Abstract
Objective
To evaluate the impact on schistosomiasis of biennial treatment with praziquantel (PZQ) among school-age children in Burkina Faso, the first country that achieved full national coverage with treatment of more than 90% of the school-age population.
Methods
A cohort of 1727 schoolchildren (6–14 years old) was monitored at yearly intervals through a longitudinal survey. Additional groups of schoolchildren were monitored in cross-sectional surveys. Parasitological examinations for Schistosoma haematobium and Schistosoma mansoni were performed, and prevalence and intensity of infection before and after treatment were analysed.
Findings
Data from the longitudinal cohort show that a single round of PZQ treatment significantly reduced prevalence of S. haematobium infection by 87% (from 59.6% to 7.7%) and intensity of infection by 92.8% (from 94.2 to 6.8 eggs/10 ml of urine) 2 years post-treatment. The impact on infection was also confirmed by a cross-sectional survey 2 years post-treatment. Importantly, the proportion of school-age children with heavy S. haematobium infection decreased from around 25% before treatment to around 2–3% 2 years post-treatment. Cross-sectional comparison of S. haematobium infection in 7-year-old children in their first year at school, who received treatment through community-based drug delivery, also showed significant reduction in both prevalence (65.9%) and intensity of S. haematobium infection (78.4%) 2 years after single treatment. A significant reduction in S. mansoni infection was also achieved.
Conclusion
Significant and sustained reduction in S. haematobium infection was achieved by biennial treatment in school-age children in Burkina Faso. This may provide a cost-effective treatment strategy for similar national schistosomiasis control programmes in sub-Saharan Africa.
Résumé
Objectif
Evaluer l’impact sur la schistosomiase du traitement biennal contre la schistosomiase par le praziquantel (PZQ) chez les enfants d’âge scolaire du Burkina Faso, premier pays ayant obtenu une couverture nationale totale par ce traitement de plus de 90% de la population d’âge scolaire.
Méthodes
Une cohorte de 1727 écoliers (6-14 ans) a été suivie à intervalles d’un an, à travers une enquête longitudinale. D’autres groupes d’écoliers ont été suivis dans le cadre d’enquêtes transversales. Des examens parasitologiques, visant à mettre en évidence Schistosoma haematobium et Schistosoma mansoni, ont été pratiqués. La prévalence et l’intensité de l’infestation avant et après le traitement ont également été analysées.
Résultats
Les données collectées pour la cohorte longitudinale montrent qu’une tournée unique de traitement par le PZQ a permis de réduire la prévalence de l’infestation par S. haematobium de 87% (baisse considérable de 59,6% à 7,7%) et l’intensité de cette infestation de 92,8% (baisse de 94,2 à 6,8 œufs/10 ml d’urine) deux ans après le traitement. Cet effet sur l’infestation a été confirmé par une enquête transversale réalisée 2 ans également après le traitement. Point important : la proportion d’enfants d’âge scolaire lourdement infestés par S. haematobium est tombée de près de 25% avant le traitement à environ 2-3% deux ans après celui-ci. Des comparaisons transversales chez les enfants de 7 ans en première année d’école primaire, ayant reçu le traitement par le biais des systèmes de délivrance communautaires, ont mis en évidence une diminution à la fois de la prévalence (65,9%) et de l’intensité (78,4%) de l’infestation par S. haematobium deux ans après le traitement unique. Ces comparaisons relevaient aussi une baisse conséquente de l’infestation par S. mansoni.
Conclusion
Une diminution importante et durable de l’infestation par S. haematobium a été obtenue par un traitement biennal des enfants d’âge scolaire au Burkina Faso. Cette démarche pourrait constituer une stratégie de traitement d’un bon rapport coût/efficacité pour d’autres programmes nationaux analogues de lutte contre la schistosomiase en Afrique sub-saharienne.
Resumen
Objetivo
Evaluar el impacto en la esquistosomiasis a los dos años del tratamiento bienal con prazicuantel (PZQ) en una población de niños en edad escolar en Burkina Faso, el primer país que logró la plena cobertura nacional tratando a más del 90% de la población en edad escolar.
Métodos
Se realizó un estudio longitudinal para seguir la evolución de una cohorte de 1727 escolares (6-14 años) a intervalos de un año. Se sometió también a seguimiento a otros grupos de escolares mediante encuestas transversales. Se realizaron exámenes parasitológicos para detectar la presencia de Schistosoma haematobium y Schistosoma mansoni, determinándose la prevalencia e intensidad de la infección antes y después del tratamiento.
Resultados
Los datos de la cohorte longitudinal muestran que, transcurridos dos años, una sola ronda de tratamiento con PZQ había reducido considerablemente la prevalencia -un 87% (de 59,6% a 7,7%)- e intensidad -92,8% (de 94,2 a 6,8 huevos/10 ml de orina)- de la infección por S. haematobium. El impacto en la infección se vio confirmado también por una encuesta transversal realizada a los 2 años del tratamiento. Un dato importante es que la proporción de niños en edad escolar con infección seria disminuyó de aproximadamente un 25% antes del tratamiento a un 2%-3% a los 2 años del mismo. La comparación transversal del estado de infección por S. haematobium entre niños de 7 años en su primer año de escolaridad, que recibieron tratamiento gracias a un sistema de suministro de medicamentos basado en la comunidad, también puso de relieve una reducción importante tanto de la prevalencia (65,9%) como de la intensidad (78,4%) de la infección por S. haematobium dos años después del tratamiento único. También se logró reducir de forma notable la infección por S. mansoni.
