Abstract
Background
An optimal technique for clinical memory fMRI is not established. Previous studies suggest activity in right parahippocampal gyrus and right hippocampus shows the strongest difference between left hippocampal sclerosis (HS) patients and normal control subjects and that the difference in activity between left and right hippocampus predicts postoperative memory change.
Methods
The authors studied 30 patients with mesial temporal lobe epilepsy (mTLE) and left HS, 12 of whom subsequently underwent surgery, and 13 normal control subjects. The patients who had surgery underwent neuropsychometric evaluation pre- and postoperatively. All subjects underwent a verbal memory encoding event-related fMRI study. Activation maps were assessed visually. Subsequently, the brain regions involved in the memory task were revealed by group averaging; these regions were used to determine regions of interest (ROIs) for subsequent analysis. By use of stepwise discriminant function and stepwise multiple regression, the ROIs that optimally discriminated between patients and normal control subjects and that optimally predicted postoperative verbal memory outcome were determined.
Results
Visual inspection of individual patient activation statistic maps revealed noisy data that did not afford visual interpretation. Stepwise discriminant function revealed the difference between left and right hippocampal activity best discriminated between patients and normal control subjects. Stepwise multiple regression revealed left hippocampal activity was the strongest predictor of postoperative verbal memory outcome; greater left hippocampal activity predicted a greater postoperative decline in memory.
Conclusions
Patients with left hippocampal sclerosis (HS) differ from normal control subjects in the distribution of memory-encoding activity between left and right hippocampus. Functional adequacy of left hippocampus best predicts postoperative memory outcome in left HS.
Hippocampal sclerosis (HS) is the most common etiology in patients with refractory epilepsy.1-3 Surgical resection of the affected hippocampus is curative in the majority of patients,4 although anterior temporal lobectomy (ATL) may worsen memory function. In particular, left ATL may lead to a disabling decline in verbal memory function.5-8 Clinical tools that can measure the functional integrity of mesial temporal structures in patients with mesial temporal lobe epilepsy (mTLE) are needed to predict the likely effect of ATL in individual patients.
Current techniques to assess the risk of memory decline following ATL include measurement of severity of HS with MRI6, assessment of baseline memory performance,7,9 and the intracarotid amobarbital test (IAT or Wada test).9-11 Structural imaging and baseline memory performance are imperfect predictors, and the IAT has major risks.12 If fMRI of memory could be shown to be reproducible and robust, then fMRI could evolve an important clinical role.
In this article, we revisit existing memory fMRI data from epilepsy patients in an attempt to identify an optimal approach to individual patient data interpretation. We anticipated that visual inspection of single-subject memory fMRI studies would be inadequate because of noise. By incorporating additional data from 14 normal control subjects, we sought to establish which mesial temporal regions of interest (ROIs) might be most clinically meaningful, allowing separation of patients from normal controls. Subsequently, we examined these ROIs for predictive value in assessing risk of post-ATL memory decline and to determine whether our data support the functional adequacy or functional reserve hypotheses.
Methods
Subjects
We studied 30 patients and 13 control subjects. All patients undergoing evaluation for epilepsy surgery at the National Hospital for Neurology and Neurosurgery, London, UK, were eligible for the study. Inclusion criteria were a clinical diagnosis of TLE; a structural MRI diagnosis of left HS with normal right hippocampal volume and T2 measurement; no other imaging lesion; fluent English speaker; and ability to give informed consent according to the Declaration of Helsinki. There were no exclusions on the basis of handedness, IQ, or subject movement during scanning. Normal control subjects were recruited from friends, colleagues, and partners of the patients (n = 10) in addition to three hospital staff.
Preoperative functional imaging
We used event-related fMRI of a verbal memory-encoding task, which allowed us specifically to identify brain regions activated by words that were subsequently remembered. We used the method described in detail previously.13,14 In brief, subjects were scanned at 2 T (Siemens VISION; Siemens, Erlangen, Germany), acquiring T2*-weighted image (echo-planar imaging) volumes, providing blood oxygenation level-dependent contrast (33 slices, whole brain, voxel dimensions 3 × 3 × 3.67 mm, echo time 40 milliseconds, repetition time 2.5 seconds). SPM99 was used for image analysis.15 The images were realigned, corrected for slice timing differences, transformed to the standard anatomic volume, and smoothed (8-mm kernel). During scanning, subjects were presented 288 single words, including 36 emotionally aversive words (e.g., “cancer,” “rape,” “terrorist”),16 1 every 4.5 seconds. Subjects pressed a right-hand button to indicate whether the word was “living” or “nonliving.” Ninety minutes after scanning, subjects performed a surprise recognition memory test that included foils (not scanned): Subjects were asked to indicate whether the word was definitely remembered (R response), if the word seemed familiar (K response), or was new (N response).17 The encoding stimuli and the associated evoked neuronal response were then conditionalized according to subjects’ recognition responses.
