission observed after OS treatment, FAC-elicited profiles were the most closely related to OS-elicited ones, as was clearly evident from the HCA dendogram and PCA projections. These results extend our appreciation of the importance of FAC signaling during attack by M. sexta larvae. Wu et al. (2007) demonstrated that the same FACs that we used were sufficient to induce similar levels of salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK) activity, which, in turn, are responsible for eliciting most of the defensive responses elicited in N. attenuata leaves when attacked by caterpillars or OS elicited. Additionally, FACs are also known to be responsible for the majority of the dramatic changes observed in the transcriptome (65%–86% of the regulated transcripts [Halitschke et al., 2001; Roda et al., 2004]) and the proteome (19 of 32 proteins elicited [Giri et al., 2006]) when leaves are OS elicited.
Many plants change the blend of volatiles released when attacked by different herbivore species (for review, see Dicke, 1999), and FACs may play a central role in tailoring the VOC blend. Whether plants integrate the whole FAC signature or only respond to dominant components remains unknown. Wu et al. (2007) demonstrated that the four most abundant FACs found in M. sexta OS are functionally redundant in terms of kinase activations. Recent analytical developments have demonstrated that the spectrum of FACs contained in the OS of many caterpillar species is more complex than previously thought (Yoshinaga et al., 2007). Clearly, additional unbiased analyses of the specific and common responses elicited by each of these FACs are needed.
FACs Suppress Particular Volatiles
One of the tantalizing discoveries from this unbiased analysis was that M. sexta OS strongly repressed the emission of particular components of the volatile bouquet (Fig. 4). Repressed metabolites included short-chain alcohol derivatives, certain hexenylesters, as well as some aromatic and terpenoid derivatives (Fig. 4; Supplemental Table S1). The analysis demonstrated that this OS-mediated repression (type II) could be largely attributed to the action of FACs (Supplemental Table S1). Treating wounded leaves with FACs reproduced, in the same range of intensity and statistical significance, approximately 80% of the alterations in volatile emission observed during the first trapping period after OS treatment. This effect was particularly well exemplified by the repression of cis-3-hexenol production detected in the first hour after OS or FAC treatment. This constitutes, to our knowledge, the first observation of a dual regulatory role for FACs on a plant's volatile release.
Factors that suppress the deployment of wound-induced defenses have been characterized in the OS of different caterpillar species. Notably, Musser et al. (2002, 2005) demonstrated that Glc oxidase (GOX), an enzyme isolated from the salivary glands of Helicoverpa zea and subsequently found in other caterpillar species, suppresses the wound-inducible accumulation of foliar nicotine in cultivated tobacco (Nicotiana tabacum) and is associated with increased survival and growth of larvae that fed on GOX-treated plants compared with those that fed on leaves treated with water. GOX enzymatic activity also decreased the emission of nicotine into the head space as well as other commonly emitted OS-induced volatiles (Delphia et al., 2007). GOX represses the transcription of genes involved in terpenoid biosynthesis in alfalfa (Medicago truncatula; Bede et al., 2006). C. Diezel and I.T. Baldwin (unpublished data) found that, when the antagonistic action of ethylene signaling is repressed, the GOX activity contained in Spodoptera exigua OS triggers the accumulation of salicylic acid in N. attenuata, which, in turn, antagonizes jasmonic acid-inducible defenses, such as the aforementioned production of nicotine. The role of ethylene signaling in the repressing effect of FACs on the emission of certain VOCs, for instance, will be particularly interesting to investigate further. Alternatively, it should be recalled that the repressing effect of FACs might not necessarily be the result of signaling cross talk. For example, FACs might directly interact with wound-released volatiles at the treatment site. This hypothesis could be tested by comparing FAC-elicited blends emitted locally and systemically.