Conclusión
El tratamiento bienal de los niños en edad escolar en Burkina Faso logró reducir considerablemente y de forma sostenida la infección por S. haematobium. Esto brindaría una estrategia de tratamiento costoeficaz para otros programas nacionales similares de control de la esquistosomiasis en el África subsahariana.
ملخص
الغرض
تقييم تأثير معالجة الأطفال في سن المدارس المصابين بالبلەارسيا لمدة عامين باستخدام البرازيكوانتيل في بوركينا فاسو، وەو أول بلد يحرز التغطية الوطنية الكاملة بمعالجة ما يربو على 90% من الأطفال في سن المدارس.
الطريقة
رُصِدَتْ مجموعة أترابية قوامەا 1727 من الأطفال في سن المدارس (بأعمار 6 – 14 عاماً) على فترات سنوية من خلال مسح طولاني. ورَصَدَتْ مسوحاتٌ متعددةُ القطاعات مجموعاتٍ إضافيةً من أطفال المدارس. وتم إجراء اختبارات للطفيليات لكشف البلەارسيات الدموية والبلەارسيات المنسونية، وتحليل معدَّل انتشار العدوى وشدتەا قبل المعالجة وبعدەا.
الموجودات
أظەرت المعطيات الخاصة بالمجموعة الأترابية الطولانية أن دورة واحدة للمعالجة باستخدام البرازيكوانتيل قد قَلَّصت معدَّل انتشار الإصابة بالبلەارسيا الدموية بنحو 87% (من 59.6% إلى 7.7%)، وخفضت شدتەا بنحو 92.8% (من 94.2 إلى 6.8 بيضة/10 مل من البول) بعد مضي عامين من المعالجة. كما تأكد التأثير على الإصابة من خلال المسح المتعدد القطاعات بعد مضي عامين من المعالجة. وما يەمنا، أن نسبة الأطفال في سن المدارس المصابين بالعدوى الثقيلة بالبلەارسية الدموية، انخفضت من 25% قبل المعالجة إلى نحو 2 – 3% بعد مضي عامين من المعالجة. كما أظەرت المقارنة المتعددة القطاعات لإصابة الأطفال في سن السابعة بالبلەارسيات الدموية في أول عام لەم في المدارس، والذين تلقوا المعالجة من خلال الإيتاء المجتمعي للأدوية، انخفاضاً كبيراً في كلٍّ من معدل الانتشار (65.9%) وشدة العدوى بداء البلەارسيا الدموية (78.4%) بعد مضي عامين على المعالجة بالبرازيكوانتيل وحدە. كما أُحْرِزَ أيضاً انخفاضٌ كبيرٌ في معدل العدوى بداء البلەارسيات المنسونية.
الاستنتاج
أحرزت معالجةُ الأطفال في سن المدارس لمدة عامين انخفاضاً كبيراً ومستمراً في معدل الإصابة بالبلەارسيات الدموية في بوركينا فاسو. وقد يطرح ەذا الإنجاز استراتيجية معالجة فعالة لقاء التكاليف للبرامج الوطنية المماثلة لمكافحة داء البلەارسيات في البلدان الواقعة جنوب الصحراء الأفريقية.
Introduction
Two main forms of human schistosomiasis or bilharzia exist in Africa – urinary schistosomiasis caused by Schistosoma haematobium infection and intestinal schistosomiasis caused by Schistosoma mansoni infection. There are around 165 million people in sub-Saharan Africa with the disease: about 112 million with urinary schistosomiasis and about 54 million with intestinal schistosomiasis.1–3 The mainstay of the current strategy recommended by WHO against schistosomiasis is morbidity control through preventive chemotherapy with praziquantel (PZQ).4,5 Schistosome morbidity is mainly caused by eggs trapped in various parts of the human body, depending on the species of schistosomes, hence the fundamental aim of morbidity control is to reduce intensity of infection by drug treatment. Several national control programmes on schistosomiasis and soil-transmitted helminthiasis (STH) are now being implemented across sub-Saharan Africa with financial and technical support from the Schistosomiasis Control Initiative.6–8 We have previously reported the successful implementation of the national control programme on intestinal schistosomiasis and STH using annual treatment strategy through school-based drug delivery for schoolchildren and community-based drug delivery for adults at high risk, and the great impact achieved on reducing morbidity and infection in Uganda in eastern Africa.9,10 We now report the impact of biennial treatment strategy on urinary schistosomiasis through both school- and community-based drug deliveries for school-age children in Burkina Faso in western Africa.
Burkina Faso is a land-locked country in western Africa with a total population of about 13 million, of which approximately 3.65 million are school-age children.11 S. haematobium is the main species prevalent throughout the country with focal prevalence of up to 100%, while S. mansoni is present mainly in the southern and western regions.12 Some small-scale control activities with treatment had taken place in some areas in the past,11,13 but the national control programme did not start until 2004. Full national coverage of treatment was achieved in 2005. A total of more than 3.3 million school-age children received their first treatment, representing 90.8% of the estimated school-age population in the country.11 Our results at one year post-treatment showed that treatment significantly reduced infection and morbidity by S. haematobium.14 The current paper presents the parasitological impact of a single treatment on schistosomiasis 2 years after treatment.
