Abstract
The chronic nature of intestinal nematode infections suggests that these parasites have evolved sophisticated immunomodulatory strategies. The induction of regulatory responses during chronic helminth infections could be advantageous to the host by minimising damage incurred by these organisms. Regulation of the host immune response to infection could however be exploited by parasites as a survival strategy. We have explored both these aspects using the murine model of whipworm infection, Trichuris muris. Of the three laboratory isolates of T. muris in use, two (the E (Edinburgh) and J (Japan - sub-cultured from E) are readily expelled by C57BL/6 mice whereas the third, the S isolate (Sobreda - isolated from wild mice in Portugal) survives for much longer. The existence of the T. muris isolates thus presents a powerful tool to explore the mechanisms underlying chronic infection in a single strain of mouse. Here we show that S isolate infected mice have increased numbers of Foxp3+ T cells in the gut compared to mice infected with the E isolate. Treatment of mice infected with the S isolate with either anti-CD25 or anti-GITR exacerbated intestinal pathology, and, in addition, mice treated with anti-GITR were able to expel worms more rapidly, implying the release of local effector mechanisms from a regulatory influence. Thus our data show for the first time that T regulatory cells protect the host from worm driven intestinal pathology. In addition, our data reveal a subversion of this damage-limiting response by the S isolate to facilitate its own survival.
Keywords: Regulatory T Cells, Parasitic-Helminth, Anti-GITR, Anti-CD25, Pathology
Introduction
Gastrointestinal nematodes, such as Trichuris trichiura (human whipworm), infect over one billion people world wide. Resistance to infection involves the generation of type 2 responses which control the sets of effector mechanisms that eliminate these types of parasites (1, 2). The observation remains however that infections with these parasites are typically chronic, with intestinal nematodes living for many years in their host. This suggests that worms possess an ability to modulate the host immune response to favour their own survival (3). More recently, evidence is accumulating that Type 2 responses are important in wound healing (4-6) but although fibrosis is a necessary part of tissue repair, unrestrained it can lead to severe scarring and organ damage. The induction of regulated Type 2 responses, or “modified” Type 2 responses (7) associated with chronic helminth infections might therefore represent a trade off, where resources have been allocated to limiting damage but also allow increased parasite survival.
Trichuris muris, the mouse model for the human infection caused by Trichuris trichiura, has contributed significantly to our understanding of the host immune response to infection over many years (2, 8, 9). In nature T. muris, probably exists in a variety of different genotypes as for most organisms including other parasites, but only three isolates have been adapted for laboratory use. The majority of studies exploiting T. muris in the mouse have focused on the E (Edinburgh) isolate; however two other isolates exist; the J (Japan) and S (Sobreda) isolates. The J isolate was subcultured from the E isolate in 1969 and the S isolate was discovered in Sobreda - Portugal in 1992.
Previous work has shown that the S isolate can survive to chronicity in CBA (10), B10BR (11) and C57BL/6 (12) mice whereas the other two isolates, E and J, are expelled from these hosts prior to reaching adulthood. Expulsion of the E and J isolates from C57BL/6 mice is associated with the development of a Type 2 response. In contrast the survival of the S isolate in C57BL/6 mice correlates with a reduced Type 2 and increased Type 1 responses (12, 13).
The differences in expulsion kinetics and cytokine profiles are likely due to isolate specific excretory/ secretory antigens, with S isolate antigens perhaps being less immunogenic or able to induce strongly regulated responses. Here we test the hypothesis that the persistent nature of S isolate infections has its basis in the induction of regulatory T (Treg) cells which minimise host pathology but also aid worm survival via the dampening down of Type 2 driven effector mechanisms. Helminth infections are known to be associated with elevated numbers of Treg cells (14), however little is known about whether this expansion is a host response to control the pathology associated with infection or a parasite induced response to aid survival, or both. Further, an observed correlation between the presence of helminth infections and a low incidence of allergy and autoimmune diseases is thought to have its basis in the induction by helminths of Treg cells (15).
Several antibodies targeted to markers of Treg cells, including anti-glucocorticoid-induced tumor necrosis factor receptor (anti-GITR), anti-CD25 and anti-CTLA4 (16) have been used individually or in combination to deplete or alter Treg function in the context of parasitic nematode infections. For example during Trichinella spiralis infection anti-CTLA4 treatment lowered the numbers of parasites recovered from skeletal muscle whereas anti-GITR had no affect on parasite numbers (17). In Litomosoides sigmodontis infection, filarial parasite numbers are only affected when a combination of anti-CTLA4 and anti-CD25 is used (18). We believe our model system to be particularly powerful in that it allows the role of Treg cells to be analysed using different strains of the same parasite species which have different survival phenotypes in the C57BL/6 host. In particular we explore for the first time the importance of Treg cells in the control of helminth driven pathology and also evaluate their contribution to intestinal nematode survival.
Materials and Methods
Mice
C57BL6 male mice were obtained from Harlan (Bicester,UK) at 6-8weeks of age. Mice were specific pathogen free and maintained in sterile conditions in individually ventilated cages by the Biological Services Faculty (BSF), University of Manchester, UK. All work was performed under the regulations of the Home Office Scientific Procedures Act (1986).
Parasites
Both the E and S isolate of T. muris were maintained as previously described (19). Excretory/ secretory (E/S) antigen was collected by incubating adult worms in RPMI media for 4 hrs. Mice were infected orally with around 150-200 embryonated eggs. Worm burden analysis was carried out as previously described (20)
Antibodies and Treatment
The antibodies used in vivo were anti-GITR (DTA-1) (a kind gift from Shimon Sakaguchi) and anti-CD25 (PC61). The treatment regime consisted of intraperitoneal injections of 1mg of anti-GITR in PBS or anti-CD25 in PBS once a week commencing at day -7. Weekly injections regimes were used as Foxp3+ cells reappear in the MLN within 2 weeks post injection once mice are infected (D’Elia and Else, unpublished data). No injection of antibody was given at day 0, when the mice were infected with T. muris, because Treg cells do not reappear within 2 weeks until under infected conditions. Therefore we found the first day -7 injection adequate until day 7 post infection to deplete Treg cells. In additionm, alternative antibody injection regimes were assessed, including a protocol of d-7, d0, d7, d14 and d21 and the efficiency of Treg depletion was found to be similar for all methods. Control injections of PBS and rat Ig followed the same regime.
Immunohistochemistry
Tissue samples, from the proximal colon, were taken and placed in optimal cutting temperature (OCT) embedding medium (R.A. Lamb, Eaxtbourne,UK). The tissue was snap frozen in liquid nitrogen and 6μm sections were cut using a Micron HM560 cryostat. Foxp3+ cells were identified by immunohistochemistry. Briefly, tissue samples were fixed in 4% formaldehyde in PBS then placed in glucose/ azide solution. Samples were then blocked with goat serum followed by an avidin/biotin step. A double antibody step followed, with the primary antibody being a anti-mouse/rat FoxP3 FJK-16s (eBioscience) at 5μg/ml and the secondary being goat anti-rat IgG F(ab)2 (Chemicon International) at 1 in 4000 dilution. ABC (Avidin Biotin Complex), DAB (3,3′-diaminobenzidine) and haemtoxylin steps were then carried out until positive staining was visualised. Immunohistochemistry on naïve, E isolate and S isolate infected samples was carried out on the same day to avoid day to day variation in background staining levels.
Foxp3+ cells were assessed by counting the number positive cells in 20 colonic crypt units (ccu) per section. 3 sections from each mouse were counted and the crypt length measured by using ImageJ analysis software (National institute of Health).
MLN cell culture and Cytokine reagents
Mesenteric lymph nodes (MLN) were removed at day 21 post infection (p.i) and placed into a 15ml falcon tube containing 10 ml of complete medium (RPMI 1640 (Invitrogen, Carlsbad, CA), 5% Foetal calf serum (FCS), 100 U/ml penicillin and 100μg/ml streptomycin, 1% L-glutamine, 0.1% MTG (Sigma- Aldrich, St Louis, MO)) on ice. MLN cell suspensions were made under sterile conditions and the cell concentration determined using a CASY-1 Coulter Counter. The final cell concentration was adjusted to give 5×106 cells/ml.
