Abstract
Sulfated polysaccharides (SP) such as heparin are known to exhibit a wide range of biological activities, e.g., anticoagulant, anti-inflammatory, and antimetastastic effects. However, since the anticoagulant activity of heparin is dominating, its therapeutic use for other medical indications is limited due to an associated risk of bleeding. Further disadvantages of heparin are its animal origin, the shortage of resources, and its complex and variable composition. However, SP without these limitations may represent a substance class with good prospects for applications other than anticoagulation. In this study, the in vitro pharmacological profiles of two nonanimal-derived SP were investigated in comparison with unfractionated heparin. One is the natural SP fraction from the red algae Delesseria sanguinea (D.s.-SP). The other one is the chemically defined PS3, a semisynthetic β-1,3-glucan sulfate with proven in vivo anti-inflammatory and antimetastatic activities. All three polysaccharides were examined in vitro for their inhibitory effects on the coagulation and complement system, polymorphonuclear neutrophil elastase, hyaluronidase, matrix metalloproteinase-1, heparanase, and p-selectin-mediated cell adhesion. Compared with heparin, the nonanimal-derived polysaccharides have a four times weaker anticoagulant activity, but mostly exhibit stronger (1.4–224 times) effects on test systems investigating targets of inflammation or metastasis. According to their different structures, PS3 and D.s.-SP differ in their pharmacological profile with PS3 being the strongest inhibitor of heparanase and cell adhesion and D.s.-SP being the strongest inhibitor of hyaluronidase and complement activation. Considering both pharmacological profile and pharmaceutical quality parameters, PS3 represents a candidate for further development as an anti-inflammatory or antimetastatic drug whereas D.s.-SP might have perspectives for cosmetic applications.
Keywords: Delesseria sanguinea, glucan sulfate, heparin, pharmacology, PS3
Introduction
Heparins still represent the drug of choice for prophylaxis and therapy of thromboembolic diseases. Their anticoagulant activity is mainly based on the antithrombin-mediated inhibition of FXa and thrombin. In addition, other antithrombin-independent mechanisms like catalysis of heparin cofactor II-mediated thrombin inhibition or release of the tissue factor pathway inhibitor contribute to their anticoagulant and antithrombotic activities (Alban 2008b). Besides the inhibition of coagulation, heparins exhibit many other biological activities which are involved in its overall therapeutic efficacy. For example, their well-known interferences with the complement system contribute to the improved biocompatibility of heparin-coated medical devices (Hsu 2001). Further, the survival benefit of tumor patients treated with low-molecular-weight heparins (LMWH) observed in clinical studies currently attracts attention (Akl et al. 2007; Lazo-Langner et al. 2007). The reduced mortality correlates with experimental animal data on antimetastatic, antiangiogenic, and anti-inflammatory effects of heparins (Smorenburg and van Noorden 2001; Lever and Page 2002; Ludwig et al. 2006; Norrby 2006). These are rather the result of a concert of actions targeting the (patho)physiological triad of hemostasis, inflammation, and metastasis than to be due to a single mechanism (Alban 2008a). One of the mechanisms currently discussed to be important (Buller et al. 2007) is the inhibition of cell adhesion to the vascular endothelium, which is initiated by selectins, and finally leads to the extravasation of inflammatory and metastasizing tumor cells (Borsig 2004). Another one is the inhibition of extracellular matrix-degrading enzymes enabling these cells to migrate through tissues, to invade into vessels, and to extravasate, and also supporting angiogenesis and tumor growth (McCachren 1991; Nelson et al. 2000; Parish et al. 2001; Girard et al. 2002; Posey et al. 2003; Taggart et al. 2005; Sato et al. 2006; Vlodavsky et al. 2006).
However, the strong anticoagulant potency limits the applicability for other therapeutic indications. A further disadvantage of heparins is their animal origin for several reasons. First, drug substances of animal origin should be avoided according to the internationally implemented “precautionary principle” (Alban 2005b). Second, heparin preparations vary considerably depending on the source of raw material and extraction process. Third, they have to be applied in units because of batch-to-batch variability of the highly complex glycosaminoglycan mixtures (Alban 2005a). Finally, the increasing demand for heparins is in conflict with the limited resources. The relevance of the latter has recently become manifested by the “heparin scandal” caused by counterfeit heparin (Food and Drug Administration 2008).
Besides the vertebrate glycosaminoglycans (GAG), marine organisms are rich sources for SP, such as carrageenans from red algae or fucoidans from brown algae. A representative of this class is the SP fraction isolated from the red algae Delesseria sanguinea (Hudson) Lamouroux (D.s.). D.s. turned out to be the dominating algae of an artificial reef installed in the Baltic Sea close to Nienhagen, Germany, several years ago. By an optimized and standardized procedure, these D.s.-SP, consisting of xylose-branched galactan sulfates, can be obtained in a reproducible quality (Grünewald et al., in preparation).
Such natural SP might be suitable as ingredients of cosmetics or food supplements but not for the development of medical products because they would hardly meet the current high requirements on pharmaceutical quality due to their complex structure composition and biovariability. In contrast, chemically defined synthetic or semisynthetic glycan sulfates may be rather promising for the development of new drug substances. A prominent example is fondaparinux, an approved antithrombotic drug. Another one is the β-1,3-glucan sulfate PS3 which showed to have both anti-inflammatory (Yvin et al. 2002; Alban et al. 2008) and antimetastatic effects on animal models (Alban et al. 2005) without the disadvantages of heparins.
In the present study, we evaluated the pharmacological profiles of D.s.-SP and PS3 in comparison with unfractionated heparin (UFH). Besides investigations of anticoagulant activity and cytotoxicity, inhibition of complement activation, inhibitory effects on extracellular matrix (ECM)-degrading enzymes (polymorphonuclear neutrophil elastase (PMNE), matrix metalloproteinase-1 (MMP-1), hyaluronidase, and heparanase) as well as inhibition of tumor cell adhesion to p-selectin are presented.
Results
The pharmacological profile of three SP – UFH, PS3, and D.s.-SP – was determined. These three selected SP differ in their origin as well as in their structural characteristics; UFH is a glucosaminoglycan derived from porcine mucosa and D.s.-SP is the non-gelling SP fraction isolated from the red algae D. sanguinea consisting of sulfated xylogalactans. In contrast to these two natural SP, PS3 is a well-defined β-1,3-glucan sulfate, which is produced by semisynthetic modification of Phycarine™ from Laminaria digitata (Table I). To estimate the anticoagulant, anti-inflammatory, and antimetastatic potential of the test compounds, corresponding in vitro test systems were established. As far as synthetic or structurally defined inhibitors of the examined targets are known, these were included in the tests: GW311616A as PMNE inhibitor, FN-439 as MMP-1 inhibitor, and escin as hyaluronidase inhibitor.
Table I.
Sources and structural characterization of UFH, PS3, and D.s.-SP
| UFH | PS3 | D.s.-SP | |
|---|---|---|---|
| Source | Porcine intestinal mucosa | Semisynthetically modified PhycarineTM from Laminaria digitata | Delesseria sanguinea |
| Molecular mass (Mr) | 5000–30,000a | 9000–11,000a | n.d. |
| Hydrodynamic volume (SEC)b | 10,000–60,000 | 16,000–20,000 | 25,000–280,000 |
| Degree of sulfationc | 1.2 | 2.2d | 0.5 |
| Basic structure | Linear glucosaminoglycan | Linear β-1,3-glucan | Branched xylogalactan |
aMr values of UFH are obtained from literature, those of PS3 were determined by ESI-MS.
bSize exclusion chromatography with neutral pullulans as molecular weight standards.
cSulfate groups per monosaccharide.