Single-subject analysis
To test for subsequent memory effects, imaging data were analyzed using an event-related design.18 Trial-specific responses were modeled, and each subject’s movement parameters were included as confounds. Scans were not excluded because of movement. Contrasts of parameter estimates were calculated to produce an image of T statistics for each subject, pertaining to the contrast (R - K) for the main effect of encoding, combining neutral and emotional items. T statistics were normalized using a Z transform. The contrast (R - K) was preferred to (R - N) because this contrast encompassed the least range of behavioral difference among subjects (discussed in detail elsewhere).13,14
Can visual inspection of activation maps reliably identify lateralization of encoding activity?
For each individual patient, a Z-statistic image of successful encoding activity thresholded at p = 0.05 (uncorrected) was produced from the first-level analysis described above; this statistic image was rendered onto an averaged patient T1-weighted MRI. An averaged structural image was used rather than individual patient T1 MRI so that specific structural characteristics would not bias the analysis of the functional data. Identifying information was removed from these images, and subsequently one investigator blindly reviewed the hippocampal regions, comparing visually the magnitude of activity in the left and right side. Only activated voxels in hippocampus proper were assessed. For each subject, one of six categories of activity could be chosen: activity strongly predominant on the left; activity slightly predominant on the left; equal activity on left and right; activity slightly predominant on the right; activity strongly predominant on the right; no activity evident. Subsequently, we compared the hippocampal volume ratio of these groups and the distribution of subjects in these groups according to postoperative memory outcome.
Group analysis
From the single-subject (first level) analysis above, for each subject, an image of the effect size (13 parameter) at each voxel for the (R - K) contrast (“contrast image”) was produced. At the second level, these contrast images were entered into a one-way T test to produce an image of effects significant at the group level. The 13 parameters are expressed in arbitrary units, relative to the mean 13 parameter across the entire image, which is set as zero. A positive 13 parameter is therefore a brain voxel with an effect size for the (R - K) contrast greater than the mean value across the whole brain; a negative 13 parameter is a brain voxel with an effect size for the (R - K) contrast less than the mean value across the whole brain.
Improving anatomic specificity: Which mesial temporal regions subserve successful encoding?
We anticipated that visual inspection of encoding activity images would reveal noisy data, with anatomically scattered regions of activation, some of uncertain relevance (nonspecific noise), as well as failure to detect activity visually due to insensitivity. We expected that it would be necessary to limit analysis to predetermined ROIs. Therefore, the next step of our analysis was to examine the group data, to establish as robustly as possible the typical mesial temporal regions that sub-served successful verbal encoding. To achieve this, the (R - K) contrast images for all subjects (patients and normal control subjects) were entered into a one-way T test; we used a large image mask to confine our analysis to hippocampus, parahippocampal gyrus, entorhinal and perirhinal cortices, fusiform gyrus, amygdale, and uncus on both sides, including the full anterior-posterior extent of all these structures. We report here all regions with a peak p value less than 0.005 (uncorrected) within this large image mask.
Single-subject analysis: Can quantified images separate patients from normal controls?
Next, we sought to establish whether single-subject memory fMRI data can reliably distinguish between a patient with left HS and a normal control subject. To do this, we used discriminant function analysis. The mean values of the voxelwise parameter estimates in each of the ROIs identified above were used. We employed a stepwise approach to determine the subset of ROIs providing optimal discrimination between the patient group and the normal controls. The stepwise methodology used p = 0.05 to enter a variable into each step and p = 0.1 to remove.
Single-subject analysis: Can quantified ROIs predict post-ATL memory outcome?