Methods
National control programme
Details about the national schistosomiasis control programme supported by the Schistosomiasis Control Initiative were described elsewhere.7,11 The control strategy adopted by the Ministry of Health was modified from the WHO guidelines and involved treatment once every two years to all school-age children (5–15 years old).4,5 Synergistic treatment for STH was also given to those who received treatment for schistosomiasis. The first treatment with PZQ and albendazole was implemented during 2004 and 2005 in a staggered two-phased campaign. Due to the low school enrolment rate in Burkina Faso, treatment was carried out both through schools and communities targeting school-age children not attending school. As described previously,11 the treatment campaign was coordinated and supervised by the Ministry of Health staff, involving education authorities and local communities. A specific national ‘treatment week’ was designated in October each year and health personnel at each level (regional, district and dispensary) were mobilized. Drug delivery in schools was carried out by trained school teachers. To reach non-enrolled children, health workers and community drug distributors formed fixed units at dispensary and mobile units that visited villages or hamlets seeking school-age children not attending school. Treatment against schistosomiasis was delivered using the WHO dose pole method for PZQ (600 mg tablets).15 A single dose of albendazole (400 mg) was used against STH.
Monitoring survey design
The monitoring survey was carried out among enrolled schoolchildren in selected schools due to logistic reasons. For the longitudinal surveys, sample size calculation and cohort design have been described elsewhere.14,16 Briefly, overall sample size was calculated with an expected reduction of 70% in mean intensity for S. haematobium over a 2-year period using the EpiSchisto software tool (available at: http://www.schoolsandhealth.org). An overall drop-out rate of 40% over the course of the monitoring period was also allowed. Sentinel schools were randomly selected from all schools in four priority regions targeted in 2004. Within each school, 180 children were selected randomly from each of the 7-, 8- and 11-year-old groups with approximately equal numbers of boys and girls in each age group. However, due to number and gender restrictions in each age group in each school, the actual age range was expanded to 6–14 years. Where the total number was not met in one school, the closest school with the same ecological conditions was selected. As a result, a cohort of 1727 schoolchildren was randomly selected at baseline from 16 schools. The cohort children were examined at baseline and followed up 1 year post-treatment (in 2005) and 2 years post-treatment (in 2006). At each follow-up, additional seven-year-old first-year new students (approximately 10 boys and 10 girls) from each sentinel school were randomly selected and examined. These children were expected to have been targeted for treatment through the community-based drug delivery before they joined the schools. Infection status in these children should represent the quality of community-based treatment.
In addition to the cohort follow-up, a cross-sectional survey was conducted during the second follow-up (2 years post-treatment), in which a group of children (7–14 years old) outside the original cohort were randomly selected and examined in the sentinel schools. The number, age and sex structures were matched to those in the cohort who were present at the second follow-up in each school. Infection status in these children should represent the quality of treatment in children outside cohorts in schools, to confirm and validate the cohort data, i.e. no preferred treatment was given to cohort children.
Parasitic infection status of each child was determined by parasitological examinations. Crosschecking was carried out for quality control. Monitoring activities received ethical clearance from the National Health System Local Research Ethics Committee of St Mary’s Hospital, London, as well as approval from the Ministry of Health of Burkina Faso. Written informed consent was obtained from head teachers at each school with prior agreements from parents or guardians.
Parasitological diagnosis
Urine examination
One urine specimen was collected from each child to determine S. haematobium infection using the filtration method and microscopy. Generally, specimens were collected around noon (between approximately 10:00 and 13:00), 10 ml of urine was filtered through a nylon filter (pore size 12 μm, Millipore, United Kingdom) and the number of eggs counted under a microscope. For specimens of less than 10 ml, the volumes were measured before filtration and the number of eggs per 10 ml calculated. Intensity of S. haematobium infection was expressed as number of eggs per 10 ml of urine (e/10 ml).
Stool examination
A single stool sample was collected from each child. Duplicate Kato–Katz slides were prepared from each sample and examined on the same day to determine S. mansoni infections. Eggs were counted and individual intensity of infection was expressed as eggs per gram of faeces (epg) calculated as the arithmetic mean of the two individual slide counts. Due to logistical reasons, at the baseline survey, only around half of cohort children were randomly selected and examined by the Kato–Katz method.
Data analysis
Of 1727 schoolchildren recruited at baseline, 763 were successfully traced and re-examined at both follow-ups with three complete sets of longitudinal parasitological data on S. haematobium. Children who dropped out or missed either of two follow-up surveys were not included in the longitudinal analysis. Baseline characteristics of children successfully followed-up showed that they had a lower mean age (9.6 years versus 11.0 years; P < 0.01), a lower proportion of boys (54.1% versus 59.1%; P < 0.05), higher S. haematobium prevalence (59.9% versus 53.1%; P < 0.01) but a similar intensity of S. haematobium infection (93.3 e/10 ml versus 91.2 e/10 ml; P > 0.05), compared with those who had dropped out. Among 763 children, 322 had valid data entry for S. mansoni at all three surveys. These longitudinal data, together with cross-sectional analysis of three sets of data from the 7-year-old children and two sets of data from the 7–14-year-olds, are presented in this paper. Baseline data from the original cohort, including those who dropped out during the follow-ups, served as the baseline data in the two cross-sectional analyses. Result tables together with 95% confidence intervals (CI) were obtained using software SAS version 9.1 (SAS Institute, Cary, NC, United States of America). Arithmetic mean intensity of infections was used in the analysis.17,18 For longitudinal cohort data the differences were tested using the McNemar’s test for prevalence and the Wilcoxon signed rank-sum test for mean intensities. For cross-sectional analysis the |² test was used to compare differences in prevalence and the Kruskal–Wallis test to compare differences in mean intensities.