1ml of MLN suspension was placed into 48 well plates and stimulated with 50μg/ml isolate-specific E/S or 2.5μg/ml Con-A as a positive control. Plates were then incubated at 37°C, 5% CO2, 95% humidity for 48 hours. The supernatant was frozen at -20 °C until analysed for cytokine content. Supernatants were assessed using a custom cytometric bead array kit (CBA- BD biosciences) for IFN-γ, IL-12p70, IL-12p40, IL-10, IL-6, TNF-α, IL-4, IL-5, IL-9 and IL-13.
FACS analysis
MLN were removed at time points p.i and placed in RPMI 1640 (Invitrogen), 5% Foetal calf serum (FCS), 100 U/ml penicillin and 100μg/ml streptomycin, 1% L-glutamine, 0.1% MTG) on ice. Cell concentration was measured on a CASY-1 Coulter Counter and adjusted to give 1×106 cells/ml. Cells were stained using the Mouse Regulatory T Cell Staining Kit (eBioscience) as per manufacturer’s instructions. Antibodies were APC anti-Foxp3 FJK-16s (eBicocience), PE anti-CD4 (L3T4) (BD Pharmigen), FITC anti-CD25 (7D4) (BD Pharmigen). Relevant isotype controls were used to verify specificity. Furthermore, an individual isotype control was carried out for each individual mouse sample. Cells were finally resuspended in equal quantities of FACS buffer and FACS Fix (2% formalydehyde in PBS). Samples were analysed on FACSCalibur (Becton Dickinson) using CellQuest Pro software (BD Biosciences, Mountain View, CA).
Statistics
Results are expressed as mean ± Standard deviations (SD).
Where data met the required assumptions, we fitted Generalised Linear models (GLM) in SPSS (vers 12.0.1 for Windows). In some cases data were normalised by Log10 (X+1) transformation. Percentages were converted to proportions and the arsine transformation of the square root of the proportion was fitted as the dependent variable. The appropriateness of all parametric models was assessed by examination of the residuals and by R2. Where data did not meet the assumptions of normality, and could not be transformed, we tested differences between groups by non-parametric methods, the Mann-Whitney U test (two factor comparisons) and Kruskal Wallis test (for more than two factors) with Dunns post test for multiple parameter comparisons. All non-parametric statistical tests were performed using GraphPad Prism software (San Diego, CA)
Results
Prolonged survival of the S isolate of T. muris compared to the E isolate in C57BL/6 mice
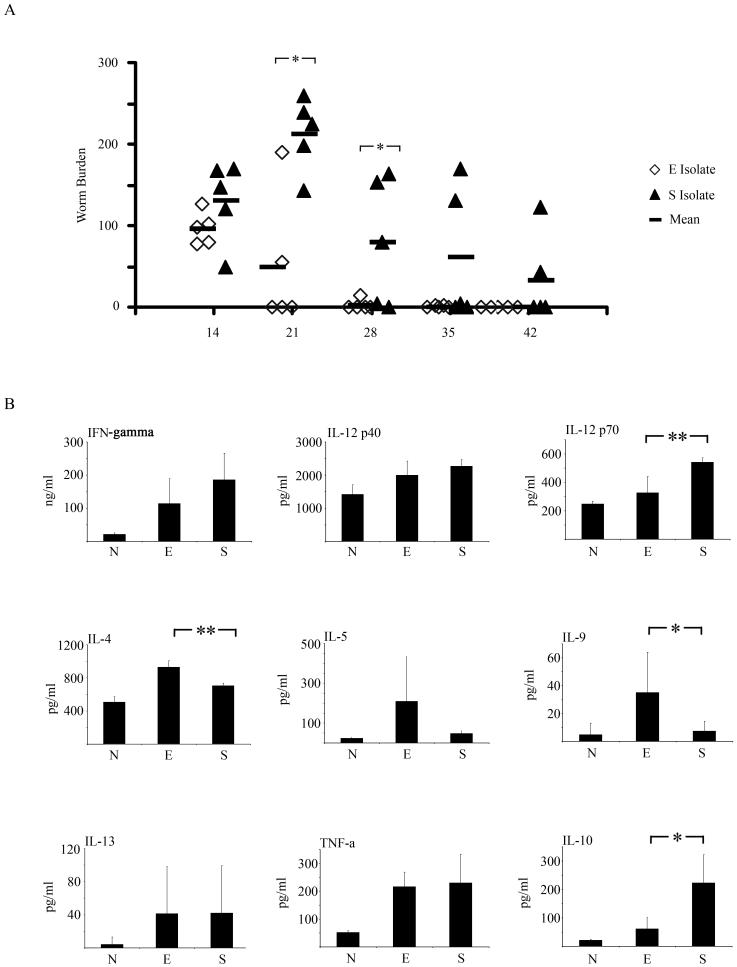
As previously reported, (12, 13), a reduction in the number of E isolate parasites is evident by d21 p.i and expulsion is almost complete by d28 p.i (Fig. 1a). In contrast, mice infected with the S isolate retained a high worm burden on day d21 p.i. and worm burdens were still elevated compared to mice infected with the E isolate on d28 p.i., although by this time some expulsion is apparent (Fig. 1a). (GLM with time (days 14, 21, 28 and 35 after infection) and strain as factors, on log (x+1) transformed worm burdens, for main effect of strain F1,40=20.4, P<0.001). In addition, MLN cytokine responses were analysed at d21 post E or S isolate infection. A general increase in Th2 cytokines is seen in supernatants following E isolate infection/re-stimulation compared to S isolate infection/re-stimulation, with significant increases in IL-4 and IL-9 (Mann Whitney U test, P= 0.0079 and 0.0159 respectively) (Fig. 1b). Further there was a significant increase in IL-12p70 (Th1 associated) following S isolate infection/re-stimulation (Mann Whitney U test, P= 0.0079) and a significant increase in IL-10 levels (Mann Whitney U test, P= 0.0159) (Fig. 1b). Interestingly at d21 p.i, two mice infected with the E isolate still retained worms unlike the other three mice in this group. These two mice had lower levels of IL-5, IL-9 and IL-13 reflected in the large Standard Deviations for these cytokines.
Figure 1.
Time course of an E and S isolate infection in C57BL/6 mice. A. C57BL/6 mice were infected with 150-200 embryonated eggs from either the E or the S isolate of T. muris. At various time points post-infection (p.i), the mice were sacrificed, caecae and proximal colons were removed and the number of worms counted. Each symbol represents an individual animal (5 mice per group) and the line represents the mean. B. Levels of IFN-γ, IL-12p40, IL-12p70, IL-4, IL-5, IL-9, IL-13, TNF- α and IL-10 in MLN supernatants were measured by CBA following MLN re -stimulation with parasite specific E/S; all cytokine data are pg/ml (B). Values represent the mean ± SD for five mice per group and were analysed at d21 p.i. *, significant difference between the E isolate and S isolate infected mice (Mann Whitney U test,*= p<0.05, **=p<0.01).