The ecophysiological consequences of this novel repression function of FACs will be exciting to explore in future work. The central question will be to determine whether this repression is advantageous for the plant or the attacking insect. GLV emissions have been shown to increase the apparency of N. attenuata plants to herbivores (Halitschke et al., 2004; Meldau et al., 2009), and getting “off the herbivore's radar” can provide a level of protection for plants that is comparable to that afforded by direct defenses. Meldau et al. (2009) observed that although most of the chemical defenses were reduced in plants rendered silenced in SIPK and WIPK signaling, these plants did not suffer more damage from herbivores when planted into a native habitat. This lack of herbivore attack was associated with a large attenuation of GLV emissions from SIPK- and WIPK-silenced plants, and the authors demonstrated that applying GLVs, particularly cis-3-hexenol, restored, under laboratory conditions, M. sexta tissue consumption and mass gain. Alternatively, some of the same VOCs that are repressed by FACs may be involved in attracting Geocoris pallens predators and thereby interfere with the defensive function of the volatile release (Kessler and Baldwin, 2001). To further complicate the matter, neighboring unattacked plants might react to these modifications of the VOC blend composition produced by attacked plants. In that direction, Paschold et al. (2006) demonstrated that VOC blends deficient in GLVs or trans-α-bergamotene regulated numerous defense-related genes in neighboring plants; this regulation did not occur when these plants were exposed to the complete VOC blends. Clearly, much more work will be required to understand the function of FAC-mediated VOC signaling and to determine the net fitness consequences for the plant of these VOCs as cues for herbivores, predators, or neighboring plants.
Regulation of trans-α-Bergamotene Emission
Consistent with the results of Halitschke et al. (2001), we found that FACs trigger trans-α-bergamotene emission and that their removal from M. sexta OS by IEX decreased the release of this sesquiterpene. We also found that 2-HOT, when applied to wounded leaves in the same range of concentration as that calculated in M. sexta OS, was able to elicit the release of the same amounts of trans-α-bergamotene as are elicited by OS and FAC treatments. We recently discovered that both 2-HOT and FACs are removed from OS by IEX purification (E. Gaquerel, A. Steppuhn, and I.T. Baldwin, unpublished data), which explains why IOS-treated plants have severely reduced emissions of trans-α-bergamotene. 2-HOT is produced from linolenic acid through the action of the α-DOX pathway and accumulates in planta during pathogen inoculation (Hamberg et al., 2002, 2003). In N. attenuata, the production of 2-HOT in the larval OS during the herbivory allows the plant to monitor the progress of the insect attack (E. Gaquerel, A. Steppuhn, and I.T. Baldwin, unpublished data). This appeared to be controlled by the striking stability of N. attenuata's α-DOX1 proteins in the alkaline and protease-filled gut of M. sexta larvae. Moreover, M. sexta larvae feeding on N. attenuata's α-dox1-silenced lines grew better than on wild-type ones and accumulated less 2-HOT in their OS when feeding on these plants. The elicitation of trans-α-bergamotene release as an indirect defense represents a new testable hypothesis for the function of 2-HOT, a function that could be readily addressed with α-dox1-silenced plants.
MATERIALS AND METHODS
Plant Material and Treatments
We used an isogenic line, obtained after 22 generations of inbreeding, of Nicotiana attenuata (synonymous with Nicotiana torreyana; Solanaceae) derived from field-collected seeds. Seeds were germinated on agar plates containing Gamborg B5 medium (Duchefa; http://www.duchefa.com) as described by Krügel et al. (2002). Plants were grown in the glasshouse in 1-L individual pots at 26°C to 28°C under 16 h of light supplied by Philips Sun-T Agro 400- or 600-W sodium lamps.
All of the eliciting treatments were performed with plants at the rosette stage. Six replicate plants for each treatment were used. Plants were divided into six groups of equal size, and each of six treatments was randomized inside each of these groups. The second fully elongated (+1 position) leaf was used. Plants were either left untreated (CTRL) or wounded using a pattern wheel to punch three rows of holes on each side of the midvein. Wounded leaves were immediately treated with 20 μL of the eliciting solution pipetted directly onto the wounded leaf and gently dispersed across the surface with a gloved finger, changing gloves between treatments.