Potential cytotoxic effects were checked with LDH (lactate dehydrogenase) and MTT assays. According to the LDH assay, no direct cytotoxic effects of UFH, PS3, or D.s.-SP on several human tumor cell lines or leukocytes were found. As revealed by the MTT assay, the test compounds did not influence tumor cell proliferation (data not shown).
The results of the activities in the various assay systems are presented as concentration-dependent curves (Figures 1–8) and doubling concentration (DC – inhibitor concentration causing a prolongation of the coagulation time to the 2-fold time of the negative control) or IC50 values (Table II), respectively. The final assay concentrations of the SP are given in μg/mL. Only in the case of the semisynthetic PS3, which consists of a homogeneously sulfated β-1,3-glucan with an average degree of polymerization of 25 and a low polydispersity, the indication of molar concentrations is acceptable. Thus, a comparison with chemically defined entities on a molar basis is possible (Figures 4B, 5B, and 6B).
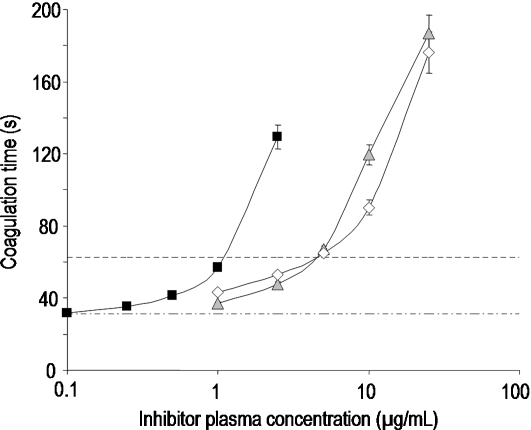
Fig. 1.
Prolongation of coagulation time in dependence on the inhibitor concentration in activated partial thromboplastin time (aPTT) by ▪ UFH,  PS3, and ⋄ D.s.-SP, concentrations given in μg/mL. All standard deviations are derived from assays performed on at least three different days. Calculated doubling concentrations differ significantly (P ≤ 0.05) for UFH–PS3, UFH–D.s.-SP, but not for PS3–D.s.-SP.
PS3, and ⋄ D.s.-SP, concentrations given in μg/mL. All standard deviations are derived from assays performed on at least three different days. Calculated doubling concentrations differ significantly (P ≤ 0.05) for UFH–PS3, UFH–D.s.-SP, but not for PS3–D.s.-SP.
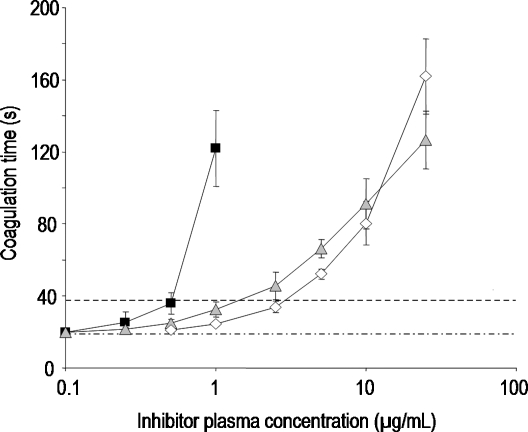
Fig. 2.
Prolongation of coagulation time in dependence on the inhibitor concentration in thrombin time (TT) by ▪ UFH,  PS3, and ⋄ D.s.-SP, concentrations given in μg/mL. All standard deviations are derived from assays performed on at least three different days. Calculated doubling concentrations differ significantly (P ≤ 0.05) for UFH–PS3, UFH–D.s.-SP, but not for PS3–D.s.-SP.
PS3, and ⋄ D.s.-SP, concentrations given in μg/mL. All standard deviations are derived from assays performed on at least three different days. Calculated doubling concentrations differ significantly (P ≤ 0.05) for UFH–PS3, UFH–D.s.-SP, but not for PS3–D.s.-SP.
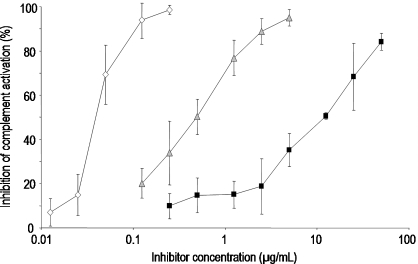
Fig. 3.
Concentration-dependent inhibition curves for the inhibition of complement activation by ▪ UFH,  PS3, and ⋄ D.s.-SP, concentrations given in μg/mL. All standard deviations are derived from assays performed on at least three different days. Calculated IC50 values differ significantly (P ≤ 0.05) for every pair of inhibitors.
PS3, and ⋄ D.s.-SP, concentrations given in μg/mL. All standard deviations are derived from assays performed on at least three different days. Calculated IC50 values differ significantly (P ≤ 0.05) for every pair of inhibitors.
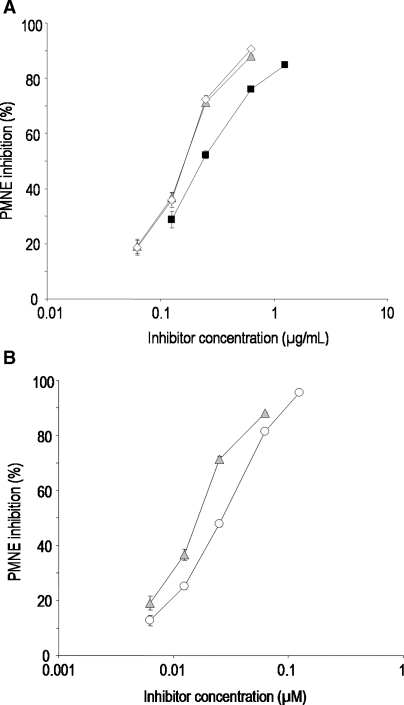
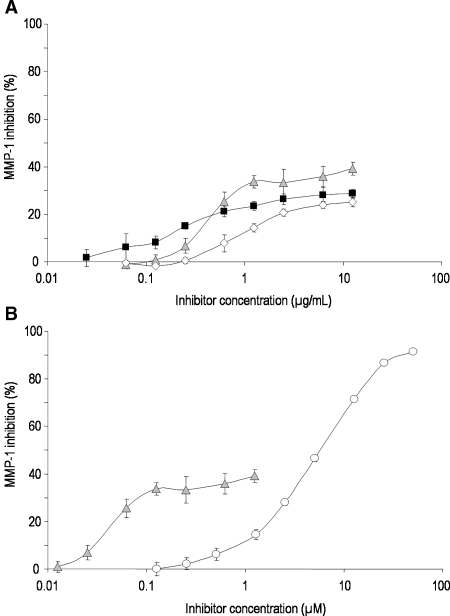
Fig. 4.
Inhibition of polymorphonuclear neutrophil elastase (PMNE) by ▪ UFH,  PS3, ⋄ D.s-SP, and the reference substance ○ GW311616A. All standard deviations are derived from assays performed on at least three different days. (A) Concentration-dependent inhibition curves of UFH, PS3, and D.s.-SP, concentrations given in μg/mL. Calculated IC50 values differ significantly (P ≤ 0.05) for UFH–PS3, UFH–D.s.-SP, but not for PS3–D.s.-SP. (B) Concentration-dependent inhibition curves of PS3 and GW311616A, concentrations given in μM.
PS3, ⋄ D.s-SP, and the reference substance ○ GW311616A. All standard deviations are derived from assays performed on at least three different days. (A) Concentration-dependent inhibition curves of UFH, PS3, and D.s.-SP, concentrations given in μg/mL. Calculated IC50 values differ significantly (P ≤ 0.05) for UFH–PS3, UFH–D.s.-SP, but not for PS3–D.s.-SP. (B) Concentration-dependent inhibition curves of PS3 and GW311616A, concentrations given in μM.