Results
S. haematobium infection
Longitudinal cohort data
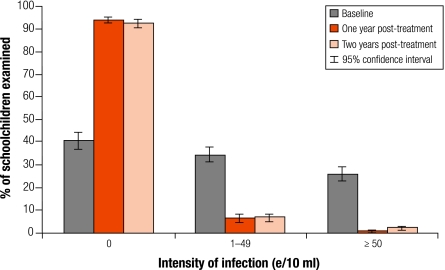
Table 1 summarizes the parasitological results from 763 cohort children successfully examined at baseline, 1 year post-treatment and 2 years post-treatment. One round of mass PZQ treatment significantly reduced prevalence in the cohort children from 59.6% at baseline to 6.2% at 1 year post-treatment and, importantly, remained at 7.7% 2 years post-treatment, an overall 87.1% reduction over 2 years (P < 0.01). The overall intensity of infection was significantly reduced from 94.2 e/10 ml at baseline to only 1.0 e/10 ml at 1 year post-treatment and 6.8 e/10 ml at 2 years post-treatment, an overall reduction of 92.8% over 2 years (P < 0.01). Significant reduction in both prevalence and intensity of infection was found in all four regions and in both boys and girls (P < 0.01; Table 1). Importantly, before treatment the proportion of heavy infections (≥ 50 e/10 ml) accounted for over 25% of the schoolchildren examined (Fig. 1). This decreased to just 0.4% at 1 year post-treatment and remained below 2% at 2 years post-treatment.
Table 1. Schistosoma haematobium prevalence and intensity of infection in the longitudinal cohort children before and after treatmenta.
| Variable | Baseline (95% CI) | 1 year post-treatment (95% CI) | 2 years post-treatment (95% CI) | Overall reduction in % |
|---|---|---|---|---|
| Prevalence in % | ||||
| Overall prevalence (n=763) | 59.6 (56.2–63.1) | 6.2 (4.5–7.9) | 7.7 (5.8–9.6) | 87.1 |
| By region | ||||
| Boucle du Mouhoun (n=238) | 48.3 (42.0–54.7) | 8.4 (4.9–11.9) | 12.2 (8.0–16.3) | 74.7 |
| Nord (n=259) | 53.7 (47.6–59.7) | 0.8 (0.0–1.8) | 2.3 (0.5–4.1) | 95.7 |
| Sahel (n=199) | 89.4 (85.2–93.7) | 12.1 (7.5–16.6) | 11.6 (7.1–16.0) | 87.0 |
| Sud Ouest (n=67) | 34.3 (23.0–45.7) | 1.5 (0.0–4.4) | 1.5 (0.0–4.4) | 95.6 |
| By sex | ||||
| Boys (n=403) | 64.3 (59.6–68.9) | 7.7 (5.1–10.3) | 11.4 (8.3–14.5)b | 82.3 |
| Girls (n=360) | 54.4 (49.3–59.6) | 4.4 (2.3–6.6) | 3.6 (1.7–5.6) | 93.4 |
| Intensity of infection in e/10 ml | ||||
| Overall mean (n=763) | 94.2 (76.4–111.9) | 1.0 (0.2–1.9) | 6.8 (1.6–12.0)b | 92.8 |
| By region | ||||
| Boucle du Mouhoun (n=238) | 76.4 (52.9–100.0) | 0.7 (0.2–1.3) | 19.2 (2.7–35.6)b | 74.9 |
| Nord (n=259) | 63.4 (38.7–88.2) | 0.0 (0.0–0.1) | 0.8 (0.0–1.7) | 98.7 |
| Sahel (n=199) | 167.7 (117.9–217.4) | 3.0 (0.0–6.1) | 2.0 (0.5–3.5) | 98.8 |
| Sud Ouest (n=67) | 57.7 (11.5–103.9) | 0.1 (0.0–0.1) | 0.1 (0.0–0.3) | 99.8 |
| By sex | ||||
| Boys (n=403) | 115.9 (91.0–140.8) | 1.6 (0.1–3.20) | 12.1 (2.4–21.8)b | 89.6 |
| Girls (n=360) | 69.9 (44.7–95.1) | 0.3 (0.1–0.6) | 0.8 (0.0–1.7) | 98.9 |
CI, confidence interval; e/10 ml, number of eggs per 10 ml urine. a All data at 1 or 2 years post-treatment were significantly lower than at baseline (all P < 0.01). b P < 0.05, significantly higher when compared with 1 year post-treatment.
Fig. 1.
Changes in the category of intensity of Schistosoma haematobium infection in schoolchildren (n=763): 3-year longitudinal data
e/10 ml, number of eggs per 10 ml urine.
Cross-sectional data
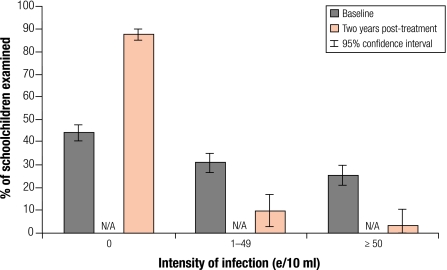
These data were compared with those of baseline children with the same ages (7–14 years old) in the original cohort before treatment. Two years after treatment, overall prevalence and intensity of S. haematobium infection were significantly lower than those at baseline by 77.4% and 80.3% respectively (P < 0.01). Significant reduction in both prevalence and intensity of infection was shown in all four regions and in both boys and girls (P < 0.01; Table 2). As in the cohort data, the proportion of heavy infections was reduced from 25% to just 3.2% (Fig. 2). However, these children outside the cohort did show a slightly higher prevalence and intensity of S. haematobium infection than those in the cohort as in Table 1 (P < 0.01) at 2 years post-treatment.