The compositions of the different eliciting solutions are summarized in Figure 1. Volatile emissions strictly induced by the mechanical wounding and/or the alkalinity of Manduca sexta's OS were assessed by applying a 0.1 m Tris, pH 9, buffer solution (B) containing 0.1% (v/v) Triton X-100. This nonionic surfactant was added to evaluate its potential eliciting effect, since it was used for the preparation of the FACs solution. OS were collected from third to fourth instar M. sexta larvae reared on wild-type plants, flushed with argon, stored at −20°C, and diluted 1:1 (v/v) before being used with a 2× concentrated buffer solution. OS purified by IEX (IOS), which are FACs and 2-HOT free, were prepared as described previously (Halitschke et al., 2001) using Amberlite IRA-400 resin (Sigma; http://www.sigmaaldrich.com/), stored at −20°C, and diluted 1:1 (v/v) before use with a 2× concentrated B solution. Synthetic N-linolenoyl-l-Gln (C18:3-Gln) and N-linolenoyl-l-Glu (C18:3-Glu), the two dominant FACs, and 2-HOT (Larodan Fine Chemicals; http://www.larodan.se/) were diluted in the buffer solution at 0.2, 0.4, and 0.1 nmol μL−1, respectively, concentrations similar to those found in M. sexta's OS (Halitschke et al., 2001; E. Gaquerel, A. Steppuhn, and I.T. Baldwin, unpublished data).
Collection of Plant Volatiles
Treated leaves were enclosed, 1 h after elicitation (the time needed to complete all of the treatments), in two 50-mL food-quality plastic containers (Huhtamaki; http://www.huhtamaki.com/) secured with miniature claw-style hair clips. Ambient air flowed into the cage primarily through a clipped-off P1000 pipette tip inserted into the bottom container and was pulled out through a self-packed glass tube (ARS, Inc.; http://www.ars-fla.com/_fpclass/fp_contact.html) containing glass wool and 20 mg of Super Q (Alltech; http://www.discoverysciences.com) and secured in a second clipped-off P1000 pipette tip inserted into the top container. Air flow was created by a manifold vacuum pump (model DAA-V114-GB; Gast Manufacturing; http://www.gastmfg.com/) as described by Halitschke et al. (2000). Plant volatiles were collected during three periods of 12 h, the second one occurring during the dark period. Background contaminants present in ambient air were also collected using empty trapping containers. Immediately after collection, traps were eluted by spiking each with 400 ng of tetralin as an internal standard and flushing the trap with 250 μL of dichloromethane into a GC vial containing a glass insert.
Instrumental Parameters
An Agilent 6890N gas chromatograph equipped with an Agilent 7683 autoinjector (Agilent Technologies; http://www.agilent.com/) coupled with a LECO Pegasus III time-of-flight mass spectrometer with a 4D thermal modulator upgrade (LECO; http://www.leco.com/) was used to collect the three-dimensional GCxGC-TOFMS data. The GC inlet and transfer line were held constant at 250°C. Splitless injections of 1 μL were made onto an RTX-5MS column (i.e. column 1 [C1] of the GCxGC system [20 m × 250 μm i.d. × 0.5 μm; Restek, Bellefonte; http://www.restek.com/]). The collected C1 effluent was transferred to a DB-17 column 2 (C2) of the GC×GC system (0.890 m × 100 μm i.d. × 0.1 μm; Agilent Technologies) every 6 s (modulation time). C1 was held at 40°C for 5 min and then increased at 5°C min−1 to 190°C and finally increased at 25°C min−1 to 250°C, where it was held for 5 min. C2 was initially set at 45°C and followed the same temperature program as C1, giving a total run of 45 min. The modulator was maintained at 30°C higher temperature than C1. The ion source was maintained at 250°C. Data were collected, after a solvent peak delay of 120 s, in the mass-to-charge ratio range 50 to 300, at a rate of 200 spectra s−1.
Data Preprocessing and Statistical Analysis
The LECO ChromaToF software version 2.21 (LECO) was used throughout to control the instruments as well as to acquire and process the data (including automatic peak deconvolution). Sample populations from each of three trapping periods were processed separately. We selected for each of the three trapping periods the largest peak table obtained from the deconvolution of OS-induced samples as a reference matrix for each of the volatile-trapping periods (Fig. 2). Processed peaks were reported at a signal-to-noise ratio of 10, since we estimated this value as the minimum required for integration and accurate identification during blasts with the NIST and homemade libraries (data not shown). Normalization (object-wise standardization) of analytical profiles is an important step in the preprocessing of metabolic profiling data. Here, we applied linear normalization by dividing all peak heights by the tetralin internal standard @ RT (846; 3.76). To minimize the effect of zero substitution, 1 was added to all areas prior to normalization.