The numbers and percentages of Foxp3+ cells in the MLN following T. muris infection increase steadily
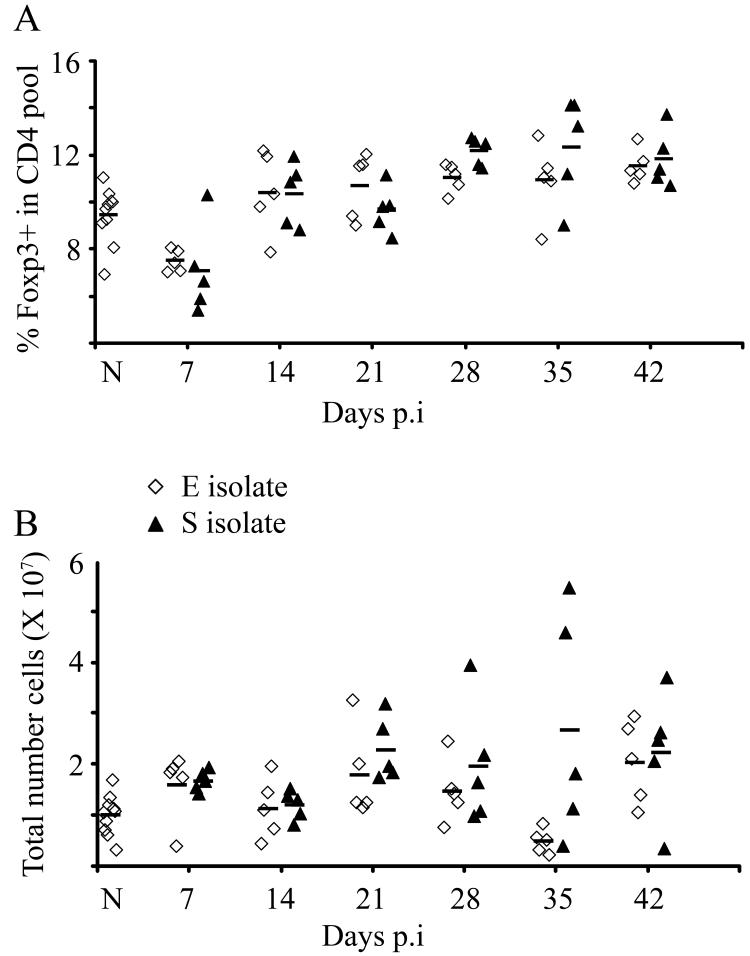
The percentage of Foxp3+ cells in the CD4+ pool increased after infection in mice exposed to both the E and S isolates as compared to their respective controls (cells from naïve mice) (Fig. 2a; GLM on arsine of square root transformed proportions with strain as factor and time (confined to days 14, 21, 28, 35 and 42) as a covariate, for effect of time F1,57=44.4, P<0.001, and β=0.002, t=6.7, P<0.001). Although on d28 and d35 p.i, the mean percentage of Foxp3+ cells in the CD4+ pool was slightly higher in mice with the S isolate infection compared to those exposed to the E isolate infection, this did not quite reach significance (for main effect of strain F1,57=0.17, P=NS) (Fig. 2a). When these percentages were converted into absolute numbers of Foxp3+ cells within the MLN a similar trend and pattern was seen, with increased numbers of Foxp3+ cells p.i in both groups of mice (infected with either the E or S isolate) and a non significant elevation in numbers of Foxp3+ cells in mice with the S isolate infection compared to the E isolate (Fig. 2b).
Figure 2.
Presence of Foxp3+ cells in the MLN following T. muris infection. C57BL/6 mice were infected with either the E or the S isolate of T. muris. At various time points mice were sacrificed and MLN cells analysed by flow cytometry (FC) for Foxp3 and CD4. (A) Percentage Foxp3+ cells within the CD4+ pool. (B) Total number of Foxp3+ cells within the MLN.
Experiments were performed at least twice and data shown is a representative experiment. Each symbol represents an individual animal (5 mice per group) and the line represents the mean. (Naïve; 10 mice); p.i (post infection)
Increased numbers of Foxp3+ cells in the gut following S isolate T. muris infection
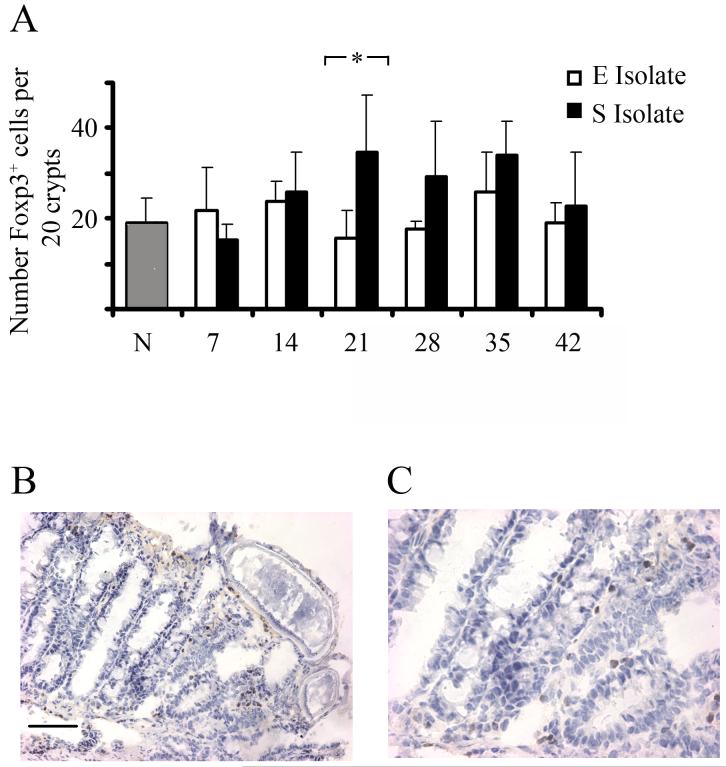
Sections of proximal colon were stained for Foxp3+ cells to quantify the number of Treg cells locally in the gut. There was little change from the background levels in naïve mice when Foxp3+ cells were counted in C57BL/6 mice infected with the E isolate but there was a significant increase in mice infected with the S isolate (GLM with strain and time as factors, F1,48=7.3, P=0.009) (Fig. 3a). Importantly at d21 p.i, a time point when expulsion of the E isolate of T. muris has begun in C57BL/6 mice, the mice infected with the S isolate had significantly more Foxp3+ cells in the proximal colon compared to the intestine of mice infected with the E isolate (Mann Whitney U test, P= 0.0317, Fig. 3a). Interestingly, mice harbouring the highest number of worms had the highest number of Foxp3+ cells in the proximal colon (data not shown). Examples of typical sections from a mouse carrying the S isolate, stained for Foxp3+, taken on d42 p.i are shown in figures 3b and 3c.
Figure 3.
Presence of Foxp3+ cells in the gut following Trichuris muris infection. C57BL/6 mice were infected with either the E or S isolate of Trichuris muris. At various time points mice were sacrificed and Foxp3+ cells were identified in colonic tissue sections via immunohistochemsisty. Data are presented as mean number of Foxp3+ cells per 20 colonic crypt units (ccu) (A), with an example at original magnification X 200 (B) X 400 (C) of brown Foxp3 staining in a mouse infected with the S isolate at d42 p.i. *, significant difference between the E isolate and S isolate infected mice (Mann Whitney U test, p<0.05). Experiments were performed at least twice and data shown is a representative experiment. Values represent the mean ± SD for five mice per group.