Fig. 5.
Inhibition of matrix metalloproteinase-1 (MMP-1) by ▪ UFH,  PS3, ⋄ D.s.-SP, and the reference substance ○ FN-439. All standard deviations are derived from assays performed on at least three different days. (A) Concentration-dependent inhibition curves of UFH, PS3, and D.s.-SP, concentrations given in μg/mL. IC50 values could not be calculated. (B) Concentration-dependent inhibition curves of PS3 and FN-439, concentrations given in μM.
PS3, ⋄ D.s.-SP, and the reference substance ○ FN-439. All standard deviations are derived from assays performed on at least three different days. (A) Concentration-dependent inhibition curves of UFH, PS3, and D.s.-SP, concentrations given in μg/mL. IC50 values could not be calculated. (B) Concentration-dependent inhibition curves of PS3 and FN-439, concentrations given in μM.
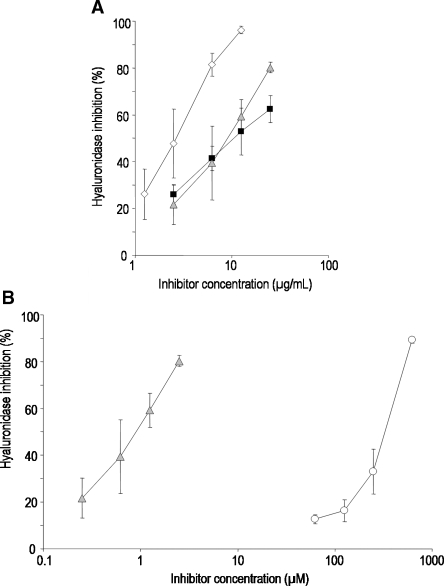
Fig. 6.
Inhibition of hyaluronidase by ▪ UFH,  PS3, ⋄ D.s.-SP, and the reference substance ○ escin. All standard deviations are derived from assays performed on at least three different days. (A) Concentration-dependent inhibition curves of UFH, PS3, and D.s.-SP, concentrations given in μg/mL. Calculated IC50 values differ significantly (P ≤ 0.05) for D.s.-SP–PS3, D.s.-SP–UFH, but not for UFH–PS3. (B) Concentration-dependent inhibition curves of PS3 and escin, concentrations given in μM.
PS3, ⋄ D.s.-SP, and the reference substance ○ escin. All standard deviations are derived from assays performed on at least three different days. (A) Concentration-dependent inhibition curves of UFH, PS3, and D.s.-SP, concentrations given in μg/mL. Calculated IC50 values differ significantly (P ≤ 0.05) for D.s.-SP–PS3, D.s.-SP–UFH, but not for UFH–PS3. (B) Concentration-dependent inhibition curves of PS3 and escin, concentrations given in μM.
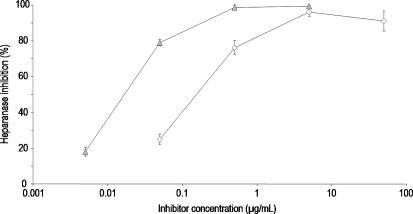
Fig. 7.
Concentration-dependent inhibition of heparanase by  PS3, and ⋄ D.s.-SP, concentrations given in μg/mL. UFH both inhibits heparanase and represents a substrate. Consequently, no valid inhibition data for UFH can be obtained. All standard deviations are derived from assays performed on at least three different days. Calculated IC50 values differ significantly (P ≤ 0.05).
PS3, and ⋄ D.s.-SP, concentrations given in μg/mL. UFH both inhibits heparanase and represents a substrate. Consequently, no valid inhibition data for UFH can be obtained. All standard deviations are derived from assays performed on at least three different days. Calculated IC50 values differ significantly (P ≤ 0.05).
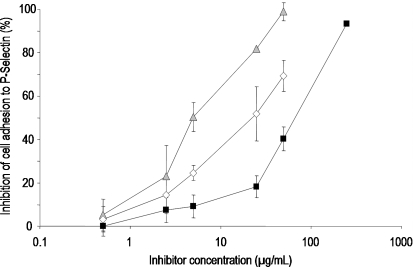
Fig. 8.
Concentration-dependent inhibition of tumor cell adhesion to p-selectin by ▪ UFH,  PS3, and ⋄ D.s.-SP, concentrations given in μg/mL. All standard deviations are derived from assays performed on at least three different days. Calculated IC50 values differ significantly (P ≤ 0.05) for every pair of inhibitors.
PS3, and ⋄ D.s.-SP, concentrations given in μg/mL. All standard deviations are derived from assays performed on at least three different days. Calculated IC50 values differ significantly (P ≤ 0.05) for every pair of inhibitors.
Table II.
Doubling concentrations (DC – inhibitor concentration causing a prolongation of the coagulation time to the 2-fold time of the negative control) and IC50 values of UFH, PS3, and D.s.-SP in the different test systems. All standard deviations are derived from assays performed on at least three different days
| UFH | PS3 | D.s.-SP | Reference substances | |
|---|---|---|---|---|
| Anticoagulant activity | 190 IU/mg | 38.2 IU/mg | 36.4 IU/mg | |
| DC (mean ± SD) (μg/mL) | ||||
| aPTT | 1.13 ± 0.01 | 4.40 ± 0.07 | 4.62 ± 0.22 | |
| TT | 0.552 ± 0.334 | 2.04 ± 0.53 | 2.80 ± 0.49 | |
| IC50 (mean ± SD) (μg/mLa or μmol/Lb) | ||||
| Complement modulation assay | 10.3 ± 2.2a | 0.502 ± 0.117a | 0.046 ± 0.012a | |
| PMNE inhibition | 0.236 ± 0.011a | 0.163 ± 0.004a 0.016 ± 0.001b | 0.164 ± 0.007a | GW311616A 0.026 ± 0.001b |
| MMP-1 inhibition | – | – | – | FN-439 5.69 ± 0.17b |
| Hyaluronidase inhibition | 9.00 ± 2.08a | 8.68 ± 3.09a 0.868 ± 0.309b | 2.75 ± 1.01a | escin 344.5 ± 92.3b |
| Heparanase inhibition | – | 0.020 ± 0.002a | 0.195 ± 0.011a | – |
| Inhibition of P-selectin binding | 65.4 ± 19.5a | 5.98 ± 2.98a | 22.7 ± 8.4a | – |
a = IC50 in μg/mL; b = IC50 in μmol/L.
To evaluate the anticoagulant activity, two coagulation assays were performed: the global activated partial thromboplastin time (aPTT) detecting any influence on the intrinsic coagulation pathway and the thrombin time (TT) measuring interferences with the thrombin-mediated fibrin formation. The DC values of PS3 and D.s.-SP were about four times higher than those of UFH reflecting only moderate anticoagulant activity (Figures 1 and 2).
Further, anti-complementary activity was examined by means of complement-induced hemolysis of antibody-sensitized erythrocytes with measurement of the released hemoglobin. All three SP reduced the extent of hemolysis and thus represent inhibitors of the classical pathway of complement activation. But compared with UFH, PS3 was 20 times and D.s.-SP even 224 times more potent (Figure 3).
To screen the inhibitory potency on ECM degradation, enzyme activity assays with two proteases, PMNE and MMP-1, and two glycosaminoglycan-degrading enzymes, hyaluronidase and heparanase, were performed.