Finally, we examined the data from patients who subsequently underwent left ATL (n = 12). Using stepwise multiple regression, we identified the ROIs most strongly predictive of post-ATL memory change. To assess verbal memory, we used the List Learning subtest of the Adult Memory and Information Processing Battery19 as part of a larger neuropsychometric test battery used for routine presurgical evaluation. The List Learning test has an initial registration component (maximum score = 75) and a delayed component (maximum score = 15). Patients were tested preoperatively and again 6 months postoperatively, using parallel tests of standardized equivalent difficulty. The difference between each of the test scores was calculated for each patient. Stepwise multiple regression was undertaken separately for each of the two sets of test score differences (immediate and delayed scores). The stepwise methodology used p = 0.05 to enter a variable into each step and p = 0.1 to remove.
Results
Clinical data for the 30 patients are included in table E-1 on the Neurology Web site at www.neurology.org. All patients were able to perform the task required during scanning. No data were omitted because of subject movement. The total number of events (R + K) included in the first-level analysis in the patients ranged from 94 to 249. Patients performed substantially above chance on the recognition test (mean recognition accuracy score 0.37, SD 0.14, difference from chance, i.e., a recognition accuracy score of 0: T = 14.0,p <0.001). Normal subjects performed better than patients (normal subjects mean 0.50; T = 2.90, p [two tailed] = 0.003).
Twelve patients underwent an ATL performed in all cases by the same neurosurgeon and in the same center. The resection included a 4-cm neocortical resection to gain access to the temporal horn of the lateral ventricle, followed by a radical removal of the hippocampus and parahippocampal gyrus to the level of the mid-brainstem.
Can visual inspection of activation maps reliably identify lateralization of encoding activity?
Single-subject Z-statistic activation maps thresholded at p = 0.05 are shown in figure E-1. As is evident from this figure, most subjects revealed activity in a number of different mesial temporal regions. Hippocampal activity was not necessarily the strongest mesial temporal activity demonstrated. The upper panel of figure 1 shows the distribution of subjects in the six visually determined categories according to left hippocampal volume. There is a trend toward larger left hippocampi in the left-lateralized patients (difference between “strongly left lateralized” and “strongly right lateralized” groups; T = 2.37, p [two tailed] = 0.05). The lower panel of figure 1 shows that visual inspection did not discriminate between subjects according to postoperative memory outcome. Subjectively, the variability of single-subject data was very considerable and not well suited to a systematic visual approach.
Figure 1.
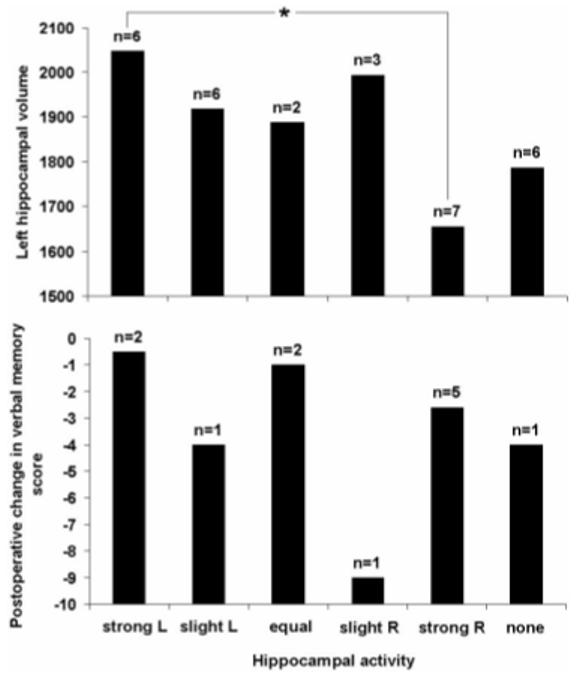
Categorization of hippocampal activity by visual inspection: The upper panel shows the relationship between visually determined hippocampal activity and left hippocampal volume (mm3, corrected for total intracranial volume); the lower panel shows the relationship between these categories and postoperative change in delayed recall of previously learned word lists. The visually determined categories are as follows: strong L (R) = strongly left (right) predominant activity; slight L (R) = slightly predominant activity on the left (right); equal activity on the two sides; no activity evident at p = 0.05. The asterisk shows a significant difference in left hippocampal volume for the strongly left and right lateral ized groups at p = 0.05. n = number of patients in each category.
Improving anatomic specificity: Which mesial temporal regions subserve successful encoding?
Examining the entire group (patients and normal control subjects), significant successful encoding-related activity was seen in left hippocampus, right hippocampus, right parahippocampus, and right amygdala (table 1; figure 2). For subsequent ROI analyses, we used the mean voxelwise parameter estimate within each of these regions.