Fewer Foxp3+ cells are observed in the MLN and intestine in mice infected with the S following treatment with anti-CD25
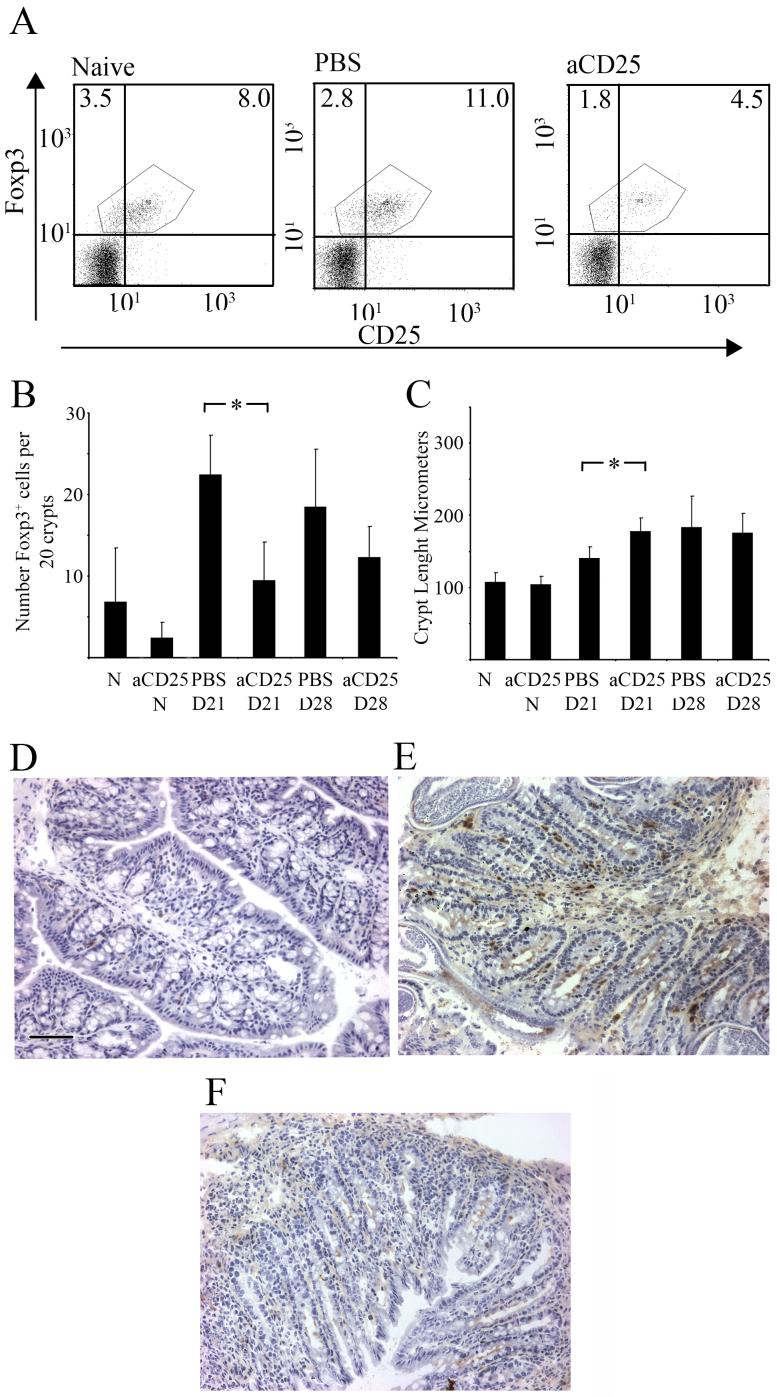
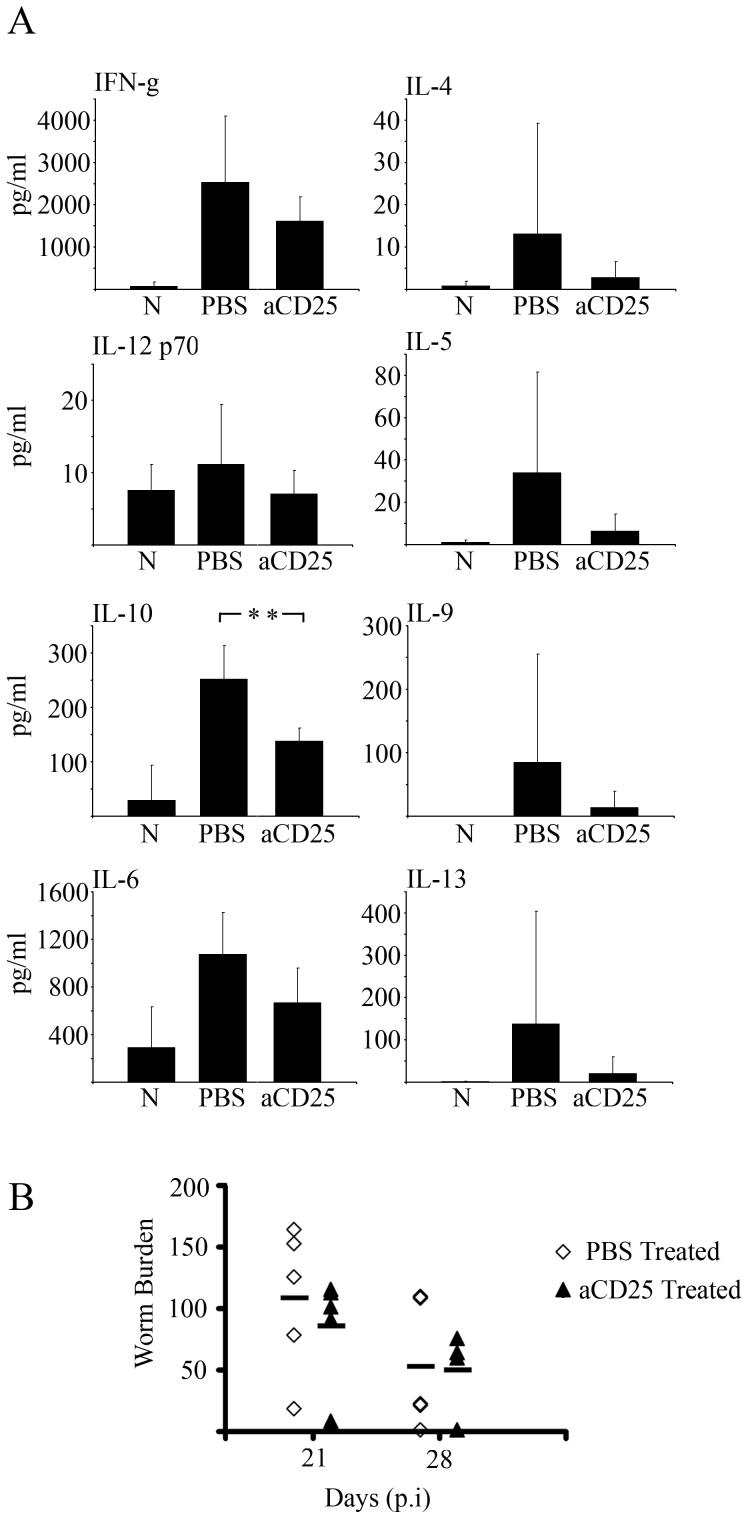
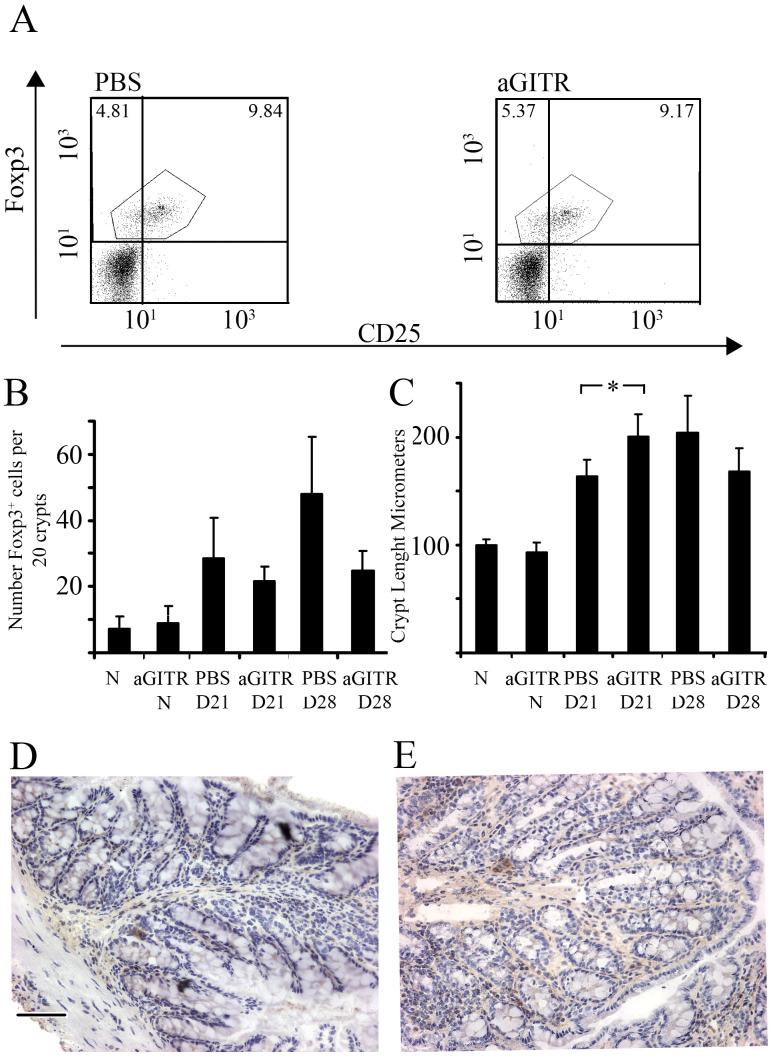
Given the increase in Foxp3+ cells in the gut of the mice infected with the S isolate on d21 p.i we investigate whether anti-CD25 treatment in vivo would reduce the number of Foxp3+ cells and alter worm associated gut pathology and/or the kinetics of worm expulsion. Mice infected with the S isolate and treated with anti-CD25 showed a significant reduction in the percentage of Foxp3+ cells in the MLN compared to infected mice given control injections (at d21 PBS control = 12.22± 0.58, anti-CD25 treated = 5.336± 0.66; at d28 PBS control = 12.0± 0.36, anti-CD25 treated = 7.7± 0.35) (GLM on arsine of square root transformed proportions, with time and treatment as factors, for main effect of treatment F1, 15=103.0, P<0.001)(Fig. 4a). A similar reduction of Foxp3+ cells to that seen in the MLN was also observed locally in the gut with a significant fall in Foxp3+ cells per 20 ccu in infected mice treated with anti-CD25 at d21 p.i compared to relevant PBS treated control samples (Fig. 4b, Mann-Whitney U test, P=0.0079) (GLM on number of Foxp3+ Cells in 20 ccus, with time and treatment as factors, F1, 15=15.0, P=0.001). Treatment of uninfected mice with anti-CD25 revealed a non significant reduction in Foxp3+ cells locally in the gut (Fig. 4b). The reduction in Foxp3+ cells in the proximal colon in anti-CD25 treated-infected mice was associated with increased gut pathology. Thus at d21 p.i infected mice treated with anti-CD25 had significantly increased crypt depth compared to PBS control treated mice (Fig. 4c, Mann-Whitney U test, P=0.028). Anti-CD25 treated uninfected mice did not show any change in gut pathology implying that the function of the depleted Treg population was to control worm driven pathology (Fig. 4c).