In the PMNE assay, the activity of the purified enzyme is determined with a synthetic substrate in a buffered system. Here, the equally active PS3 and D.s.-SP are significantly but not much stronger inhibitors than UFH exhibiting a 1.4 times higher activity (Figure 4A). Similarly, PS3 is 1.6 times more active than the synthetic inhibitor GW311616A (Figure 4B). The superiority to UFH was confirmed by a fluorimetric assay using FITC-labeled elastin as a substrate (data not shown).
MMP-1 activity was examined in an assay based on the same principle as the PMNE assay. While the synthetic inhibitor FN-439 reveals inhibitions of up to 90% with an IC50 of 5.69 ± 0.17 μmol/L (Figure 5B, Table II), the SP UFH, PS3, and D.s.-SP show only low maximal inhibitions of less than 50% (Figure 5A) and higher interassay variations.
For the determination of inhibitory effects on hyaluronidase, the cleavage of its natural substrate hyaluronic acid was quantified by converting the resulting fragments into colored products. While the potency of PS3 does not significantly differ from that of UFH, D.s.-SP is a 3.3 times stronger inhibitor than UFH (Figure 6A, Table II). According to the observed superiority of PS3 to escin (Figure 6B), the investigated SP represent by several orders of magnitude stronger hyaluronidase inhibitors.
In the heparanase assay, PS3 and D.s.-SP cannot be compared with UFH since the latter both inhibits the enzyme and represents a substrate. Consequently, no valid inhibition data for UFH can be obtained. Inversely to the activity against hyaluronidase, PS3 is a stronger heparanase inhibitor than D.s.-SP (Figure 7).
As a simple screening method to assess influences on cell adhesion to p-selectin, a static microplate assay was used. Again, both PS3 and D.s.-SP exhibit stronger inhibitory effects than UFH with PS3 being even stronger than D.s-SP (Figure 8).
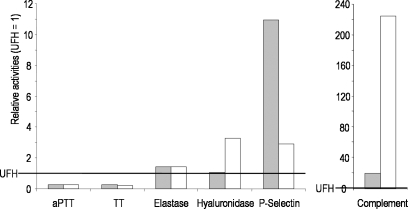
As obvious from Figure 9, each of the tested SP has an individual pharmacological profile. In that of UFH, the anticoagulant activity clearly dominates. This is known to be mediated by 30–50% of its molecules containing the specific pentasaccharide sequence (Mulloy 2005; Alban 2008a) which binds to antithrombin and thus catalyzes the inhibition of thrombin and factor Xa. According to a chromogenic substrate assay on the antithrombin-mediated inhibition of factor Xa, neither D.s.-SP nor PS3 have any antithrombin-mediated activity (data not shown), which explains their moderate anticoagulant activity. But concerning the other activities, they represent generally more or less stronger inhibitors than UFH. D.s.-SP, which has a relatively low degree of sulfation (DS), but a high molecular weight (Mr), impresses by its inhibitory activity on complement activation and hyaluronidase whereas PS3, the SP with the highest DS and the lowest Mr of the three examined SP, is the most potent in the cell adhesion and heparanase inhibition assays.
Fig. 9.
Activities of  PS3 and ⋄ D.s.-SP in relation to UFH. UFH activity is equated with 1. Relative activities >1 express a higher potency and those <1 a lower potency than UFH.
PS3 and ⋄ D.s.-SP in relation to UFH. UFH activity is equated with 1. Relative activities >1 express a higher potency and those <1 a lower potency than UFH.
Discussion
In this study, several screening assays were performed to evaluate the potential of PS3 and D.s.-SP as new compounds with anti-inflammatory and antimetastatic activities. The results suggest that these two SP exhibit a wide range of biological activities like heparins do (Alban 2008b). However, compared to UFH, their pharmacological profile is clearly shifted in favor of effects that are considered to be important for in vivo anti-inflammatory and antimetastatic activities.
In this way, our findings exemplarily demonstrate that the activities of SP are not based on unspecific electrostatic interactions with positively charged proteins, but depend on their individual structure. They confirm studies on structure–activity relationships of structurally defined semisynthetic glucan sulfates. The latter revealed that important factors for their pharmacological activities are charge density, chain length, as well as the sulfation pattern and the basic polysaccharide structure (Alban and Franz 1994, 2001; Becker et al. 2003; Fritzsche et al. 2006). Depending on the target, different structural aspects are decisive for the inhibitory potency. Whereas the anticoagulant and PMNE inhibiting activity depend on both DS and Mr, for inhibition of complement activation and hyaluronidase, a high Mr is more important than a high DS. Inversely, to inhibit heparanase, a high DS seems to be more important than high Mr as PS3 is much more potent than D.s.-SP, and also the highly sulfated oligosaccharide mixture PI-88 is a very potent inhibitor of heparanase (Parish et al. 2001).
In addition, sometimes other structural characteristics than Mr and DS are crucial for the activity. For example, the anticoagulant activity of heparin is caused by the interaction with antithrombin via a specific pentasaccharide sequence. Further, the anticoagulant activity of D.s.-SP was superior to that of a κ-carrageenan with similar DS and Mr (data not shown). This could be due to the branched structure of D.s.-SP (Grünewald et al., in preparation) since branched glucan sulfates showed to be stronger anticoagulants than linear ones with similar DS and Mr (Alban and Franz 1994). Concerning PMNE inhibition, the β-1,3-glucan sulfate PS3 was found to be a more potent inhibitor than pentosan polysulfate (PPS), although this sulfated glucuronoxylan has similar Mr and DS (Becker et al. 2003).
These observations agree with the description of cell-surface heparan sulfates as structures specifically interacting with their numerous binding partners by exposing distinct three-dimensional patterns of sulfate substitution (Lindahl et al. 1998; Mulloy 2005). Analogously, heparins and SP, which are structurally related to heparan sulfate, may – in dependence on their individual structure – interfere more or less with processes, where the GAG heparan sulfate or other glycan structures are involved (Alban 2008b). Heparan sulfate shows pronounced structural diversity and has manifold regulatory functions by interactions with a large number of biomolecules such as enzymes, enzyme inhibitors, growth factors, extracellular matrix proteins, cytokines, adhesion molecules, and receptor proteins (Lindahl et al. 1998). In addition, also other GAG and negatively charged glycan structures such as mucins binding to p- and l-selectin are involved in cell–cell and cell–matrix interactions (McEver 1997). This may partly explain why heparins and SP do not principally display only one specific activity, but rather act as multivalent biomodulators. In this study, we found that PS3 not only inhibits the p-selectin-mediated cell adhesion, but also represents a potent heparanase and elastase inhibitor. Although extensive mechanistic studies including experiments with knock-out mice support the relevance of antagonization of p-selectin (Alban et al. 2008), the in vivo anti-inflammatory activity may not only be based on this effect.
Another reason for the multiple activities of heparins and SP is that hemostasis, immune system, and tumor progression including metastasis and angiogenesis represent a (patho)physiological network (Altieri 1995; Coussens and Werb 2002; Opal and Esmon 2003; Bierhaus and Nawroth 2005; Polgar et al. 2005; Rak et al. 2006; Buller et al. 2007). They are closely connected by mechanisms which play a role in several of these processes. This can be exemplified by most targets investigated in this study. The ECM-degrading enzymes PMNE, MMP-1, hyaluronidase, and heparanase are expressed by both inflammatory and tumor cells (McCachren 1991; Nelson et al. 2000; Parish et al. 2001; Girard et al. 2002; Posey et al. 2003; Taggart et al. 2005; Sato et al. 2006; Vlodavsky et al. 2006). They thus enable these cells to pass basal membranes and/or to migrate through tissues. This is essential for inflammatory cells to extravasate and reach the center of inflammation, but is also important for tumor growth and metastasis. p-Selectin, which is expressed by activated endothelial cells and platelets, mediates the rolling of inflammatory cells and thus initiates their extravasation. In addition, it has been recognized to be involved in tumor metastasis (Borsig 2004) as well as in hemostasis and vascular pathologies (André 2004). The complement system, an important part of the innate immune defense, has strong influences on inflammatory processes but also enhances coagulation by activation of platelets, increasing of tissue factor expression in several cell types, etc. which often contributes to life-threatening complications during inflammatory diseases (Markiewski et al. 2007). Interference with any step within this network may therefore be beneficial for the therapy of numerous diseases.