The variations in intensity across treatments were analyzed by univariate ANOVA (P < 0.05) on log2-transformed normalized peak areas. Hierarchical clustering analyses were done with The Institute for Genomic Research MultiExperiment Viewer 3.1 software (http://www.tm4.org/mev.html) on autoscale using the Euclidean distance as a clustering metric and the complete linkage aggregation method. The statistical robustness of the dendogram structures was tested by the bootstrap resampling procedure. PCA was performed on log2-transformed and mean-centered values using an Excel add-in developed by the Bristol Chemometrics group (http://www.chm.bris.ac.uk/org/chemometrics/). Projection plots were obtained from the coordinates calculated for the PCs extracted.
VOCs markers underlying treatment-based discriminations were searched against a custom spectrum library constructed from authentic standards and the NIST98 standard and identified based on retention index and spectrum similarity match. For determination of the retention index, a C8 to C24 n-alkanes series was used. Only the first-dimension RT was used to calculate the retention index according to Kováts (1958), given that the second RT value was negligible.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. PCA of differentially emitted volatiles.
Supplemental Figure S2. Regulation of trans-α-bergamotene emission.
Supplemental Table S1. Induced changes in volatile emission.
Supplemental Table S2. Evaluation of injection, RT, and calibration precisions.
Supplementary Material
Acknowledgments
We thank Prof. Dr. R. Brereton for providing the Chemometric Excel add-in used for PCA, Dr. N. Heinzel for his help with the identification of plant volatiles, A. Weber and A. Schüenzel for growing the plants, E. Wheeler for editorial assistance, and two anonymous reviewers for comments on an earlier version of the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ian T. Baldwin (baldwin@ice.mpg.de).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Adams RP (2004) Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing Corporation, Carol Stream, IL
- Alborn HT, Turlings TC, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276 945–948 [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabyashi J (2000. a) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406 512–515 [DOI] [PubMed] [Google Scholar]
- Arimura G, Tashiro K, Kuhara S, Nishioka T, Ozawa R, Takabayashi J (2000. b) Gene responses in bean leaves induced by herbivory and by herbivore-induced volatiles. Biochem Biophys Res Commun 277 305–310 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA (2006) Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 311 812–815 [DOI] [PubMed] [Google Scholar]
- Bede JC, Musser RO, Felton GW, Korth KL (2006) Caterpillar herbivory and salivary enzymes decrease transcript levels of Medicago truncatula genes encoding early enzymes in terpenoid biosynthesis. Plant Mol Biol 60 519–531 [DOI] [PubMed] [Google Scholar]
- Bernasconi ML, Turlings TCJ, Ambrosetti L, Bassetti P, Dorn S (1998) Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Entomol Exp Appl 87 133–142 [Google Scholar]
- Bernasconi Ockroy ML, Turlings TJC, Edwards PJ (2001) Response of natural populations of predators and parasitoids to artificially induced volatile emissions in maize plants (Zea mays L.). Agric For Entomol 3 1–10 [Google Scholar]
- Boccard J, Grata E, Thiocone A, Gauvrit JY, Lanteri P, Carrupt PA, Wolfender JL, Rudaz S (2007) Multivariate data analysis of rapid LC-TOF/MS experiments from Arabidopsis thaliana stressed by wounding. Chemom Intell Lab Syst 86 189–197 [Google Scholar]
- Carroll MJ, Schmelz EA, Teal PEA (2008) The attraction of Spodoptera frugiperda neonates to cowpea seedlings is mediated by volatiles induced by conspecific herbivory and the elicitor inceptin. J Chem Ecol 34 291–300 [DOI] [PubMed] [Google Scholar]
- Colazza S, McElfresh JS, Millar JG (2004) Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J Chem Ecol 30 945–964 [DOI] [PubMed] [Google Scholar]