Table 2. Cross-sectional analysis of Schistosoma haematobium infection in the 7–14-year-old schoolchildren before and after treatment.
| Variable | Baseline (95% CI) | 2 years post-treatment (95% CI) | Overall reduction in % |
|---|---|---|---|
| Prevalence in % | |||
| Overall prevalence | 55.8 (53.4–58.2) | 12.6 (10.2–15.0) | 77.4 |
| (n = 1644) | (n = 761) | ||
| By region | |||
| Boucle du Mouhoun | 58.6 (53.8–63.3) | 15.8 (11.7–20.0) | 73.0 |
| (n = 413) | (n = 297) | ||
| Nord | 61.2 (56.5–65.8) | 2.0 (0.0–4.8) | 96.7 |
| (n = 417) | (n = 99) | ||
| Sahel | 84.2 (80.7–87.7) | 30.0 (21.8–38.2) | 64.4 |
| (n = 412) | (n = 120) | ||
| Sud Ouest | 18.4 (14.6–22.2) | 4.5 (1.9–7.1) | 75.5 |
| (n = 402) | (n = 245) | ||
| By sex | |||
| Boys | 59.8 (56.7–63.0) | 16.5 (12.9–20.1) | 72.4 |
| (n = 936) | (n = 406) | ||
| Girls | 50.6 (46.9–54.2) | 8.2 (5.3–11.0) | 83.8 |
| (n = 708) | (n = 355) | ||
| Intensity of infection in e/10 ml | |||
| Overall mean | 91.3 (80.0–102.7) | 18.0 (9.4–26.6) | 80.3 |
| (n = 355) | (n = 761) | ||
| By region | |||
| Boucle du Mouhoun | 106.7 (86.0–127.5) | 37.4 (16.9–58.0) | 64.9 |
| (n = 413) | (n = 297) | ||
| Nord | 91.0 (67.3–114.6) | 0.1 (0.0–0.2) | 99.9 |
| (n = 417) | (n = 99) | ||
| Sahel | 126.9 (99.3–154.4) | 14.5 (0.0–31.2) | 88.6 |
| (n = 412) | (n = 120) | ||
| Sud Ouest | 39.4 (22.8–56.1) | 3.4 (0.0–7.3) | 91.4 |
| (n = 402) | (n = 245) | ||
| By sex | |||
| Boys | 111.8 (95.6–128.1) | 26.8 (12.3–41.3) | 76.0 |
| (n = 936) | (n = 406) | ||
| Girls | 64.2 (49.1–79.3) | 7.9 (0.0–15.8) | 87.7 |
| (n = 708) | (n = 355) | ||
CI, confidence interval; e/10 ml, number of eggs per 10 ml urine; n, sample size in the group.
Fig. 2.
Changes in the category of intensity of Schistosoma haematobium infection in schoolchildren: cross-sectional data 2 years post-treatment (n=761) compared with baseline data (n=1644)
e/10 ml, number of eggs per 10 ml urine; N/A, not available.
Comparison of 3-year data from the 7-year-old schoolchildren
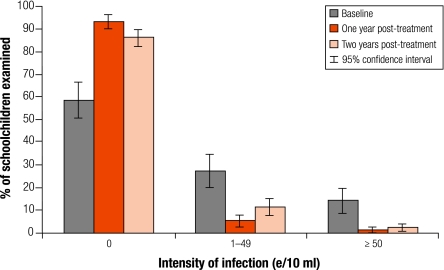
Only S. haematobium infection is presented here, as the infection level for other helminths was low. As in Table 3 (available at: http://www.who.int/bulletin/volumes/86/10/07-048694/en/index.html), similar to the cohort data, 1 year after treatment overall both prevalence and intensity of S. haematobium infection in the 7-year-old children showed a dramatic reduction, by an average of 82.9% and 92.3% respectively (P < 0.01). There was a significant uptrend in both overall prevalence and intensity of infection 2 years post-treatment compared with 1 year post-treatment (P < 0.01), but the overall level of prevalence and intensity of infection was still far below the original level by 65.9% and 78.4% respectively (P < 0.01). More importantly, the proportion of heavy infections (≥ 50 e/10 ml) remained lower at 2.5% 2 years later compared with 14% before treatment (Fig. 3). The trend in different regions and in both genders was generally similar with prevalence and intensity of infection post-treatment being significantly lower than at baseline (P < 0.01) except in the south-west (Sud Ouest) region. There, the boys showed a significant increase in both prevalence and intensity of infection 2 years post-treatment compared to one year post-treatment, while girls did not.
Table 3. Cross-sectional analysis of Schistosoma haematobium infection in the 7-year-old first-year schoolchildren before and after treatmenta.