Table 1. Mesial temporal lobe regions showing significant activation at p< 0.005 in analysis of whole group.
| Region | Coordinate | T score | Z score | p Value |
|---|---|---|---|---|
| L hippocampus | −30 −20 −18 | 4.35 | 3.93 | <0.001 |
| R hippocampus | 32 −18 −12 | 3.76 | 3.47 | <0.001 |
| R amygdala | 20 −8 −20 | 2.92 | 2.77 | 0.003 |
| R parahippocampus | 30 −30 −22 | 3.17 | 2.98 | 0.001 |
Figure 2.
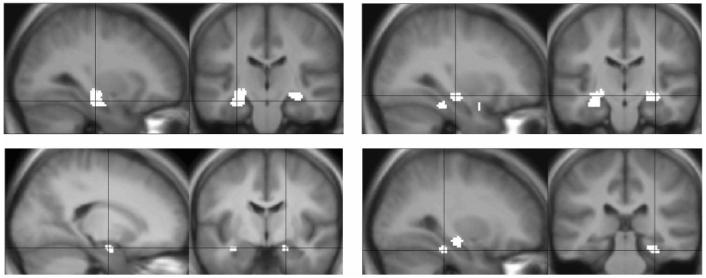
Group analysis: mesial temporal lobe regions showing significant activation at p <0.005 in the analysis of the whole group. Brain left is on the figure left. (Top left) left hippocampus; (top right) right hippocampus; (bottom left) right amygdale; (bottom right) right para-hippocampal gyrus. Coordinates and Z scores for these regions are given in table 1.
Single-subject analysis: Can quantified images separate patients from normal controls?
We examined activity in left and right hippocampus, right parahippocampus, and right amygdala. Stepwise discriminant function analysis revealed that the following function discriminated optimally between normal control subjects and the patient group (Wilks X = 0.831, x2 = 7.423,p = 0.024): (parameter estimate in left hippocampus ROI X −1.199) + (parameter estimate in right hippocampus ROI X 1.569). All other combinations of ROI values led to a less significant result.
Single-subject analysis: Can quantified ROIs predict post-ATL memory outcome?
We examined activity in left and right hippocampus, right parahippocampus, and right amygdala. Activity in each of these regions was correlated with each of the memory change scores (table 2). Significant correlations were found between score change in list learning (delayed) and left hippocampal activity (R = 0.72, p = 0.0087) and between score change in list learning (delayed) and right hippocampal activity (R = 0.71, p = 0.010). Stepwise multiple regression, using values from left and right hippocampus, right parahippocampus, and right amygdala, revealed that only left hippocampus made a significant independent prediction of score change in list learning (delayed). Note that activity in left and right hippocampus was correlated, with greater activity in left hippocampus predicting greater activity in right hippocampus (R = 0.741, p <0.001). Figure 3 illustrates the correlation between left and right hippocampal ROI activity and postoperative memory outcome.
Table 2. Correlations between preoperative memory-encoding fMRI activity in regions of interest and postoperative verbal memory score change.
| L hippocampus | R hippocampus | R parahippocampus | R amygdala | |
|---|---|---|---|---|
| Verbal learning (delayed) | ||||
| Pearson r | −0.717 | −0.705 | −0.355 | −0.205 |
| p | 0.009 | 0.010 | 0.258 | 0.523 |
| Spearman p | −0.789 | −0.754 | −0.460 | −0.085 |
| p | 0.002 | 0.005 | 0.132 | 0.793 |
| Verbal learning (immediate) | ||||
| Pearson r | −0.063 | 0.054 | 0.054 | −0.062 |
| p | 0.847 | 0.868 | 0.868 | 0.848 |
| Spearman p | 0.039 | 0.210 | −0.396 | −0.105 |
| p | 0.905 | 0.512 | 0.203 | 0.745 |
Figure 3.