Although this study focuses on effects in simple in vitro assays, the proven in vivo activities of heparin (Smorenburg and van Noorden 2001; Lever and Page 2002; Ludwig et al. 2006; Akl et al. 2007; Lazo-Langner et al. 2007) and PS3 (Yvin et al. 2002; Alban et al. 2005, 2008) strengthen the significance of our observations and support the hypothesis that both PS3 and D.s.-SP may have better anti-inflammatory and antimetastatic activities in vivo than heparin. However, there are big differences between the two test compounds. This concerns not only their structural characteristics and their pharmacological profile, but also their origin and consequently the options for potential applications.
D.s.-SP is a natural product like heparins, fucoidans isolated from brown algae (Berteau and Mulloy 2003), or other biologically active algae polysaccharides such as sulfated glucuronogalactans (SGG) isolated from the red alga Schizymenia dubyi. The latter were shown to have in vitro effects against several viruses, to inhibit the proliferation of NSCLC-N6 tumor cells and to exhibit anticoagulant and anticomplementary activities (Bourgougnon et al. 1994; Alban et al. 1997). However, the SGG revealed high batch-to-batch variability in both structural parameters and pharmacological activities (Bourgougnon et al. 1996). Also fucoidans vary in both their structural composition and their pharmacological profile depending on their origin (Cumashi et al. 2007). Variability represents a common problem of complex structured SP from natural origin, and is, e.g., the cause why heparins have to be applied in International Units based on their in vitro anticoagulant activity instead of milligrams in clinical practice. It might also be one of the reasons that so far fucoidans have not been tested in clinical studies despite numerous promising pharmacological activities such as anticoagulant and antithrombotic effects as well as anti-inflammatory, antiproliferative, antiadhesive, and antiviral ones (Berteau and Mulloy 2003; Mourão 2004).
Although for the production of D.s.-SP only algae from a certain artificial reef are used and D.s.-SP can be obtained in reproducible quality by a specific extraction procedure, it is assumed to be not suitable for drug development, but may be a candidate with good prospects for other applications. Already in 1987, a patent was filed on an emulsion containing 1–5% of an aqueous extract of D. sanguinea, which has been supposed to improve peripheral blood circulation and to relieve vein complaints (Herve et al. 1987). Recently, a cosmetic composition for the treatment and prevention of skin stretch marks was patented, which contains – among other components – an extract of D. sanguinea (Montanari and Guglielmo 2008). Except for the anticoagulant activity of extracts from D. sanguinea (Potin et al. 1992), so far no further pharmacological properties are however known justifying these applications. In this study, we identified some effects of D.s.-SP, i.e., a major component of the extracts, which support the intended use. The inhibition of ECM-degrading enzymes and especially hyaluronidase may suit the claimed purposes by reduction of vascular permeability. Interestingly, D.s.-SP turned out to be considerably more active than escin, a known hyaluronidase inhibitor (Facino et al. 1995). Escin is the active ingredient in horse chestnut seed extract, which has been acknowledged as an efficacious treatment for chronic venous insufficiency (Pittler and Ernst 2006)
In contrast to D.s.-SP, PS3 is a structurally well-defined semisynthetic SP, which is produced according to the good manufacturing practice (GMP) guidelines. It can be compared with PPS and PI-88 as examples for other semisynthetic sulfated poly- or oligosaccharides, respectively, and accordingly represents a promising candidate for drug development. PPS is obtained by sulfation of glucuronoxylans extracted from the bark of Fagus sylvatica. It has been licensed by the FDA for the treatment of interstitial cystitis. Like PS3, PPS has manifold activities which may contribute to its therapeutic efficacy in this indication (Anderson and Perry 2006). In Germany, it is approved for oral supporting treatment of peripheral arterial disease, for parenteral postoperative prophylaxis of thromboembolism, and for topic application in diseases like superficial thrombophlebitis. Compared to PPS, the advantage of PS3 is its more homogenous composition and its lower polydispersity. PI-88 is obtained by hydrolysis and sulfation of yeast-derived phosphomannan (Khachigian and Parish 2004). It has strong inhibitory potency toward heparanase (Parish et al. 2001) and is being developed as an antiangiogenic anticancer agent (Ferro et al. 2007) with approved clinical effects (Lewis et al. 2008). PPS and PI-88 exemplify the potential of semisynthetic SP like PS3 to be successfully introduced as drugs.
Examinations of structurally defined SP in a wide range of in vitro assays together with studies on structure–activity relationships and in vivo experiments may help to identify those modes of action which are most important for the in vivo anti-inflammatory and/or antimetastatic activity of heparins and other SP.
Our results suggest that PS3 would be worth being examined in clinical studies as an anti-inflammatory and/or antimetastatic agent. It resulted as the most promising candidate from extensive structure–activity relationship studies, has been shown to be active in vivo, is produced under GMP-conditions, and already passed preclinical toxicological tests. But also for D.s.-SP, there might be a perspective of application in cosmetics as an anti-inflammatory agent with potential anti-ageing effects due to inhibition of ECM-degrading enzymes.
Material and methods
Test compounds
Unfractionated heparin (UFH) from porcine mucosal origin (Lot-No 73508019, a purity of ≥99% has been verified by the certificate of analysis by Biochemie GmbH Kundl, Austria) was a kind gift from Novartis (Nürnberg, Germany). PS3, a linear β-1,3-glucan sulfate with the in vivo anti-inflammatory activity (US Patent No. US7008931-B2, Mr = 10 kDa, produced under GMP conditions, purity ≥ 99%), was synthesized as previously described (Yvin et al. 2002; Alban et al. 2008). The source of the sulfated polysaccharide fraction D.s.-SP was the red algae D. sanguinea (Hudson) Lamouroux harvested in June 2006 from an artificial reef situated in the Baltic Sea (Nienhagen, Germany). D.s.-SP was isolated following a standard procedure (purity ≥ 95%) (Grünewald et al., in preparation). Important characteristics of these test compounds are shown in Table I.
As reference substances, the synthetic enzyme inhibitors GW311616A (Mr = 433.99) for PMNE (Sigma, Taufkirchen, Germany), FN-439 (Mr = 490.6) for MMP-1 (Calbiochem, Merck KGaA, Darmstadt, Germany), and escin (Mr = 1101.23) for hyaluronidase (Sigma, Taufkirchen, Germany) were examined.
Materials
Microplates were obtained from Nunc (Wiesbaden, Germany). Coagulation assays were performed using the Amelung-coagulometer KC10 macro (Lemgo, Germany), microplates in fluorescence or absorbance assays were read out by the microplate reader Polarstar Optima, BMG Labtech, Jena.
LDH-assay
For the detection of any cytotoxicity, the lactate dehydrogenase (LDH) kit from Roche Diagnostics (Mannheim, Germany) was performed using the tumor cell lines U-937, MONO-MAC-6, and LS-180 as well as with monocytes and polymorphonuclear neutrophils freshly isolated from human blood following the recommended procedure.