- Cui X, Churchill GA (2003) Statistical tests for differential expression in cDNA microarray experiments. Genome Biol 4 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delphia CM, Mescher MC, De Moraes CM (2007) Induction of plant volatiles by herbivores with different feeding habits and the effects of induced defenses on host-plant selection by thrips. J Chem Ecol 33 997–1012 [DOI] [PubMed] [Google Scholar]
- De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393 570–573 [Google Scholar]
- Dicke M (1994) Local and systemic production of volatile herbivore-induced terpenoids: their role in plant-carnivore mutualism. J Plant Physiol 143 465–472 [Google Scholar]
- Dicke M (1999) Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol Exp Appl 91 131–142 [Google Scholar]
- Dicke M, Sabelis MW, Dejong M (1988) Analysis of prey preference in phytoseiid mites by using an olfactometer, predation models and electrophoresis. Exp Appl Acarol 5 225–241 [Google Scholar]
- Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA (1990. a) Plant strategies of manipulating predator–prey interactions through allelochemicals: prospects for application in pest-control. J Chem Ecol 16 3091–3118 [DOI] [PubMed] [Google Scholar]
- Dicke M, Vanbeek TA, Posthumus MA, Bendom N, Vanbokhoven H, Degroot AE (1990. b) Isolation and identification of volatile kairomone that affects acarine predator–prey interactions: involvement of host plant in its production. J Chem Ecol 16 381–396 [DOI] [PubMed] [Google Scholar]
- Duffey SS, Stout MJ (1996) Antinutritive and toxic components of plant defense against insects. Arch Insect Biochem Physiol 32 3–37 [Google Scholar]
- Engel E, Baty C, Le Corre D, Souchon I, Martin N (2002) Flavor-active compounds potentially implicated in cooked cauliflower acceptance. J Agric Food Chem 50 6459–6467 [DOI] [PubMed] [Google Scholar]
- Feeny P, Stadler E, Ahman I, Carter M (1989) Effects of plant odor on oviposition by the black swallowtail butterfly, Papilio polyxenes (Lepidoptera, Papilionidae). J Insect Behav 2 803–827 [Google Scholar]
- Frost CJ, Appel M, Carlson JE, De Moraes CM, Mescher MC, Schultz JC (2007) Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol Lett 10 490–498 [DOI] [PubMed] [Google Scholar]
- Giri AP, Wunsche H, Mitra S, Zavala JA, Muck A, Svatos A, Baldwin IT (2006) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant's proteome. Plant Physiol 142 1621–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth H, Grosch W (1991) A comparative-study of the potent odorants of different virgin olive oils. Fett Wissenschaft Technologie/Fat Science Technology 93 335–339 [Google Scholar]
- Hagan SO, Dunn WB, Knowles JD, Broadhurst D, Williams R, Ashworth JJ, Cameron M, Kell DB (2007) Closed-loop, multiobjective optimization of two-dimensional gas chromatography/mass spectrometry for serum metabolomics. Anal Chem 79 464–476 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Gase K, Hui DQ, Schmidt DD, Baldwin IT (2003) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiol 131 1894–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Kessler A, Kahl J, Lorenz A, Baldwin IT (2000) Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia 124 408–417 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 125 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Ziegler J, Keinanen M, Baldwin IT (2004) Silencing of hydroperoxide lyase and allene oxide synthase reveals substrate and defense signaling crosstalk in Nicotiana attenuata. Plant J 40 35–46 [DOI] [PubMed] [Google Scholar]
- Hamberg M, de Leon IP, Sanz A, Castresana C (2002) Fatty acid alpha-dioxygenases. Prostaglandins Other Lipid Mediat 68-69 363–374 [DOI] [PubMed] [Google Scholar]
- Hamberg M, Sanz A, Rodriguez MJ, Calvo AP, Castresana C (2003) Activation of the fatty acid alpha-dioxygenase pathway during bacterial infection of tobacco leaves: formation of oxylipins protecting against cell death. J Biol Chem 278 51796–51805 [DOI] [PubMed] [Google Scholar]
- Holopainen JK (2004) Multiple functions of inducible plant volatiles. Trends Plant Sci 9 529–533 [DOI] [PubMed] [Google Scholar]
- Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M (2002) Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J Chromatogr A 976 265–275 [DOI] [PubMed] [Google Scholar]