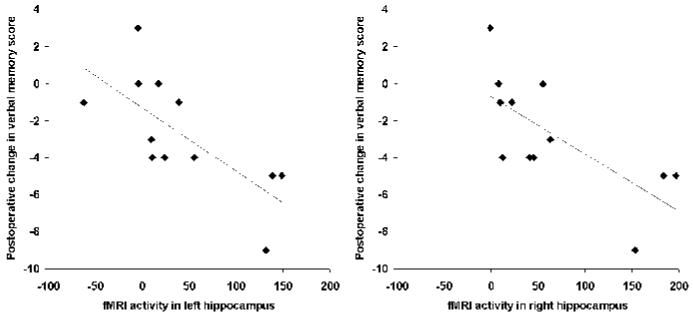
Preoperative measurement of verbal memory-encoding fMRI activity in left and right hippocampal regions of interest correlated with postoperative change in delayed recall of previously learned word lists. Memory-encoding fMRI activity is expressed as effect size in arbitrary units (13 parameters), relative to the mean 13 parameter across the entire image, which was set as 0. A positive 13 parameter is therefore a brain voxel with an effect size for the (R - K) contrast greater than the mean value across the whole brain; a negative 13 parameter is a
Discussion
We report here 30 patients with left HS and 13 normal control subjects undergoing fMRI of successful verbal memory encoding, with no exclusions on the basis of IQ, handedness, first language, or subject movement during scanning. We attempt to optimize the utility of individual patient data, by a pragmatic approach to the anatomic regions to be examined. We show that fMRI of successful verbal memory encoding is feasible for a wide range of patients and produces reliable and meaningful data at the single-subject level when a quantitative analysis of hippocampal ROIs is undertaken.
Single-subject fMRI data are prone to errors in localization of activation due to a number of factors including head movement, thermal noise, pulsatile brain movement, image distortion, signal dropout, and detection of activity in draining veins rather than brain itself; these factors may be variable across brain regions and between individuals. A consequence of these factors is “false-positive” activation. Such activations are likely to be anatomically randomly distributed in individual subjects, hence not prominent in group results, explaining the marked difference between the highly variable single patients illustrated in figure E-1 and the highly anatomically specific group result illustrated in figure 2. Studies of language using fMRI in epilepsy patients have also shown highly variable patterns between individuals, but clinically meaningful findings have been obtained when quantified data were derived from ROIs based on group results.20-22 An alternative explanation for variability between patients is that the activations seen are “true” and the variability in site of activity is due to reorganization in the context of localized lesions; there is some supportive evidence for this in studies of simple motor function.23
In this study, visually inspecting single-subject activation maps proved difficult because of the scattered foci of activation seen throughout the brain at the lenient threshold chosen (p = 0.05, uncorrected). Even at this threshold, one in five patients showed no hippocampal activity. Nonetheless, where hippocampal activity was seen, the lateralization of such activity correlated with hippocampal volume. The data presented here suggest that visual inspection of activation Z-statistic maps is not an adequate procedure to fully characterize the memory function of the hippocampus in HS patients.
Our group analysis showed highly anatomically specific and robust activity in left and right midante rior hippocampus proper. Whereas in the past, there has been debate regarding the interpretation of foci of activity in functional imaging data of memory encoding (e.g., whether encoding function is subserved in hippocampus proper or other mesial temporal regions, or whether this function is subserved in anterior or posterior mesial temporal lobe),24 our own data seem unambiguous in this regard. Hence, we feel confident to ascribe a primary role in verbal memory encoding to the midanterior hippocampus and to use the hippocampal ROIs identified in this group analysis to characterize the single-subject data. Other fMRI studies of memory in TLE patients have revealed activity outside the hippocampus or in very posterior regions.25,26 Anterior hippocampus proper is the target of resection for HS, and functional evaluation of this specific region is more relevant than other regions.
The patients performed above chance on the recognition test, resulting in an adequate number of events for statistical analysis for all patients. Although we made no attempt to exclude patients with poor verbal memory, we concede that there must be a lower limit of performance below which an adequate event-related memory study could not be produced. Nonetheless, our patients, who were unselected as regards memory function and IQ, did not reach this lower limit. Empirically, we showed in these data that the magnitude of activity in the mesial temporal regions did not correlate with performance variables, either when examining the whole group or when separately examining patients and normal subjects. We examined activity in left and right hippocampus, right parahippocampus, and right amygdala. None of these parameters correlated with the number of R events included in the first-level analysis for each subject, the number of K events, the total number of events (R + K), or the recognition accuracy score. This suggests our approach would not be confounded by memory performance factors. Similarly, a previ study from another group27 ous showed no correlation between their memory performance variable and activity in any ROI.