| Variable | Baseline (95% CI) | 1 year post-treatment (95% CI) | 2 years post-treatment (95% CI) | Overall reduction in % |
|---|---|---|---|---|
| Prevalence in % | ||||
| Overall prevalence | 41.6 (33.8–49.3) | 7.1 (4.3–10.1) | 14.2 (10.4–18.0) | 65.9 |
| (n = 154) | (n = 307) | (n = 317)b | ||
| By region | ||||
| Boucle du Mouhoun | 35.6 (23.4–47.8) | 0 (N/A) | 6.7 (0.4–13.0) | 81.2 |
| (n = 59) | (n = 20) | (n = 60) | ||
| Nord | 51.4 (35.3–67.5) | 4.0 (0.2–7.9) | 2.0 (0.0–4.8) | 96.1 |
| (n = 37) | (n = 99) | (n = 98) | ||
| Sahel | 92.0 (81.4–100.0) | 16.7 (9.6–23.7) | 30.0 (21.8–38.2) | 67.4 |
| (n = 25) | (n = 108) | (n = 120)b | ||
| Sud Ouest | 0 (N/A) | 0 (N/A) | 7.7 (0.0–16.1) | – |
| (n = 33) | (n = 80) | (n = 39) | ||
| By sex | ||||
| Boys | 39.8 (29.2–50.3) | 6.0 (2.2–9.8) | 18.4 (12.5–24.4) | 53.8 |
| (n = 83) | (n = 150) | (n = 163)b | ||
| Girls | 43.7 (32.1–55.2) | 8.3 (4.0–12.6) | 9.7 (5.1–14.4) | 77.8 |
| (n = 71) | (n = 157) | (n = 154) | ||
| Intensity of infection in e/10 ml | ||||
| Overall mean | 63.5 (30.0–97.0) | 4.9 (0.0–11.4) | 13.7 (2.5–24.8) | 78.4 |
| (n = 154) | (n = 307) | (n = 317)b | ||
| By region | ||||
| Boucle du Mouhoun | 63.7 (22.2–105.3) | 0 (N/A) | 33.6 (0.0–80.3) | 47.3 |
| (n = 59) | (n = 20) | (n = 60) | ||
| Nord | 44.0 (0.0–100.5) | 1.1 (0.0–2.7) | 0.1 (0.0–0.2) | 99.8 |
| (n = 37) | (n = 99) | (n = 98) | ||
| Sahel | 175.6 (10.9–340.2) | 13.0 (0.0–31.5) | 14.5 (0.0–31.3) | 91.7 |
| (n = 25) | (n = 108) | (n = 120)b | ||
| Sud Ouest | 0 (N/A) | 0 (N/A) | 14.5 (0.0–38.5) | – |
| (n = 33) | (n = 80) | (n = 39) | ||
| By sex | ||||
| Boys | 91.2 (33.4–148.9) | 1.0 (0.0–2.1) | 24.9 (3.3–46.6) | 72.7 |
| (n = 83) | (n = 150) | (n = 163)b | ||
| Girls | 31.2 (4.1–58.2) | 8.7 (0.0–21.4) | 1.7 (0.5–3.0) | 94.6 |
| (n = 71) | (n = 157) | (n = 154) | ||
CI, confidence interval; e/10 ml, number of eggs per 10 ml urine; N/A, not available; n, sample size in the group. a Except in Sud Ouest, all data at 1 year or 2 years post-treatment were significantly lower than at baseline (all P < 0.01). b P < 0.05, significantly higher when compared with 1 year post-treatment.
Fig. 3.
Changes in the category of intensity of Schistosoma haematobium infection in the 7-year-old first-year schoolchildren before treatment (n=154), 1 year post-treatment (n=307) and 2 years post-treatment (n=317)
e/10 ml, number of eggs per 10 ml urine.
S. mansoni infection
Longitudinal cohort data
Parasitological results on S. mansoni infection in the longitudinal cohort children successfully examined at baseline and followed-up one year and two years post-treatment are summarized in Table 4 (available at: http://www.who.int/bulletin/volumes/86/10/07-048694/en/index.html). S. mansoni infection was detected only in the Sud Ouest region in sentinel schools with prevalence of 13.6% and intensity of infection of 22.4 epg in the region. Two years after treatment these were significantly reduced to 1.5% and 2.9 epg respectively (P < 0.05).
Table 4. Schistosoma mansoni prevalence and intensity of infection in the longitudinal cohort children before and after treatmenta.
| Variable | Baseline (95% CI) | 1 year post-treatment (95% CI) | 2 years post-treatment (95% CI) |
|---|---|---|---|
| Prevalence in % | |||
| Overall (n=322) | 2.8 (1.0–4.6) | 1.6 (0.2–2.9) | 0.3 (0.0–0.9) |
| Sud Ouest (n=66) | 13.6 (5.4–21.9) | 7.6 (1.2–14.0) | 1.5 (0.0–4.5) |
| Intensity of infection in epg | |||
| Overall (n=322) | 4.6 (0.0–9.7) | 7.0 (0.0–15.1) | 0.6 (0.0–1.8) |
| Sud Ouest (n=66) | 22.4 (0.0–47.3) | 33.8 (0.0–73.7) | 2.9 (0.0–8.7) |
CI, confidence interval; epg, number of eggs per gram of faeces. a All data at 2 years post-treatment were significantly lower than at baseline (all P < 0.05). No significant difference was found between 1 year post-treatment and baseline or between 2 years and 1 year post-treatment (P > 0.05).
Cross-sectional data
In the cross-sectional data, S. mansoni infection was also shown only in the Sud Ouest region. In baseline children (7–14 years old) in the original cohort in this region, prevalence of S. mansoni infection was 14.2% (95% CI: 10.8–17.6; n = 408) and intensity of infection was 23.0 epg (95% CI: 11.8–34.2; n = 408) before treatment. Two years after treatment, S. mansoni prevalence in this region was 7.6% (95% CI: 4.4–11.0; n = 248) and intensity of infection was 16.5 epg (95% CI: 1.9–31.0; n = 248) (both P < 0.05).