- Johne AB, Weissbecker B, Schutz S (2006) Volatile emissions from Aesculus hippocastanum induced by mining of larval stages of Cameraria ohridella influence oviposition by conspecific females. J Chem Ecol 32 2303–2319 [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT (1997) Induced Responses to Herbivory. University of Chicago Press, Chicago
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Diezel C, Baldwin IT (2006) Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 148 280–292 [DOI] [PubMed] [Google Scholar]
- Kost C, Heil M (2006) Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol 94 619–628 [Google Scholar]
- Kováts E (1958) Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1. Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv Chim Acta 41 1915–1932 [Google Scholar]
- Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT (2002) Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12 177–183 [Google Scholar]
- Kusano M, Fukushima A, Kobayashi M, Hayashi N, Jonsson P, Moritz T, Ebana K, Saito K (2007) Application of a metabolomic method combining one-dimensional and two-dimensional gas chromatography-time-of-flight/mass spectrometry to metabolic phenotyping of natural variants in rice. J Chromatogr B Analyt Technol Biomed Life Sci 855 71–79 [DOI] [PubMed] [Google Scholar]
- Landolt PJ, Tumlinson JH, Alborn DH (1999) Attraction of Colorado potato beetle (Coleoptera: Chrysomelidae) to damaged and chemically induced potato plants. Environ Entomol 28 973–978 [Google Scholar]
- Matsui K (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9 274–280 [DOI] [PubMed] [Google Scholar]
- Mattiacci L, Dicke M, Posthumus MA (1995) β-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA 92 2036–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldau S, Wu J, Baldwin IT (2009) Silencing two herbivory-activated MAP kinases, SIPK and WIPK, does not increase Nicotiana attenuata's susceptibility to herbivores in the glasshouse and in nature. New Phytol 181 161–173 [DOI] [PubMed] [Google Scholar]
- Mithöfer A, Wanner G, Boland W (2005) Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol 137 1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser RO, Cipollini DF, Hum-Musser SM, Williams SA, Brown JK, Felton GW (2005) Evidence that the caterpillar salivary enzyme glucose oxidase provides herbivore offense in solanaceous plants. Arch Insect Biochem Physiol 58 128–137 [DOI] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW (2002) Herbivory: caterpillar saliva beats plant defences. A new weapon emerges in the evolutionary arms race between plants and herbivores. Nature 416 599–600 [DOI] [PubMed] [Google Scholar]
- Pare PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121 325–331 [PMC free article] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT (2006) Using ‘mute’ plants to translate volatile signals. Plant J 45 275–291 [DOI] [PubMed] [Google Scholar]
- Quiroz A, Pettersson J, Pickett JA, Wadhams LJ, Niemeyer HM (1997) Semiochemicals mediating spacing behavior of bird cherry-oat aphid, Rhopalosiphum padi feeding on cereals. J Chem Ecol 23 2599–2607 [Google Scholar]
- Roda A, Halitschke R, Steppuhn A, Baldwin IT (2004) Individual variability in herbivore-specific elicitors from the plant's perspective. Mol Ecol 13 2421–2433 [DOI] [PubMed] [Google Scholar]
- Ruther J (2000) Retention index database for identification of general green leaf volatiles in plants by coupled capillary gas chromatography-mass spectrometry. J Chromatogr A 890 313–319 [DOI] [PubMed] [Google Scholar]
- Sabelis MW, van de Baan HE (1983) Location of distant spider-mite colonies by phytoseiid predators: demonstration of specific kairomones emitted by Tetranychus urticae and Panonychus ulmi. Entomol Exp Appl 33 303–314 [Google Scholar]