MTT assay
This test was performed with the tumor cell lines U-937, MONO-MAC-6, and LS-180 as described by Lindl (2002). Cells were adjusted to a concentration of 105 cells/mL. Hundred milliliters of this cell suspension was mixed with 100 μL of different concentrations of the test substance in a microplate. After incubation at 37°C (5% CO2) for 24 h, 20 μL of sterile MTT solution (MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide, solved in PBS at 5 mg/mL) per well was added. During further 2 h of incubation at 37°C (5% CO2), a blue product emerges. The microplate was centrifuged (250 × g, 5 min), the supernatant removed, and 100 μL of lysis mixture (consisting of 99.4 mL DMSO, 0.6 mL glacial acetic acid, 10 g SDS) was pipetted into each well. After 5 min of incubation at room temperature and 5 min of thorough mixing, the optical density at 570 nm was measured.
Activated partial thromboplastin time
Pooled human platelet-poor plasma from at least 10 healthy volunteers, stored at −80°C, was thawed at 37°C and mixed thoroughly. An aliquot of 90 μL of plasma was added to 10 μL of sample (diluted in NaCl 0.9%). After 60 s of incubation at 37°C, 100 μL of Pathromtin SL® (Dade Behring, Marburg, Germany) was added followed by another incubation time of 120 s. Finally, 100 μL of preheated (37°C) CaCl2 solution (0.025 mol/L, Dade Behring) was added. The time until fibrin clot formation was recorded, and the “doubling concentration” (inhibitor concentration causing a prolongation of the coagulation time to the 2-fold time of the negative control) was determined. NaCl 0.9% without inhibitor served as negative control; UFH was used as reference substance.
Further, the specific activities (IU/mg) of the test compounds were determined according to the European Pharmacopoeia 6.0 by means of a calibration curve of the “5th International Standard for Unfractionated Heparin” (NIBSC code: 97/578).
Thrombin time
Pooled human platelet-poor plasma from at least 10 healthy volunteers, stored at −80°C, was thawed at 37°C and mixed thoroughly. An aliquot of 90 μL of plasma was added to 10 μL of sample (diluted in NaCl 0.9%). After 60 s of incubation at 37°C, 200 μL of preheated (37°C) Test Thrombin Reagent (Dade Behring, Marburg, Germany) was added. The time which passes until the mixture coagulates was recorded. Again the doubling concentration was determined. NaCl 0.9% without inhibitor served as negative control; UFH was used as reference substance.
Elastase inhibition assay
For the inhibition assay, human PMNE (EC 3.4.21.37) from Calbiochem (Merck KGaA, Darmstadt, Germany) was used. Tests were performed in black 96-well microplates. An aliquot of 25 μL of Tris buffer (50 mM Tris, 155 mM NaCl, pH 8.3) was mixed with 25 μL of inhibitor (diluted in NaCl 0.9%) and 25 μL of PMNE (c = 100 nM) in Na acetate buffer (50 mM Na acetate, 200 mM NaCl, 1% BSA, pH 5.5). After 5 min of incubation at 37°C, 25 μL of substrate solution (MeOSuc-Ala-Ala-Pro-Val-7-amido-4-methylcoumarin, Bachem [Weil am Rhein, Germany]), diluted in Tris buffer (c = 3 mM), was added. Fluorescence was measured after 5 min incubation time at 37°C at excitation (Ex) 370 nm, emission (Em) 450 nm. The fluorescence values from blanks were subtracted from all other values. The resulting values were used to determine the concentration-dependent inhibition (%) in relation to the positive control (100%). The synthetic inhibitor GW311616A was used as reference substance.
Hyaluronidase inhibition assay
Tests were performed in 96-well microplates. For the enzyme reaction, 25 μL of phosphate buffer (pH 5.0), 25 μL of inhibitor in 0.9% NaCl, 25 μL hyaluronic acid in water (4 mg/mL), and 25 μL bovine hyaluronidase (80 U/mL) from Sigma (Taufkirchen, Germany) (EC 3.2.1.35) were incubated for 120 min at 37°C. To cleave the terminal N-GlcNAc units from the resulting hyaluronan oligosaccharides, 10 μL of K2B4O7 buffer (pH 10.0) were added, and the covered microplate was incubated for 30 min at 105°C. After cooling, 170 μL of 4-dimethylaminobenzaldehyde (2%) was added, followed by incubation for 30 min at 40°C (Morgan–Elson reaction). The OD at 570 nm was measured versus an enzyme-free blank. The absorbance values from blanks were subtracted from all other values. The resulting values were used to determine the concentration-dependent inhibition (%) in relation to the positive control (100%). Escin was used as reference substance.
MMP-1 inhibition assay
For the inhibition assay, recombinant human MMP-1 (EC 3.4.24.7) (R&D Systems, Wiesbaden, Germany) was used. For activation of MMP-1, it was incubated for 3 h at 37°C with the 1 mM p-aminophenylmercuric acetate solution (Sigma, Taufkirchen, Germany). Enzyme inhibition tests were performed in black 96-well microplates. An aliquot of 25 μL of Tris buffer (50 mM Tris, 150 mM NaCl, 10 mM CaCl2, 0.05% Brij 35, pH 7.5) was mixed with 25 μL of inhibitor (diluted in NaCl 0.9%) and 25 μL of activated MMP-1 (c = 0.5 μg/mL in Tris buffer). After incubation for 5 min at 37°C, 25 μL of substrate solution (ES001 = Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2, c = 20 μM in Tris buffer, R&D Systems) was added. After another incubation time of 60 min at 37°C, fluorescence intensity was measured at Ex 320 nm, Em 405 nm. The fluorescence values from blanks were subtracted from all other values. The resulting values were used to determine the concentration-dependent inhibition (%) in relation to the positive control (100%). The synthetic inhibitor 4-Abz-Gly-Pro-d-Leu-d-Ala-NHOH (FN-439, Calbiochem, Merck KGaA, Darmstadt, Germany) was used as reference substance.
Hemolytic complement modulation assay
An aliquot of 75 μL of inhibitor dilution in the veronal buffer (Virion Serion, Würzburg, Germany) was mixed with 50 μL of antibody-sensitized sheep erythrocytes (Virion-Serion) and 25 μL of a dilution of human pooled serum in a V-microplate. After incubation for 45 min at 37°C, the microplate was centrifuged, and the supernatants were transferred to a flat bottom microplate. The optical density was determined at 405 nm after dilution with 100 μL deionized water per well.
Heparanase inhibition assay
The assay was performed in microplates using a novel test principle developed by Schiemann and Alban (in preparation).
P-Selectin binding assay
This test was performed with the monocytic cell line U937. Microplates (Maxisorp™) were coated with 50 μL p-selectin (human, recombinant, R&D-Systems) in PBS without Ca2+/Mg2+ (c = 3 μg/mL) for 1 h. After blocking with BSA 1% in PBS without Ca2+/Mg2+ for 1 h, microplates were washed with PBS without Ca2+/Mg2+. Then, 100 μL of inhibitor in PBS without Ca2+/Mg2+ was added followed by centrifugation (250 × g, 2 min, 20°C) and an incubation time of 10 min at room temperature (RT). The addition of 100 μL of cell suspension (c = 0.5 × 106 cells/mL in RPMI without phenol red) was followed by another incubation time of 10 min at 37°C. Unbound cells and buffer were removed, and microplates were washed twice with PBS without Ca2+/Mg2+. The cells which bound to p-selectin were quantified by the addition of 50 μL Triton X-100 solution 0.2% and subsequent detection of released LDH with the Cytotoxicity Kit® from Roche Diagnostics (Mannheim, Germany).