Discussion
In line with previous findings,12 S. haematobium infection was found prevalent throughout cohort schools in the country with very high levels of infection in the north, particularly in the Sahel region where infection was nearly universal in some schools (details not shown), and a moderate level of infection in the Sud Ouest region. Although S. haematobium infection was relatively low in the Sud Ouest compared with other regions, S. mansoni infection was also found to be prevalent. Therefore, the combined prevalence of schistosomiasis was actually very high, reaching nearly 50% in the longitudinal data and more than 30% in the cross-sectional data at baseline. The baseline data confirmed the significant burden caused by schistosomiasis to school-age children in Burkina Faso. We also found universally low STH infections (data not shown). This may be due to the fact that the national control programme on lymphatic filariasis using albendazole and ivermectin started in 2001, and several rounds of treatment had already been implemented in some areas of the country.19,20
Impact of treatment
A single treatment significantly reduced S. haematobium infection in the country and kept it low for the following 2 years. Two years after treatment, prevalence of S. haematobium infection in school-age children was still at a significantly lower level than at baseline and, more importantly, intensity of infection remained at a low level. The proportion of heavy infections was greatly reduced. This is particularly important as high intensity of S. haematobium infection has been shown to contribute to morbidity, including anaemia, in children.14 The reduction in prevalence and intensity of infection was confirmed by both longitudinal cohort follow-up and cross-sectional survey. In previous small-scale studies on S. haematobium control in eastern Africa, an annual treatment strategy was predominantly used, with varying results.21,22 However, in western Africa, one study in the Niger showed that, 3 years after a single PZQ treatment, prevalence and intensity of S. haematobium infection remained significantly lower than at baseline.23,24 In another study in Ghana, with one single PZQ treatment, intensity of S. haematobium infection was reduced by 80–99% 12 months after treatment and remained very low in two of three study areas 24 months after treatment.25 Our results are in line with these studies, suggesting that a more spaced treatment strategy, as implemented in Burkina Faso, is highly effective on S. haematobium infections, even in highly-endemic areas. In addition, we also observed a significant reduction in S. mansoni infection during the 2 years after treatment.
One of the factors likely to have contributed to the great impact demonstrated in Burkina Faso is the high nationwide treatment coverage (over 90%) achieved in a relatively short space of time by the control programme.11 Another factor is that 2004 was a very dry year and treatment was delivered in the dry season. These two important factors together may have helped to reduce transmission26 and, therefore, should be considered when implementing a national control programme in other sub-Saharan countries to maximize the treatment impact. Nevertheless, the general uptrend of the prevalence and intensity of S. haematobium infection shown 2 years post-treatment, compared with 1 year post-treatment, could also signal a potential rebound of S. haematobium infection should drug distribution be interrupted. This therefore highlights the importance of continued effort in monitoring disease transmission and of repeated treatment when and where necessary.
Throughout the 2 years, the drop-out rate of cohort children was high, particularly in the Sud Ouest region where very few original cohort children were traced and re-examined at both follow-ups. One of the main reasons for the loss of cohort children was family migration, which is very common in the country and particularly in this region (Seydou Touré, personal observation). Another of the main reasons was that a proportion of the original cohort (older age groups) had naturally progressed to secondary schools over the study period. High drop-out is indeed a relatively common problem in the monitoring activities of our programmes in sub-Saharan Africa.
Treatment strategy
Unlike in Uganda,9,10 a biennial treatment of school-age children (5–15 years old) through school-based and community-based drug deliveries was implemented in Burkina Faso. This decision was based on previous experience with S. haematobium in western Africa23–25 and on the fact that the ongoing monitoring and evaluation activities showed a low level of infection 1 year post-treatment.14 Current results, together with previous findings by others,23–25 suggest that the WHO-recommended treatment strategies on urinary schistosomiasis should be adopted with some degree of flexibility according to different epidemiological and geographical settings. Provided that coverage is high and is implemented in a dry season, treatment once every two years may be sufficient, even in highly-endemic regions such as Sahel and Boucle du Mouhoun, and in less endemic regions perhaps treatment every three or more years could equally prove sufficient. It is however more difficult to make inferences on the appropriate interval of treatment on intestinal schistosomiasis from the current data because of the relatively small number of individuals with such infection enrolled in our survey, and the lower levels of infection registered. Our study also suggests that monitoring and evaluation is a crucial component of the national control programme for fine-tuning a treatment strategy according to national and local epidemiological conditions.
Previous analysis has shown that in Burkina Faso, the total costs per child treated against schistosomiasis and STH, including drug and delivery, was US$ 0.32.11 A possible further reduction of treatment frequency for urinary schistosomiasis, where applicable, is expected to further reduce the overall costs of the control activities. The current national control programme in Burkina Faso has recently entered a new phase – integrated control of neglected tropical diseases.27,28 The next rounds of treatment are planned to be delivered in an integrated manner to include schistosomiasis, STH, lymphatic filariasis, onchocerciasis and trachoma.5 In this framework, the best way to tackle schistosomiasis is being assessed. Treatment with reduced frequency plus integrated control strategies may significantly increase the sustainability of national control programmes in Burkina Faso and in the rest of sub-Saharan Africa.
Conclusion
This study showed that a significant impact on urinary schistosomiasis was achieved by biennial distribution of PZQ with high coverage rates in Burkina Faso. We demonstrated, for the first time on a national scale, that such treatment frequency can be successfully applied to control urinary schistosomiasis in sub-Saharan Africa. This may provide a cost-effective treatment strategy for similar national schistosomiasis control programmes in resource-limited settings. ■
Acknowledgements
We thank all the staff at the Programme National de Lutte contre la Schistosomiase et les Vers Intestinaux, Ministère de la Santé, Burkina Faso, and Ruairidh Crawford for his data analysis during his student project.
Footnotes
Funding: The Schistosomiasis Control Initiative is sponsored by the Bill and Melinda Gates Foundation, which had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: None declared.
References
- 1.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/S0001-706X(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–39. doi: 10.1016/S0001-706X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 3.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 4.Prevention and control of schistosomiasis and soil-transmitted helminthiasis Report of a WHO expert committee. Geneva: WHO; 2002 (WHO Technical Report Series No. 912). pp. 1-57. [PubMed]
- 5.Preventive chemotherapy in human helminthiasis Geneva: WHO; 2006.