- Schmelz EA, Carroll MJ, LeClere S, Phipps SM, Meredith J, Chourey PS, Alborn HT, Teal PEA (2006) Fragments of ATP synthase mediate plant perception of insect attack. Proc Natl Acad Sci USA 103 8894–8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwachtje J, Minchin PEH, Jahnke S, van Dongen JT, Schittko U, Baldwin IT (2006) SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc Natl Acad Sci USA 103 12935–12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellie R, Marriott P, Morrison P (2001) Concepts and preliminary observations on the triple-dimensional analysis of complex volatile samples by using GCxGC-TOFMS. Anal Chem 73 1336–1344 [Google Scholar]
- Shellie RA, Welthagen W, Zrostlikova J, Spranger J, Ristow M, Fiehn O, Zimmermann R (2005) Statistical methods for comparing comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry results: metabolomic analysis of mouse tissue extracts. J Chromatogr A 1086 83–90 [DOI] [PubMed] [Google Scholar]
- Shimoda T, Takabayashi J, Ashihara W, Takafuji A (1997) Response of predatory insect Scolothrips takahashii toward herbivore-induced plant volatiles under laboratory and field conditions. J Chem Ecol 23 2033–2048 [Google Scholar]
- Shulaev V, Cortes D, Miller G, Mittler R (2008) Metabolomics for plant stress response. Physiol Plant 132 199–208 [DOI] [PubMed] [Google Scholar]
- Spiteller D, Dettner K, Boland W (2000) Gut bacteria may be involved in interactions between plants, herbivores and their predators: microbial biosynthesis of N-acylglutamine surfactants as elicitors of plant volatiles. Biol Chem 381 755–762 [DOI] [PubMed] [Google Scholar]
- Spiteller D, Pohnert G, Boland W (2001) Absolute configuration of volicitin, an elicitor of plant volatile biosynthesis from lepidopteran larvae. Tetrahedron Lett 42 1483–1485 [Google Scholar]
- Stowe KA, Marquis RJ, Hochwender CG, Simms EL (2000) The evolutionary ecology of tolerance to consumer damage. Annu Rev Ecol Syst 31 565–595 [Google Scholar]
- Triqui R, Reineccius GA (1995) Changes in flavor profiles with ripening of anchovy (Engraulis encrasicholus). J Agric Food Chem 43 1883–1889 [Google Scholar]
- Tu BP, Mohler RE, Liu JC, Dombek KM, Young ET, Synovec RE, McKnight SL (2007) Cyclic changes in metabolic state during the life of a yeast cell. Proc Natl Acad Sci USA 104 16886–16891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TCJ, Bernasconi M, Bertossa R, Bigler F, Caloz G, Dorn S (1998) The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol Control 11 122–129 [Google Scholar]
- Turlings TCJ, Tumlinson JH, Eller FJ, Lewis WJ (1991. a) Larval-damaged plants: source of volatile synomones that guide the parasitoid Cotesia marginiventris to the microhabitat of its hosts. Entomol Exp Appl 58 75–82 [Google Scholar]
- Turlings TCJ, Tumlinson JH, Heath RR, Proveaux AT, Doolittle RE (1991. b) Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J Chem Ecol 17 2235–2251 [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250 1251–1253 [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Wäckers F (2004) Recruitment of predators and parasitoids by herbivore-injured plants. Insect Chemical Ecology 2 21–75 [Google Scholar]
- Van Dam NM, Poppy GM (2008) Why plant volatile analysis needs bioinformatics: detecting signal from noise in increasingly complex profiles. Plant Biol 10 29–37 [DOI] [PubMed] [Google Scholar]
- van Poecke RMP, Dicke M (2004) Indirect defence of plants against herbivores: using Arabidopsis thaliana as a model plant. Plant Biol 6 387–401 [DOI] [PubMed] [Google Scholar]
- von Dahl CC, Havecker M, Schlogl R, Baldwin IT (2006) Caterpillar-elicited methanol emission: a new signal in plant-herbivore interactions? Plant J 46 948–960 [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Meldau S, Baldwin IT (2007) Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 19 1096–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga N, Aboshi T, Ishikawa C, Fukui M, Shimoda M, Nishida R, Lait CG, Tumlinson JH, Mori N (2007) Fatty acid amides, previously identified in caterpillars, found in the cricket Teleogryllus taiwanemma and fruit fly Drosophila melanogaster larvae. J Chem Ecol 33 1376–1381 [DOI] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Baldwin IT (2004) Constitutive and inducible trypsin proteinase inhibitor production incurs large fitness costs in Nicotiana attenuata. Proc Natl Acad Sci USA 101 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.