ROI methods require an a priori decision regarding the anatomic regions to be examined, guided by previous data, but nonetheless this a priori decision may be somewhat arbitrary and ROI placement may not be optimal for the question being addressed. Evaluating ROIs in a univariate sense may allow significant effects to be detected but may overlook the contribution that multiple anatomically distant ROIs may make jointly to determining a significant effect; examining asymmetries is one simple approach to using data from more than one ROI simultaneously, but other approaches also exist to accommodate data from multiple ROIs to determine the most relevant subset to best address the question, such as discriminant function analysis.
The strongest activations seen in our group data were in the left and right hippocampus. Furthermore, the discriminant function analysis identified left and right hippocampal ROI values as the most important parameters to discriminate between normal subjects and left HS patients. Our previous study that made a voxelwise “mass univariate” categorical comparison of left HS patients and normal controls’3 identified a significant difference between patients and normal subjects in right parahippocampal gyrus and right hippocampus, but no significant difference in left hippocampus. The discriminant function coefficients were negative for left and positive for right hippocampus, showing that difference in hippocampal activity of opposite sign (decreased in left, increased in right) in patients compared with controls is a characteristic difference. Contrasting the findings of our previous study’3 and this study points to important differences between a univariate approach and even the simplest multivariate approach. Moreover, our demonstration of a difference between patients and normal subjects that is partly dependent on left hippocampal activity provides justification for including left hippocampus ROI in clinical fMRI studies of patients with left HS, despite this region alone showing no difference between patients and normal subjects.
In the context of postsurgical memory decline in ATL, two hypotheses have been put forward to provide a framework for assessing risk: hippocampal functional reserve and hippocampal functional adequacy.28 Hippocampal reserve suggests that it is the reserve or capacity of the contralateral hippocampus to support memory after surgery that determines whether there will be a decline in memory function; on the other hand, hippocampal adequacy suggests that memory decline post ATL is inversely proportional to the functional adequacy of the tissue to be resected. Our previous data show that during verbal memory encoding measured using fMRI, the most significant difference between normal subjects and left HS patients is encoding activity in right parahippocampal gyrus, right hippocampus, and right amygdala’3 and that the redistribution of encoding activity between left and right hippocampus is a function of left hippocampal volume.’4 These findings suggest that right hippocampal functional reserve might play an important part in determining verbal memory outcome post ATL.
In our previous study,29 we examined 10 patients with left HS. Prior to ATL, all underwent a memory-encoding fMRI study. We showed that asymmetry of memory-encoding activity in the left and right hippocampus predicted postoperative memory decline; we did not examine the two sides separately, so cannot address the functional adequacy/functional reserve hypotheses. Although this study clearly demonstrates the importance of measuring hippocampal activity preoperatively to predict the consequences of surgery for memory function, our results were reported as a group effect at the voxel level; this approach is not readily translated into clinical use. An alternative approach addressing the same clinical problem27 employed ROIs measured from visual memory-encoding fMRI in individual patients prior to ATL; 10 patients had left ATL, 14 right. Overall, asymmetry of memory-encoding activity in a large medial temporal ROI predicted postoperative memory decline. However, in the left ATL group, only memory-encoding activity in the left-sided ROI predicted memory decline; asymmetry did not. This finding provides strong support for the hippocampal functional adequacy model. A further fMRI study30 used a visual memory-recall task to assess hippocampal function preoperatively in 16 patients with right mTLE. This study also found that asymmetry of activity between mesial temporal ROIs on the left and right side predicted postoperative change in visual memory; like our own prior study,29 this study did not report data from individual left and right ROIs, so also cannot address the functional adequacy/functional reserve hypotheses.
Using stepwise multiple regression, we showed that activity in left hippocampus is the strongest independent predictor of decline in postoperative verbal memory, specifically in delayed recall of previously learned word lists. This finding provides strong support for the functional adequacy model, that is, that memory outcome is determined by residual function in the to-be-resected hippocampus, as evidenced by the correlation between activity and outcome, with greater left hippocampal activity predicting a poorer memory outcome. Of note, activity in the right hippocampus is strongly correlated with activity in the left hippocampus in our data (R = 0.741) and also shows a significant correlation between activity and outcome, with greater activity in right hippocampus predicting a poorer memory outcome, providing no support whatever for the functional reserve model. Although paradoxical and counterintuitive, it is important to point out here that a study from another group27 also found left and right ROI values to be correlated (hippocampus ROI R = 0.408, p = 0.053; hippocampus/parahippocampus/fusiform ROI R = 0.598, p = 0.003).