- 6.Fenwick A. New initiatives against Africa’s worms. Trans R Soc Trop Med Hyg. 2006;100:200–7. doi: 10.1016/j.trstmh.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Garba A, Toure S, Dembele R, Bosque-Oliva E, Fenwick A. Implementation of national schistosomiasis control programmes in West Africa. Trends Parasitol. 2006;22:322–6. doi: 10.1016/j.pt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Kabatereine NB, Fleming FM, Nyandindi U, Mwanza JC, Blair L. The control of schistosomiasis and soil-transmitted helminths in East Africa. Trends Parasitol. 2006;22:332–9. doi: 10.1016/j.pt.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Kabatereine NB, Brooker S, Koukounari A, Kazibwe F, Tukahebwa EM, Fleming FM, et al. Impact of a national helminth control programme on infection and morbidity in Ugandan schoolchildren. Bull World Health Organ. 2007;85:91–9. doi: 10.2471/BLT.06.030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Koukounari A, Kabatereine N, Fleming F, Kazibwe F, Tukahebwa E, et al. Parasitological impact of two-year preventive chemotherapy on schistosomiasis and soil-transmitted helminthiasis in Uganda. BMC Med. 2007;5:27. doi: 10.1186/1741-7015-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabrielli AF, Toure S, Sellin B, Sellin E, Ky C, Ouedraogo H, et al. A combined school- and community-based campaign targeting all school-age children of Burkina Faso against schistosomiasis and soil-transmitted helminthiasis: performance, financial costs and implications for sustainability. Acta Trop. 2006;99:234–42. doi: 10.1016/j.actatropica.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Poda JN, Traore A, Sondo BK. Schistosomiasis endemic in Burkina Faso. Bull Soc Pathol Exot. 2004;97:47–52. [PubMed] [Google Scholar]
- 13.Sellin B, Simonkovich E, Sellin E, Rey JL, Mouchet F. Course of urinary schistosomiasis over 3 consecutive years after treatment with metrifonate in a dry savanna village in Upper Volta. Med Trop. 1984;44:357–9. [PubMed] [Google Scholar]
- 14.Koukounari A, Gabrielli AF, Touré S, Bosqué-Oliva E, Zhang Y, Sellin B, et al. Schistosoma haematobium infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. J Infect Dis. 2007;196:659–69. doi: 10.1086/520515. [DOI] [PubMed] [Google Scholar]
- 15.Montresor A, Engels D, Chitsulo L, Bundy DAP, Brooker S, Savioli L. Development and validation of a “tablet pole” for the administration of praziquantel in sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2001;95:542–4. doi: 10.1016/S0035-9203(01)90034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooker S, Whawell S, Kabatereine NB, Fenwick A, Anderson RM. Evaluating the epidemiological impact of national control programs for helminths. Trends Parasitol. 2004;20:537–45. doi: 10.1016/j.pt.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Fulford AJ. Dispersion and bias: can we trust geometric means? Parasitol Today. 1994;10:446–8. doi: 10.1016/0169-4758(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 18.Montresor A. Arithmetic or geometric means of eggs per gram are not appropriate indicators to estimate the impact of control measures in helminth infections. Trans R Soc Trop Med Hyg. 2007;101:773–6. doi: 10.1016/j.trstmh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molyneux DH, Bradley M, Hoerauf A, Kyelem D, Taylor MJ. Mass drug treatment for lymphatic filariasis and onchocerciasis. Trends Parasitol. 2003;19:516–22. doi: 10.1016/j.pt.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Burkina Faso Global Alliance to Eliminate Lymphatic Filariasis. Available from: http://www.filariasis.org/resources/burkina_faso.htm [accessed on 20 August 2008].
- 21.Magnussen P. Treatment and re-treatment strategies for schistosomiasis control in different epidemiological settings: a review of 10 years’ experiences. Acta Trop. 2003;86:243–54. doi: 10.1016/S0001-706X(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 22.Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. 2006;75:83–92. [PMC free article] [PubMed] [Google Scholar]
- 23.Campagne G, Garba A, Barkire H, Vera C, Sidiki A, Chippaux JP. Continued ultrasonic follow-up of children infected with Schistosoma haematobium after treatment with praziquantel. Trop Med Int Health. 2001;6:24–30. doi: 10.1046/j.1365-3156.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- 24.Garba A, Campagne G, Tassie JM, Barkire A, Vera C, Sellin B, et al. Long-term impact of a mass treatment by praziquantel on morbidity due to Schistosoma haematobium in two hyperendemic villages of Niger. Bull Soc Pathol Exot. 2004;97:7–11. [PubMed] [Google Scholar]
- 25.Nsowah-Nuamah NN, Aryeetey ME, Jolayemi ET, Wagatsuma Y, Mensah G, Dontwi IK, et al. Predicting the timing of second praziquantel treatment and its effect on reduction of egg counts in southern Ghana. Acta Trop. 2004;90:263–70. doi: 10.1016/j.actatropica.2002.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Gurarie D, King CH. Heterogeneous model of schistosomiasis transmission and long-term control: the combined influence of spatial variation and age-dependent factors on optimal allocation of drug therapy. Parasitology. 2005;130:49–65. doi: 10.1017/S0031182004006341. [DOI] [PubMed] [Google Scholar]
- 27.Molyneux DH, Hotez PJ, Fenwick A. “Rapid-impact interventions”: how a policy of integrated control for Africa’s neglected tropical diseases could benefit the poor. PLoS Med. 2005;2:e336. doi: 10.1371/journal.pmed.0020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–27. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]