Statistical analysis
All liquid assay measurements were done in duplicate and repeated at least three times on different days (n ≥ 6); cell assay measurements were done in triplicate and repeated at least three times as well (n ≥ 9). All data are presented as mean ± standard deviation (SD) of the mean from n experiments. For determination of doubling concentrations and IC50 values, data were analyzed by nonlinear curve fitting using the program Sigma Plot 8.0. IC50 values were determined for each experiment (n > = 6), resulting in the indicated mean values ± SD. Statistical analysis was performed using Student's t-test; P ≤ 0.05 was considered as statistically significant.
Funding
The EU (FIFG – Financial Instrument for Fisheries Guidance); the LFALF (Landesforschungsanstalt für Landwirtschaft und Fischerei) Mecklenburg-Vorpommern; GOEMAR Laboratories (St. Malo, France). Funding to pay the Open Access publication charges for this article were provided by the EU (Financial Instrument for Fisheries Guidance); the Landesforschungsanstalt für Landwirtschaft und Fischerei Mecklenburg-Vorpommern.
Acknowledgments
We are grateful to Thomas Mohr and Dr. Christof Schygula for delivery of the Delesseria sanguinea algae material. We thank Simone Schiemann for the determination of heparanase inhibitory activities.
Conflict of interest statement
None declared.
Abbreviations
- aPTT
activated partial thromboplastin time
- DC
doubling concentration
- DS
degree of sulfation
- D.s.
Delesseria sanguinea (Hudson) Lamouroux
- D.s.-SP
sulfated polysaccharides from Delesseria sanguinea
- ECM
extracellular matrix
- FDA
food and drug administration
- GAG
glycosaminoglycans
- GMP
good manufacturing practice
- LDH
lactate dehydrogenase
- LMWH
low-molecular-weight heparins
- MMP-1
matrix metalloproteinase-1
- Mr
molecular weight
- PG
proteoglycans
- PMN
polymorphonuclear neutrophils
- PMNE
polymorphonuclear neutrophil elastase
- PPS
pentosan polysulfate
- SGG
sulfated glucuronogalactans
- SP
sulfated polysaccharides
- TT
thrombin time
- UFH
unfractionated heparin
References
- Akl EA, van Doormaal FF, Barbara M, Kamath G, Kim SY, Kuipers S, Middeldorp S, Yosuico V, Dickinson HO, Schünemann HJ. Parenteral anticoagulation for prolonging survival in patients with cancer who have not other indication for anticoagulation. Cochrane Database Syst Rev. 2007;(3):CD006652. doi: 10.1002/14651858.CD006652. [DOI] [PubMed] [Google Scholar]
- Alban S. From heparins to factor Xa inhibitors and beyond. Eur J Clin Invest. 2005a;35(Suppl 1):12–20. doi: 10.1111/j.0960-135X.2005.01452.x. [DOI] [PubMed] [Google Scholar]
- Alban S. The “precautionary principle” as a guide for future drug development. Eur J Clin Invest. 2005b;35(Suppl 1):33–44. doi: 10.1111/j.0960-135X.2005.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alban S. Natural and synthetic glycosaminoglycans. Molecular characteristics as the basis of distinct drug profiles. Hämostaseologie. 2008a;28(1–2):51–61. [PubMed] [Google Scholar]
- Alban S. Pharmacological strategies for inhibition of thrombin activity. Curr Pharm Des. 2008b;14(12):1152–1175. doi: 10.2174/138161208784246135. [DOI] [PubMed] [Google Scholar]
- Alban S, Becker M, Bendas G, Gille J, Ludwig R. A concert of actions contributes to the antimetastastic activity of the semisynthetic glucan sulfate PS3. Haematol Rep. 2005;1:74. [Google Scholar]
- Alban S, Bourgougnon N, Franz G. Anticoagulant activity of an antiviral sulfated glucuronogalactan from Schizymenia dubyi (Rhodophyta, Gigartinales) Thromb Haemost. 1997;78(Suppl):PS2836. [Google Scholar]
- Alban S, Franz G. Gas-liquid chromatography-mass spectrometry analysis of anticoagulant active curdlan sulfates. Semin Thromb Hemost. 1994;20(2):152–158. doi: 10.1055/s-2007-1001898. [DOI] [PubMed] [Google Scholar]
- Alban S, Franz G. Partial synthetic glucan sulfates as potential new antithrombotics: A review. Biomacromolecules. 2001;2(2):354–361. doi: 10.1021/bm010032u. [DOI] [PubMed] [Google Scholar]
- Alban S, Ludwig R, Bendas G, Schön MP, Oostingh G, Radeke HH, Fritzsche J, Pfeilschifter J, Kaufmann R, Boehncke W-H. PS3, a novel semisynthetic β-1,3 glucan-sulfate, diminishes contact hypersensitivity responses through inhibition of l- and p-selectin functions. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.358. 2008 Dec 4 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Altieri DC. Inflammatory cell participation in coagulation. Semin Cell Biol. 1995;6(5):269–274. doi: 10.1006/scel.1995.0036. [DOI] [PubMed] [Google Scholar]
- Anderson VR, Perry CM. Pentosan polysulfate: A review of its use in the relief of bladder pain or discomfort in interstitial cystitis. Drugs. 2006;66(6):821–835. doi: 10.2165/00003495-200666060-00006. [DOI] [PubMed] [Google Scholar]
- André P. P-Selectin in haemostasis. Br J Haematol. 2004;126(3):298–306. doi: 10.1111/j.1365-2141.2004.05032.x. [DOI] [PubMed] [Google Scholar]
- Becker M, Franz G, Alban S. Inhibition of PMN-elastase activity by semisynthetic glucan sulfates. Thromb Haemost. 2003;89(5):915–925. [PubMed] [Google Scholar]
- Berteau O, Mulloy B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003;13(6):29R–40R. doi: 10.1093/glycob/cwg058. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Nawroth PP. Coagulation, inflammation and immune response – An evolutionary conserved plan as cause for disseminated intravasal coagulation? Hämostaseologie. 2005;25(1):23–32. doi: 10.1267/hämo05010023. [DOI] [PubMed] [Google Scholar]
- Borsig L. Selectins facilitate carcinoma metastasis and heparin can prevent them. News Physiol Sci. 2004;19:16–21. doi: 10.1152/nips.01450.2003. [DOI] [PubMed] [Google Scholar]
- Bourgougnon N, Lahaye M, Quemener B, Chermann JC, Rimbert M, Cormaci M, Furnari G, Kornprobst JM. Annual variation in composition and in vitro anti-HIV-1 activity of the sulfated glucuronogalactan from Schizymenia dubyi (Rhodophyta, Gigartinales) J Appl Phycol. 1996;8:155–161. [Google Scholar]
- Bourgougnon N, Roussakis C, Kornprobst JM, Lahaye M. Effects in vitro of sulfated polysaccharide from Schizymenia dubyi (Rhodophyta, Gigartinales) on a non-small-cell bronchopulmonary carcinoma line (NSCLC-N6) Cancer Lett. 1994;85(1):87–92. doi: 10.1016/0304-3835(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Buller HR, van Doormaal FF, van Sluis GL, Kamphuisen PW. Cancer and thrombosis: From molecular mechanisms to clinical presentations. J Thromb Haemost. 2007;5(Suppl 1):246–254. doi: 10.1111/j.1538-7836.2007.02497.x. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17(5):541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- Facino RM, Carini M, Stefani R, Aldini G, Saibene L. Anti-elastase and anti-hyaluronidase activities of saponins and sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus: Factors contributing to their efficacy in the treatment of venous insufficiency. Arch Pharm (Weinheim) 1995;328(10):720–724. doi: 10.1002/ardp.19953281006. [DOI] [PubMed] [Google Scholar]
- Ferro V, Dredge K, Liu L, Hammond E, Bytheway I, Li C, Johnstone K, Karoli T, Davis K, Copeman E, et al. PI-88 and novel heparan sulfate mimetics inhibit angiogenesis. Semin Thromb Hemost. 2007;33(5):557–568. doi: 10.1055/s-2007-982088. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration Information on heparin. 2008. Available at http://www.fda.gov/cder/drug/infopage/heparin/default.htm. (Accessed on September 16, 2008)
- Fritzsche J, Alban S, Ludwig RJ, Rubant S, Boehncke WH, Schumacher G, Bendas G. The influence of various structural parameters of semisynthetic sulfated polysaccharides on the p-selectin inhibitory capacity. Biochem Pharmacol. 2006;72(4):474–485. doi: 10.1016/j.bcp.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Girard N, Maingonnat C, Bertrand P, Tilly H, Vannier JP, Delpech B. Human monocytes synthesize hyaluronidase. Br J Haematol. 2002;119(1):199–203. doi: 10.1046/j.1365-2141.2002.03733.x. [DOI] [PubMed] [Google Scholar]
- Herve R, Percehais S, Yvin JC. Topical pharmaceutical containing the extract (protoexoplasma) of red algae for the treatment of leg circulation disorders. 1987. Patent No. FR2593067-A1.