Figure 4.
S isolate infected mice treated with anti-CD25 have reduced numbers of Foxp3+ cells in the MLN and an increased gut pathology. S isolate infected or uninfected C57BL/6 mice were treated with 1mg of anti-CD25 antibody or PBS i.p weekly commencing at day -7, with no injection given on day 0. Mice were sacrificed at 21 or 28 days p.i. MLN were analysed by FC for CD4, CD25 and Foxp3. (A) Plots are gated on CD4+ T cells and show Foxp3 vs CD25 for a naïve, a PBS infected control and an anti-CD25 treated infected mice. Experiments were performed at least twice with five mice per group; data are from one representative mouse per group. (B) Foxp3+ cells were detected in colonic tissue sections via immunohistochemsisty, data are presented as mean number of Foxp3+ cells per 20 ccu. (C) Crypt length was measured to determine gut pathology and data are presented as mean crypt lengths in μm. Experiments were performed at least twice and data shown is a representative experiment. Values represent the mean ± SD for five mice per group. *, significant difference between the PBS control and anti-CD25 S isolate infected mouse (Mann Whitney U * = p<0.05, **=p<0.01). D21, D28; day 21 or day 28 post infection. aCD25, anti-CD25; N,Naïve.
(D, E and F) Representative example of Foxp3 staining in a naïve (D) a PBS injected S isolate infected control (E) and an anti- CD25 treated S isolate infected mouse at d21 p.i. Scale Bar = 100 μm
Immunohistochemistry of a naïve (Fig. 4d), PBS injected and infected control (Fig. 4e) and an infected anti-CD25 treated mouse (Fig. 4f) highlighted clearly the differences in the number of Foxp3+ cells present in the proximal colon and how these correlated with disrupted crypt architecture, leading to an increase in crypt length following reduction of Treg cells.
Reduction in Th2 cytokine profile following anti-CD25 treatment, and unaltered worm expulsion kinetics
Anti-CD25 treatment significantly reduced IL-10 production by MLNC at d21 p.i in mice infected with the S isolate (Mann Whitney U test, P= 0.0079), however treatment also decreased the Th2 associated cytokines IL-4, IL-5, IL-9 and IL-13 (Fig. 5a). The decrease in Th2 associated cytokines may explain the non significant difference in worm burden between PBS infected controls and anti-CD25 infected mice at d21 and d28 p.i (Fig. 5b).
Figure 5.
Mesenteric Lymph Node (MLN) cells from S isolate infected mice treated with anti-CD25 secrete a reduced level of the IL-10 and levels of the Th2 cytokines. S isolate infected C57BL/6 mice were treated with 1mg anti-CD25 (mAB) or PBS weekly from d-7 excluding d0. At day 21 p.i MLN cells from naïve, PBS control and anti-CD25 treated S isolate infected mice were isolated and cultured with parasite specific ES antigen for 48hrs. A. Levels of IFN-γ, IL-12p70, IL-10, IL-6, IL-4, IL-5, IL-9 and IL-13 in MLN supernatants were measured by CBA; all cytokine data are pg/ml (A). Values represent the mean ± SD for five mice per group. *, significant difference between the PBS control and anti-CD25 S isolate infected mice (Mann Whitney U * = p<0.05, **=p<0.01)
B. Worm numbers in mice from both PBS control and anti-CD25 treated groups were counted at days 21 and 28p.i. (B) Each symbol represents an individual animal and the line represents the mean with 5 mice per group. Experiments were performed at least twice and data shown is a representative experiment.
Anti- GITR treatment does not alter the numbers of Foxp3+ cells in the MLN and gut post S isolate infection
Since anti-CD25 treatment reduced the number of Foxp3+ cells in the MLN and gut of S isolate infected mice, but also altered the Th2 cytokine profile and failed to enhance worm clearance, an alternative approach was used in vivo to manipulate the Treg cell population. Mice infected with the S isolate were injected with anti-GITR following the same regime as anti-CD25. Anti-GITR has been shown to have varying effects on Treg populations (21), the principal consequence being the disruption of Treg function (22).
Anti-GITR treatment of infected mice did not alter the percentage of Foxp3+ cells in the MLN compared to PBS treated infected mice (at d21 PBS control = 12.566± 1.83, anti-GITR treated = 11.918± 1.76) (Fig. 6a). This lack of depletion of Foxp3+ cells in the MLN was also seen at d21 p.i in the proximal colon (Fig. 6b), with no significant difference in Foxp3+ cells in 20 ccu between anti-GITR treated infected mice and PBS control infected mice. An increase in Foxp3+ cells in PBS treated infected mice was seen on d28 p.i and likely reflected worm persistence in this group (see Fig. 7b). Although the numbers of Foxp3+ cells in the guts of infected mice were similar, anti-GITR treatment did appear to enhance gut pathology. Thus at d21 p.i there was a significant increase in crypt length between control and anti-GITR treated groups (Fig. 6c Mann-Whitney U test, P=0.0317). Naïve mice injected with anti-GITR had similar numbers of resident Foxp3+ cells and gut pathology compared to naïve untreated samples (Fig. 6b and 6c), Immunohistochemistry of PBS infected control (Fig. 6d) and infected anti-GITR treated mice (Fig. 6e) showed no differences in the number of Foxp3+ cells present in the proximal colon, however, a disrupted crypt architecture, leading to increase in crypt length following anti-GITR treatment was evident.
Figure 6.
The number and percentage of Foxp3+ cells in the MLN of S isolate infected C57BL/6 mice are not affected by anti-GITR treatment but gut pathology is increased. S isolate infected or uninfected C57BL/6 mice were treated i.p with 1mg anti-GITR or PBS weekly from d-7 excluding d0. Mice were sacrificed at 21 or 28 days p.i. MLN were analysed by FC for CD4, CD25 and Foxp3. (A) Plots are gated on CD4+ T cells and show Foxp3 vs CD25 for a naïve, PBS infected control and an anti-GITR treated infected mouse. Experiments were performed at least twice with five mice per group; data are from one representative mouse per group. (B) Foxp3+ cells, detected in proximal colon tissue sections via immunohistochemsisty, are presented as mean number of Foxp3+ cells per 20 ccu. (C) Crypt length was measured to quantify gut pathology. Data are presented as mean crypt length in μm (C). Experiments were performed at least twice and data shown is a representative experiment. Values represent the mean ± SD for five mice per group. *, significant difference between the PBS treated, S isolate infected and anti-GITR treated S isolate infected mice (Mann Whitney U test, p<0.05). D21, D28; day 21 or day 28 post infection. aGITR, anti-GITR; N, Naïve.