A possible explanation for the lack of support for the functional reserve model may be found in our previous data14 showing that relatively greater memory-encoding activity in right hippocampus in these patients correlates with poorer memory. This might suggest that verbal memory function sub-served by right hippocampus in left HS patients is “dysfunctional.” Although such right hippocampal activity might protect against postoperative amnesia (not examined here), such “dysfunctional” activity does not provide an adequate postoperative reserve to maintain memory function at the preoperative level.
The strong correlation between left and right ROI values and the correlation between right hippocampal activity and postoperative memory outcome suggest that a combined measure of hippocampal activity might provide the best prediction. However, our stepwise linear regression model showed that including left and right hippocampal activity separately in the model did not add to the predictive value of left hippocampal activity alone. Similarly, mean hippocampal activity did not provide a clearly better prediction of postoperative memory change than left hippocampal activity alone (correlation between mean hippocampal activity and postoperative memory change R = 0.74). Nonetheless, we do not rule out the possibility that in a larger dataset, activity from combined ROIs might have better predictive value.
Despite the strong predictive value for objective memory outcome provided by the method described here and in other studies, there remains a gap between objective measurement of postoperative change and the patient’s own subjective experience.31 As yet, techniques do not exist to predict the patient’s subjective experience of postoperative memory change.
In this study, we have identified and implemented a cognitive fMRI study that our patients were able to perform adequately. This is an important aspect of functional imaging in patients: Failure to perform the task required in scanning will result in failure of the neuronal network performing this task in normal subjects to be activated in patients.32 In this context, we are interested in the residual functional capacity of an epileptogenic left hippocampus; it is essential to use a task not only that the patients can perform but also that activates the anatomic ROI. Furthermore, the midanterior hippocampal regions activated in this study fall within the typical resection margins of a standard ATL. As noted above, other memory fMRI approaches applied to epilepsy patients have revealed activity posterior to typical resection margins, in regions of brain that would remain intact following ATL.25,26
Our approach is technically possible in a purely clinical context, requiring 22 minutes of scanning, followed by a 90-minute break, leading to a 35-minute recognition memory test. The 90-minute period could be used to obtain further imaging or other neuropsychometric data. Subsequent data analysis used standard tools; total processing time was about 2 hours per subject, with interaction required for a total of about 15 minutes for an experienced user.
Supplementary Material
Acknowledgment
The authors thank their colleagues (Profs. Ley Sander and Simon Shorvon, Drs. Matthias Koepp, Sanjay Sisodiya, Hannah Cock, Matthew Walker, and Shelagh Smith) for patient referrals.
References
- 1.Plate KH, Wieser HG, Yasargil MG, Wiestler OD. Neuropathological findings in 224 patients with temporal lobe epilepsy. Acta Neuropathol Berl. 1993;86:433–438. doi: 10.1007/BF00228577. [DOI] [PubMed] [Google Scholar]
- 2.Kuzniecky R, Murro A, King D, et al. Magnetic resonance imaging in childhood intractable partial epilepsies: pathologic correlations. Neurology. 1993;43:681–687. doi: 10.1212/wnl.43.4.681. [DOI] [PubMed] [Google Scholar]
- 3.Wolf HK, Campos MG, Zentner J, et al. Surgical pathology of temporal lobe epilepsy. Experience with 216 cases. J Neuropathol Exp Neurol. 1993;52:499–506. doi: 10.1097/00005072-199309000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 5.Katz A, Awad IA, Kong AK, et al. Extent of resection in temporal lobectomy for epilepsy. II. Memory changes and neurologic complications. Epilepsia. 1989;30:763–771. doi: 10.1111/j.1528-1157.1989.tb05336.x. [DOI] [PubMed] [Google Scholar]