- Hsu LC. Heparin-coated cardiopulmonary bypass circuits: Current status. Perfusion. 2001;16(5):417–428. doi: 10.1177/026765910101600512. [DOI] [PubMed] [Google Scholar]
- Khachigian LM, Parish CR. Phosphomannopentaose sulfate (PI-88): Heparan sulfate mimetic with clinical potential in multiple vascular pathologies. Cardiovasc Drug Rev. 2004;22(1):1–6. doi: 10.1111/j.1527-3466.2004.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost. 2007;5(4):729–737. doi: 10.1111/j.1538-7836.2007.02427.x. [DOI] [PubMed] [Google Scholar]
- Lever R, Page CP. Novel drug development opportunities for heparin. Nat Rev Drug Discov. 2002;1(2):140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- Lewis KD, Robinson WA, Millward MJ, Powell A, Price TJ, Thomson DB, Walpole ET, Haydon AM, Creese BR, Roberts KL, et al. A phase II study of the heparanase inhibitor PI-88 in patients with advanced melanoma. Invest New Drugs. 2008;26(1):89–94. doi: 10.1007/s10637-007-9080-5. [DOI] [PubMed] [Google Scholar]
- Lindahl U, Kusche-Gullberg M, Kjellén L. Regulated diversity of heparan sulfate. J Biol Chem. 1998;273(39):24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- Lindl T. Zell- und Gewebekultur: Einführung in die Grundlagen sowie ausgewählte Methoden und Anwendungen. 5th ed. Heidelberg, Berlin: Spektrum, Akad. Verl. 2002 [Google Scholar]
- Ludwig RJ, Alban S, Bistrian R, Boehncke WH, Kaufmann R, Henschler R, Gille J. The ability of different forms of heparins to suppress p-selectin function in vitro correlates to their inhibitory capacity on blood-borne metastasis in vivo. Thromb Haemost. 2006;95(3):535–540. doi: 10.1160/TH05-07-0515. [DOI] [PubMed] [Google Scholar]
- Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: Strangers or partners in crime? Trends Immunol. 2007;28(4):184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- McCachren SS. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- McEver RP. Selectin-carbohydrate interactions during inflammation and metastasis. Glycoconj J. 1997;14(5):585–591. doi: 10.1023/a:1018584425879. [DOI] [PubMed] [Google Scholar]
- Ménard R, Alban S, de Ruffray P, Jamois F, Franz G, Fritig B, Yvin JC, Kauffmann S. Beta-1,3 glucan sulfate, but not beta-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. Plant Cell. 2004;16(11):3020–3032. doi: 10.1105/tpc.104.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanari D, Guglielmo M. Cosmetic composition for the treatment and/or prevention of skin stretch marks. 2008. Patent No. WO 2008 080443.
- Mourão PA. Use of sulfated fucans as anticoagulant and antithrombotic agents: Future perspectives. Curr Pharm Des. 2004;10(9):967–981. doi: 10.2174/1381612043452730. [DOI] [PubMed] [Google Scholar]
- Mulloy B. The specificity of interactions between proteins and sulfated polysaccharides. An Acad Bras Cienc. 2005;77(4):651–664. doi: 10.1590/s0001-37652005000400007. [DOI] [PubMed] [Google Scholar]
- Nelson AR, Fingleton B, Rothenberg M. Matrix metalloproteinases: Biologic activity and clinical implications. J Clin Oncol. 2000;18(5):1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- Norrby K. Low-molecular-weight heparins and angiogenesis. APMIS. 2006;114(2):79–102. doi: 10.1111/j.1600-0463.2006.apm_235.x. [DOI] [PubMed] [Google Scholar]
- Opal SM, Esmon CT. Bench-to-bedside review: Functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit Care. 2003;7(1):23–38. doi: 10.1186/cc1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CR, Freeman C, Hulett MD. Heparanase: A key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471(3):M99–M108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev. 2006;(1):CD003230. doi: 10.1002/14651858.CD003230.pub3. [DOI] [PubMed] [Google Scholar]
- Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3(8):1590–1596. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- Posey JT, Soloway MS, Ekici S, Sofer M, Civantos F, Duncan RC, Lokeshwar VB. Evaluation of the prognostic potential of hyaluronic acid and hyaluronidase (HYAL1) for prostate cancer. Cancer Res. 2003;63(10):2638–2644. [PubMed] [Google Scholar]
- Potin P, Patier P, Floc’h JY, Yvin JC, Rochas C, Kloareg B. Chemical characterization of cell-wall polysaccharides from tank-cultivated and wild plants of Delesseria sanguinea (Hudson) Lamouroux (Ceramiales, Delesseriaceae): Culture patterns and potent anticoagulant activity. J Appl Phycol. 1992;4:119–128. [Google Scholar]
- Rak J, Milsom C, May L, Klement P, Yu J. Tissue factor in cancer and angiogenesis: The molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin Thromb Hemost. 2006;32(1):54–70. doi: 10.1055/s-2006-933341. [DOI] [PubMed] [Google Scholar]
- Sato T, Takahashi S, Mizumoto T, Harao M, Akizuki M, Takasugi M, Fukutomi T, Yamashita J. Neutrophil elastase and cancer. Surg Oncol. 2006;15(4):217–222. doi: 10.1016/j.suronc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Smorenburg SM, van Noorden CJF. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol Rev. 2001;53(1):93–105. [PubMed] [Google Scholar]
- Taggart CC, Greene CM, Carroll TP, O’Neill SJ, McElvaney NG. Elastolytic proteases: Inflammation resolution and dysregulation in chronic infective lung disease. Am J Respir Crit Care Med. 2005;171(10):1070–1076. doi: 10.1164/rccm.200407-881PP. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Abboud-Jarrous G, Elkin M, Naggi A, Casu B, Sasisekharan R, Ilan N. The impact of heparanase and heparin on cancer metastasis and angiogenesis. Pathophysiol Haemost Thromb. 2006;35(1–2):116–127. doi: 10.1159/000093553. [DOI] [PubMed] [Google Scholar]
- Yvin J, Alban S, Franz G. Anti-inflammatory and healing medicine based on laminarin sulfate. 2002 PCT Int Appl Patent No. WO 2002 036 132. [Google Scholar]