(D and E) Representative example of Foxp3 staining in (D) a PBS treated, infected control and (E) an anti-GITR treated mouse infected with the S isolate at (d21 p.i)/N= naïve; D21,D28 = day 21 or 28 post infection. Scale Bar = 100 μm
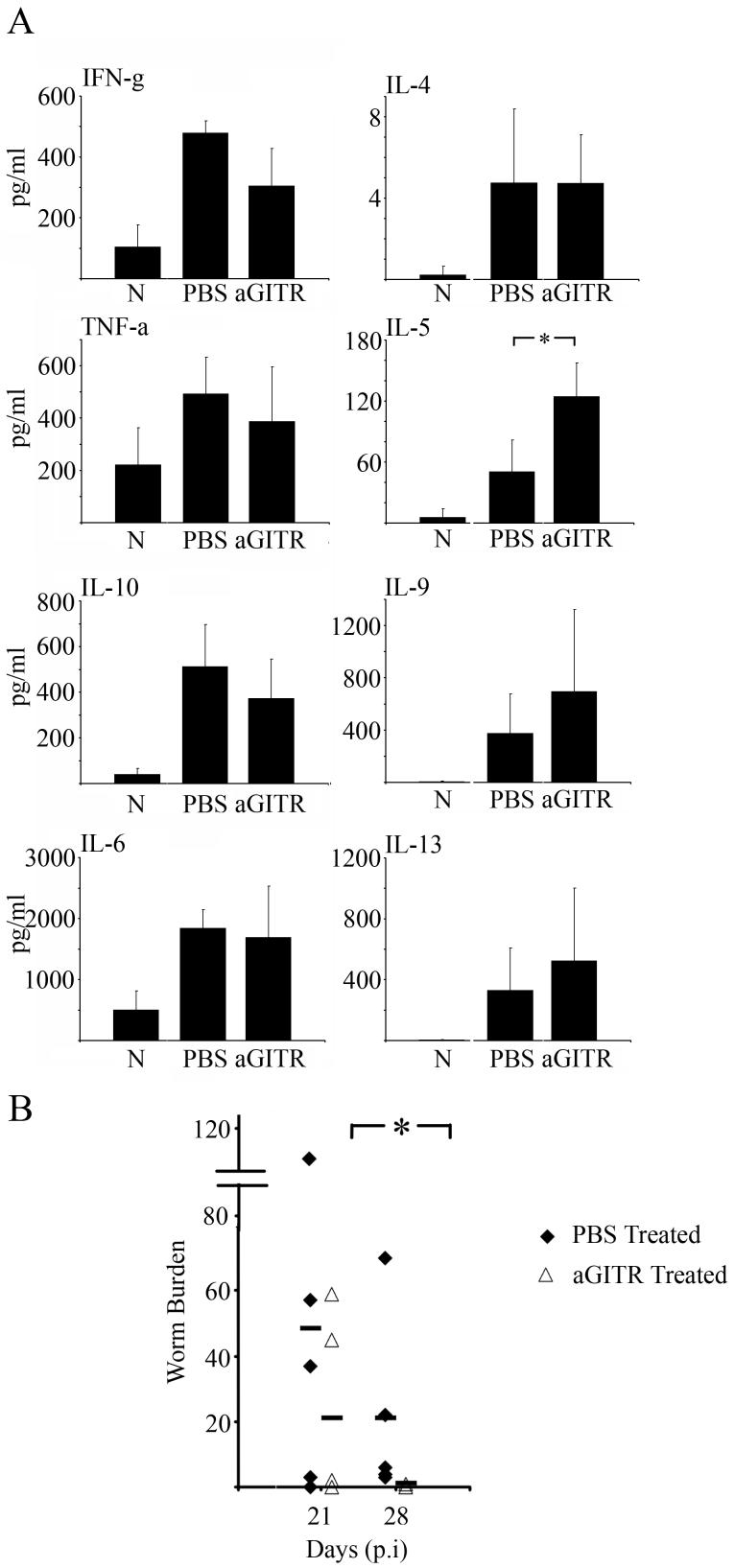
Figure 7.
Treatment of S isolate infected mice with anti-GITR does not affect IL-10 or Th1 associated cytokines but does increase some Th2 cytokines. S isolate infected C57BL/6 mice were treated i.p with 1mg anti-GITR or PBS weekly from d-7 excluding d0. At day 21 p.i MLN cells from naïve, PBS control and anti-GITR treated S isolate infected mice were isolated and cultured with parasite specific ES antigen for 48hrs. Supernatant levels of IFN-γ, TNF-α, IL-10, IL-6, IL-4, IL-5, IL-9 and IL-13 were measured by CBA; all cytokine data are pg/ml (A). Values represent the mean ± SD for five mice per group. *, significant difference between the PBS control and anti-GITR S isolate infected mouse (Mann Whitney U test, p<0.05).
Worm numbers in mice from both PBS control and anti-GITR treated groups were analysed at days 21 and 28p.i. (B) Each symbol represents an individual animal (5 mice per group) and the line represents the mean. Experiments were performed at least twice and data shown is a representative experiment.
Increased Th2 cytokine profile following anti-GITR treatment, and significantly faster worm expulsion
As previously stated, anti-GITR treatment did not affect the percentage or number of Foxp3+ cells in the MLN or gut but did exacerbate gut pathology. This implies that the anti-GITR antibody weakened a regulatory influence, and had a similar qualitative effect observed with anti-CD25 treatment where the reduction in Treg function was quantitative. Analyses of the MLN cell cytokine profiles and worm burdens in anti-GITR treated infected mice revealed important differences between anti-CD25 and anti-GITR treatments. Thus IL-10 was not significantly different between PBS treated infected controls and anti-GITR treated infected groups (Fig. 7a). Interestingly, in contrast to anti-CD25 treatment, anti-GITR treated infected mice had significantly elevated IL-5 (Fig. 7a, Mann-Whitney U test, P=0.0317) as compared to PBS treated infected controls. Other Th2 associated cytokines, IL-9 and IL-13 also showed a similar trend with higher values in the anti-GITR treated groups (Fig. 7a) although IL-4 levels were not increased. There was no significant alteration in the Th1 associated cytokine IFN-g (Fig. 7a) or IL-12 p70 (levels extremely low, data not shown) and also no changes in TNF-α and IL-6 levels (Fig. 7a). The increase in the general Th2 profile following anti-GITR treatment correlated with a significant decrease in worm burdens at d28 compared to the PBS infected controls (Fig. 7b, Mann-Whitney U test, P=0.0159). By d28 p.i only one anti-GITR treated mouse harboured one worm, all the other 4 mice in the group having completely expelled their worms. In contrast all the mice in the PBS infected controls harboured some worms.
To validate the use of our PBS injection control through out, we conducted an experiment to directly compare cytokines levels, gut pathology and FOXP3+ cells in S-isolate infected mice treated with rat Ig, PBS or anti-GITR. Our data shows that the cytokine levels in the rat Ig treated and PBS-treated groups are not significantly different (IFN-γ: rat Ig treated = 79.3±6.5 versus PBS treated = 93.6± 23.7; IL-10: 40.45±52.13 versus 44.6± 45.87; IL-12p70: 0 versus 5.31± 11.76; IL-13: 3.92±4.81 versus 10.24± 12.4; IL-4: 0.75±0.83 versus 0.27± 0.66; IL-5: 2.51±3.54 versus 1.27± 1.5; IL-6: 146.6±219.0 versus 706.51± 848.89; IL-9: 2.806±6.87 versus 27.41± 25.3 and TNF-α: 165.19±259.75 versus 327.05± 278.92).
The changes in cytokine levels in the anti-GITR treated group were similar to data shown in Fig. 7a (IFN-γ: 178±121.6; IL-10: 136.05±144.9; IL-12p70: 0.65± 1.6; IL-13: 2.93±2.94; IL-4: 3.395±2.72; IL-5: 21.39±16.34 IL-6: 455.05±502.4; IL-9: 7.42±11.627 and TNF-α: 625.65±1018.7), with IL-5 levels again being significantly elevated above rat Ig and PBS controls (P<0.05 for both).