- 6.Trenerry MR, Jack CR, Ivnik RJ, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800–1805. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- 7.Helmstaedter C, Elger CE. Cognitive consequences of two-thirds anterior temporal lobectomy on verbal memory in 144 patients: a three-month follow-up study. Epilepsia. 1996;37:171–180. doi: 10.1111/j.1528-1157.1996.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 8.Saykin AJ, Gur RC, Sussman NM, O’Connor MJ, Gur RE. Memory deficits before and after temporal lobectomy: effect of laterality and age of onset. Brain Cogn. 1989;9:191–200. doi: 10.1016/0278-2626(89)90029-8. [DOI] [PubMed] [Google Scholar]
- 9.Jokeit H, Ebner A, Holthausen H, et al. Individual prediction of change in delayed recall of prose passages after left-sided anterior temporal lobectomy. Neurology. 1997;49:481–487. doi: 10.1212/wnl.49.2.481. [DOI] [PubMed] [Google Scholar]
- 10.Lee GP, Park YD, Westerveld M, Hempel A, Blackburn LB, Loring DW. Wada memory performance predicts seizure outcome after epilepsy surgery in children. Epilepsia. 2003;44:936–943. doi: 10.1046/j.1528-1157.2003.05003.x. [DOI] [PubMed] [Google Scholar]
- 11.Loring DW, Meador KJ, Lee GP, et al. Wada memory asymmetries predict verbal memory decline after anterior temporal lobectomy. Neurology. 1995;45:1329–1333. doi: 10.1212/wnl.45.7.1329. [DOI] [PubMed] [Google Scholar]
- 12.Meador KJ, Loring DW. The Wada test: controversies, concerns, and insights. Neurology. 1999;52:1535–1536. doi: 10.1212/wnl.52.8.1535. [DOI] [PubMed] [Google Scholar]
- 13.Richardson MP, Strange BA, Duncan JS, Dolan RJ. Preserved verbal memory function in left medial temporal pathology involves reorganisation of function to right medial temporal lobe. Neuroimage. 2003;20:S112–S119. doi: 10.1016/j.neuroimage.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Richardson MP, Strange BA, Dolan RJ. Emotional memory encoding depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- 15.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Map. 1995;2:189–210. [Google Scholar]
- 16.Strange BA, Henson RN, Friston KJ, Dolan RJ. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. Neuroimage. 2000;12:425–433. doi: 10.1006/nimg.2000.0637. [DOI] [PubMed] [Google Scholar]
- 17.Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- 18.Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 19.Coughlan A, Hollows S. The Adult Memory and Information Processing Battery. Psychology Department, St. James Hospital; Leeds: 1986. [Google Scholar]
- 20.Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 21.Benson RR, FitzGerald DB, LeSueur LL, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52:798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- 22.Sabsevitz DS, Swanson SJ, Hammeke TA, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- 23.Krings T, Topper R, Willmes K, Reinges MH, Gilsbach JM, Thron A. Activation in primary and secondary motor areas in patients with CNS neoplasms and weakness. Neurology. 2002;58:381–390. doi: 10.1212/wnl.58.3.381. [DOI] [PubMed] [Google Scholar]
- 24.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 25.Dupont S, Van de Moortele PF, Samson S, et al. Episodic memory in left temporal lobe epilepsy: a functional MRI study. Brain. 2000;123:1722–1732. doi: 10.1093/brain/123.8.1722. [DOI] [PubMed] [Google Scholar]
- 26.Jokeit H, Okujava M, Woermann FG. Memory fMRI lateralizes temporal lobe epilepsy. Neurology. 2001;57:1786–1793. doi: 10.1212/wnl.57.10.1786. [DOI] [PubMed] [Google Scholar]
- 27.Rabin ML, Narayan VM, Kimberg DY, et al. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain. 2004;127:2286–2298. doi: 10.1093/brain/awh281. [DOI] [PubMed] [Google Scholar]
- 28.Chelune GJ. Hippocampal adequacy versus functional reserve: predicting memory functions following temporal lobectomy. Arch Clin Neuropsychol. 1995;10:413–432. [PubMed] [Google Scholar]
- 29.Richardson MP, Strange BA, Thompson PJ, Baxendale SA, Duncan JS, Dolan RJ. Preoperative verbal memory fMRI predicts postoperative memory decline after left temporal lobe resection. Brain. 2004;127:2419–2426. doi: 10.1093/brain/awh293. [DOI] [PubMed] [Google Scholar]
- 30.Janszky J, Jokeit H, Kontopoulou K, et al. Functional MRI predicts memory performance after right mesiotemporal epilepsy surgery. Epilepsia. 2005;46:244–250. doi: 10.1111/j.0013-9580.2005.10804.x. [DOI] [PubMed] [Google Scholar]
- 31.Baxendale S, Thompson P. Defining meaningful postoperative change in epilepsy surgery patients: measuring the unmeasurable? Epilepsy Behav. 2005;6:207–211. doi: 10.1016/j.yebeh.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Map. 1999;8:102–108. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