Thus the changes in Trichuris-specific cytokine responses in anti-GITR-treated mice and anti-CD25 treated mice (Figure 5 and 7) are not due to an accumulating anti-rat Ig response.
Further, Foxp3+ staining for cells in gut tissue and MLN of S-isolate infected mice treated with rat Ig, PBS or anti-GITR likewise showed identical numbers in the two control groups (Gut: cells per 20 crypt units, Rat Ig treated = 20.83±7.37 versus PBS =21.49±7.89); (MLN Rat Ig treated =11.55±1.2, PBS =11.66±1.27) and these were not significantly different to the anti-GITR treated mice, as before (gut: cells per 20 crypt units 22.14±7.9; MLN:11.4±1.19). In addition the crypt lengths in S-isolate infected mice treated with rat Ig or PBS were identical (crypt Length, rat Ig treated = 137.28±15.9 versus PBS treated = 138.63±23.8) and significantly increased in the anti-GITR (crypt Length 197.4±30.71) (Kruskal-Wallis Test, p<0.05 vs PBS treated and p<0.01 vs Rat Ig treated)
Thus an anti-rat Ig response is not responsible for the observed changes in Foxp3+ cell numbers or crypt length post treatment with either anti-CD25 or anti-GITR.
Discussion
The S isolate of T. muris has the ability to survive in strains of mice, including CBA (13) and C57BL/6 mice (12) which expel the E isolate. In this study we confirm the ability of the S isolate to survive for longer in C57BL/6. Importantly we also provide evidence that Treg cells limit S isolate induced intestinal pathology and that this regulation also underlies the ability of the S isolate to persist beyond the fourth week of infection, a time when the E isolate has been expelled.
The more chronic nature of the S isolate compared to the E isolate may reflect its more recent adaptation to passage in laboratory mice (13). In contrast the E isolate has been passaged for many years in immunosuppressed or SCID mice and thus may have lost the ability to induce the mechanisms required to support its survival in the absence of any immunological pressure.
Helminth induced regulation is currently an area of intense research and there is evidence of increased Treg numbers in many helminth infections (1, 14, 15, 23). Despite a large body of work existing on the immunology of T. muris infection, analysis of the role of Treg cells in worm persistence and in the control of worm driven pathology has not previously been evaluated. The breadth of immunomodulatory strategies employed by intestinal helminths to survive within their hosts is astonishing (3). T. muris for instance, is believed to express an IFN-g like molecule (24) and other cytokine-like molecules (25) which are able to drive the Th response inappropriately towards Th1 dominance. Adding to this literature, we here demonstrate a functional association between the presence of Foxp3+ cells and the survival of T. muris. We describe three novel findings. Firstly we show that the number of Foxp3+ cells in the proximal colon of C57BL/6 mice infected with the S isolate is higher than those infected with the E isolate. Secondly, the reduction in the number or function of Treg cells in the MLN and gut following anti-CD25 or anti-GITR treatment results in increased gut pathology following T. muris infection. Thirdly, anti-CD25 and anti-GITR treatments have contrasting effects; although both treatments increase gut pathology only anti-GITR treatment leads to earlier worm expulsion.
The increased number of Treg cells found in the proximal colon of S isolate infected C57BL/6 may be due to an increased recruitment of Foxp3+ cells from the intestine-draining MLN or an increased induction of Treg cells by S isolate antigens locally in the mucosa. Moreover, in support of the flow cytometric and immunohistochemical data, microarray data showed an increase in gene expression of Foxp3 (probeset 1420765) in the MLN and gut of mice infected with the S isolate compared to mice infected with the E isolate. (D’Elia and Else, unpublished data).
The differences in the survival time of the two isolates and the elevated numbers of Foxp3+ cells in the gut of S isolate infected mice lead us to hypothesise that the S isolate is able to exploit a regulatory response evolved to limit host damaging pathology. Thus removal of regulation in the context of an S isolate infection predicts the observed exacerbation in gut pathology and facilitation of worm expulsion due to a reduction in the regulation of effector responses.
Changes in parasite numbers have been reported previously after the removal Treg cells. Thus, when Treg cells were depleted in Litomosoides sigmodontis infection using combinations of anti-CD25 and anti-CTLA4 (18) or anti-CD25 and anti-GITR (26), parasite numbers were reduced. More recently it has been reported that the use of anti-CTLA4 antibodies in vivo resulted in reduced numbers of Trichinella spiralis muscle larvae in female NIH mice, whereas anti-GITR antibodies had no affect (17). In addition, during Trypanosoma cruzi infection, in BALB/c mice, anti-GITR or anti-CD25 treatment increased mortality, with anti-GITR also increasing myocarditis (27). Thus manipulation of Treg cell populations by different antibody treatments has varied effects on parasite survival and the host inflammatory response.
In the current study we use anti-CD25 or anti-GITR individually to determine whether altering Treg cell numbers / function in vivo can alter the survival of and pathology induced by S isolate, the more long-lived of the two isolates. Anti-CD25 does not completely deplete the percentage of Foxp3+ cells in the MLN but does reduce numbers by around 50%. Although higher levels of depletion have been reported this may reflect differences in the methodology of detecting the presence of CD25+ cells by flow cytometry. Other studies have used the same antibody for detection and depletion. Here we deplete with one antibody, PC61 and stain with another, 7D4 which we believe gives a more realistic measure of effectiveness.
The reduction in numbers of Foxp3+ MLN cells after anti-CD25 treatment was also seen in the gut. This reduction, from the elevated levels typically seen after infection to levels comparable to those observed in naïve animals, was associated with exacerbated gut pathology in infected mice, compared to controls. Although Treg have been shown to play important roles in the control of gut inflammation in the context of inflammatory bowel disease ( IBD )(28), this is the first time that these cells have been shown to be important in the control of worm induced intestinal pathology.
Treatment with antibodies to Treg markers, and in particular anti-CD25, can also affect activated T cells expressing CD25 (29, 30) In our T. muris model, anti-CD25 reduced the amount of secreted IL-10, from isolated MLN cells cultured with parasite specific E/S, however the antibody treatment also seemed to have an effect on the overall Th2 profile. Thus cytokine levels of IL-4, IL-5, IL-9 and IL-13 were all lower in anti-CD25 treated mice compared to control mice. Given the importance of Th2 cytokines in the resistance to T. muris infection (31-33) it is not surprising therefore that anti-CD25 treatment did not facilitate worm expulsion even though Treg populations had been reduced.
GITR is a member of the tumor necrosis factor receptor (TNFR) superfamily and is expressed on regulatory T cells (34, 35). Anti-GITR antibodies have been used to alter Treg cell function, although the mode of action of anti-GITR antibodies is still controversial. It has been shown to deplete Treg numbers, affect Treg function (22) and even to increase regulatory populations (21, 36). It may also act in a co-stimulatory way to increase T effector cell function (37, 38).
Unlike the anti-CD25 treatment; anti-GITR treatment did not reduce the percentage of Foxp3+ cells in the MLN or the number of Foxp3+ cells in the gut at d21 p.i. This is consistent with other studies (34). However, despite this lack of a quantitative difference in Treg cells, exacerbated gut pathology and an increase in crypt length were seen at d21 p.i suggesting a change in local Treg cell function. The lower number of Treg cells seen in anti-GITR treated mice at d28 p.i could be due to the completion of worm expulsion and thus the cellular environment of the gut returning to normal.
In contrast to anti-CD25 treatment of infected mice, anti-GITR treatment did not result in a reduction in IL-10 production by MLNC. However IL-10 is produced by a number of other cell types including Th1, Th2 and B cells (39, 40) and the cellular source of the IL-10 was not identified in this study. In addition Th2 cytokines were elevated in anti-GITR treated mice consistent with the faster expulsion of the S isolate. These data, together with the exacerbated gut pathology, suggest that anti-GITR treatment released Th2 effector mechanisms from a regulatory influence (41). Currently it is unclear how depletion of Tregs from the host facilitates worm expulsion. It is likely that decreased regulation of a Th2-controlled effector mechanism allows expulsion to proceed with greater efficacy. For example, a depressed rate of epithelial cell turnover results in an inability to expel T.muris (42) and thus one exciting possibility is that Tregs are able to regulate the so called “epithelial escalator”.
A number of studies have demonstrated the ability of helminths to induce Treg cells to dampen inflammatory disorders and furthermore, this ability forms the basis of therapeutic use of helminths to treat IBD (43, 44). The data presented reveal a role for Treg cells in the control of worm induced gut pathology and moreover, suggest the exploitation of Treg cells by a helminth to facilitate its own survival within the host. Comparisons of parasite derived antigens between the S and E isolates will facilitate the identification of those antigens unique to the S isolate which drive the observed increase in Treg cell numbers post infection
Acknowledgements
We thank Professor Shimon Sakaguchi for the kind donation of the DTA-1 hybridoma. In addition we would like to thank Dr. Mathew Taylor, University of Edinburgh, for advice on anti-CD25 injection regimes.
GRANT SUPPORT:
RD is supported by PhD Studentship from the BBSRC and KJE is supported by a grant from the Wellcome Trust (Grant no. 081120).
NON-STANDARD ABBREVIATIONS
- d
day
- ccu
colonic crypt units
- E/S
Excretory/ Secretory
- MLN
mesenteric lymph node
- p.i
post infection
Footnotes
Publisher's Disclaimer: Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nature reviews. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Advances in parasitology. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- 3.Else KJ. Have gastrointestinal nematodes outwitted the immune system? Parasite immunology. 2005;27:407–415. doi: 10.1111/j.1365-3024.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 4.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 5.Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- 6.Reiman RM, Thompson RW, Feng CG, Hari D, Knight R, Cheever AW, Rosenberg HF, Wynn TA. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infection and immunity. 2006;74:1471–1479. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson MS, Maizels RM. Regulatory T cells induced by parasites and the modulation of allergic responses. Chemical immunology and allergy. 2006;90:176–195. doi: 10.1159/000088892. [DOI] [PubMed] [Google Scholar]
- 8.Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. International journal for parasitology. 1998;28:1145–1158. doi: 10.1016/s0020-7519(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 9.Roach TI, Wakelin D, Else KJ, Bundy DA. Antigenic cross-reactivity between the human whipworm, Trichuris trichiura, and the mouse trichuroids Trichuris muris and Trichinella spiralis. Parasite immunology. 1988;10:279–291. doi: 10.1111/j.1365-3024.1988.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 10.Bellaby T, Robinson K, Wakelin D, Behnke JM. Isolates of Trichuris muris vary in their ability to elicit protective immune responses to infection in mice. Parasitology. 1995;111(Pt 3):353–357. doi: 10.1017/s0031182000081907. [DOI] [PubMed] [Google Scholar]
- 11.Koyama K, Ito Y. Comparative studies on immune responses to infection in susceptible B10.BR mice infected with different strains of the murine nematode parasite Trichuris muris. Parasite immunology. 1996;18:257–263. doi: 10.1046/j.1365-3024.1996.d01-92.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnston CE, Bradley JE, Behnke JM, Matthews KR, Else KJ. Isolates of Trichuris muris elicit different adaptive immune responses in their murine host. Parasite immunology. 2005;27:69–78. doi: 10.1111/j.1365-3024.2005.00746.x. [DOI] [PubMed] [Google Scholar]
- 13.Bellaby T, Robinson K, Wakelin D. Induction of differential T-helper-cell responses in mice infected with variants of the parasitic nematode Trichuris muris. Infection and immunity. 1996;64:791–795. doi: 10.1128/iai.64.3.791-795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nature reviews. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 15.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites--masters of regulation. Immunological reviews. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 16.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature immunology. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 17.Furze RC, Culley FJ, Selkirk ME. Differential roles of the co-stimulatory molecules GITR and CTLA-4 in the immune response to Trichinella spiralis. Microbes and infection / Institut Pasteur. 2006;8:2803–2810. doi: 10.1016/j.micinf.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Taylor MD, Harris A, Babayan SA, Bain O, Culshaw A, Allen JE, Maizels RM. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J Immunol. 2007;179:4626–4634. doi: 10.4049/jimmunol.179.7.4626. [DOI] [PubMed] [Google Scholar]
- 19.Wakelin D. Acquired immunity to Trichuris muris in the albino laboratory mouse. Parasitology. 1967;57:515–524. doi: 10.1017/s0031182000072395. [DOI] [PubMed] [Google Scholar]
- 20.Else KJ, Wakelin D. Genetically-determined influences on the ability of poor responder mice to respond to immunization against Trichuris muris. Parasitology. 1990;100(Pt 3):479–489. doi: 10.1017/s0031182000078793. [DOI] [PubMed] [Google Scholar]
- 21.Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: more than an effector T cell co-stimulatory system. European journal of immunology. 2007;37:1165–1169. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annual review of immunology. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 23.Maizels RM. Infections and allergy - helminths, hygiene and host immune regulation. Current opinion in immunology. 2005;17:656–661. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Grencis RK, Entwistle GM. Production of an interferon-gamma homologue by an intestinal nematode: functionally significant or interesting artefact? Parasitology. 1997;115(Suppl):S101–106. doi: 10.1017/s0031182097002114. [DOI] [PubMed] [Google Scholar]
- 25.Pennock JL, Behnke JM, Bickle QD, Devaney E, Grencis RK, Isaac RE, Joshua GW, Selkirk ME, Zhang Y, Meyer DJ. Rapid purification and characterization of L-dopachrome-methyl ester tautomerase (macrophage-migration-inhibitory factor) from Trichinella spiralis, Trichuris muris and Brugia pahangi. The Biochemical journal. 1998;335(Pt 3):495–498. doi: 10.1042/bj3350495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 27.Mariano FS, Gutierrez FR, Pavanelli WR, Milanezi CM, Cavassani KA, Moreira AP, Ferreira BR, Cunha FQ, Cardoso CR, Silva JS. The involvement of CD4(+)CD25(+) T cells in the acute phase of Trypanosoma cruzi infection. Microbes and infection / Institut Pasteur. 2008 doi: 10.1016/j.micinf.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Control of immune pathology by regulatory T cells. Novartis Foundation symposium. 2003;252:92–98. discussion 98-105, 106-114. [PubMed] [Google Scholar]
- 29.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. The Journal of experimental medicine. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bancroft AJ, Artis D, Donaldson DD, Sypek JP, Grencis RK. Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. European journal of immunology. 2000;30:2083–2091. doi: 10.1002/1521-4141(200007)30:7<2083::AID-IMMU2083>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Bancroft AJ, Grencis RK. Th1 and Th2 cells and immunity to intestinal helminths. Chemical immunology. 1998;71:192–208. doi: 10.1159/000058711. [DOI] [PubMed] [Google Scholar]
- 33.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. The Journal of experimental medicine. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nature immunology. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 35.McHugh RS, Shevach EM. The role of suppressor T cells in regulation of immune responses. The Journal of allergy and clinical immunology. 2002;110:693–702. doi: 10.1067/mai.2002.129339. [DOI] [PubMed] [Google Scholar]
- 36.Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. European journal of immunology. 2005;35:1016–1022. doi: 10.1002/eji.200425818. [DOI] [PubMed] [Google Scholar]
- 37.Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. European journal of immunology. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 39.Rutz S, Janke M, Kassner N, Hohnstein T, Krueger M, Scheffold A. Notch regulates IL-10 production by T helper 1 cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3497–3502. doi: 10.1073/pnas.0712102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rieger A, Bar-Or A. B-cell-derived interleukin-10 in autoimmune disease: regulating the regulators. Nature reviews. 2008;8:486–487. doi: 10.1038/nri2315-c1. [DOI] [PubMed] [Google Scholar]
- 41.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 42.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: A new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 43.Moreels TG, Pelckmans PA. Gastrointestinal parasites: potential therapy for refractory inflammatory bowel diseases. Inflammatory bowel diseases. 2005;11:178–184. doi: 10.1097/00054725-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Ruyssers NE, De Winter BY, De Man JG, Loukas A, Herman AG, Pelckmans PA, Moreels TG. Worms and the treatment of inflammatory bowel disease: are molecules the answer? Clinical & developmental immunology. 2008;2008:567314. doi: 10.1155/2008/567314. [DOI] [PMC free article] [PubMed] [Google Scholar]