Abstract
Elimination of endemic measles transmission is the culmination of a range of control measures at a national level. Current documentation of elimination proposed by WHO’s regional offices requires achieving specific targets for surveillance process indicators. We demonstrate how Australia, although not meeting these specific targets, has satisfied multiple criteria that justify the formal declaration of measles elimination. Our review shows that few countries previously declaring measles elimination have satisfied the current WHO surveillance targets. We argue that the requirements for recognition of measles elimination should not restrict countries to a particular type of surveillance system or surveillance criteria.
Résumé
L’élimination de la transmission endémique de la rougeole est le point culminant d’une série de mesures de lutte contre cette maladie au niveau national. Pour attester de l’élimination de la rougeole, les Bureaux régionaux de l’OMS proposent actuellement que les indicateurs servant à la surveillance aient atteint des objectifs spécifiques. Nous démontrons comment l’Australie, bien que n’ayant pas atteint ces objectifs, a rempli plusieurs critères justifiant la déclaration formelle de l’élimination de la rougeole. Notre analyse montre que peu de pays ayant antérieurement déclaré la rougeole comme éliminée ont rempli les objectifs actuels en matière de surveillance de l’OMS. A notre avis, les exigences pour reconnaître l’élimination de la rougeole ne devraient pas imposer aux pays l’utilisation d’un type particulier de système ou de critère pour la surveillance.
Resumen
La eliminación de la transmisión endémica del sarampión supone la culminación de toda una serie de medidas de control desplegadas a nivel nacional. La actual documentación sobre la eliminación propuesta por las oficinas regionales de la OMS requiere que se alcancen metas concretas para los indicadores del proceso de vigilancia. Explicamos aquí de qué manera Australia, si bien no ha alcanzado esas metas específicas, ha satisfecho muchos criterios que justifican la declaración oficial de eliminación del sarampión. Nuestro análisis muestra que, entre los países que han declarado haber eliminado esta enfermedad, son pocos los que han alcanzado las actuales metas de vigilancia de la OMS. Se argumenta que, entre los requisitos para el reconocimiento de la eliminación del sarampión, no se debe exigir a los países que apliquen sólo un sistema de vigilancia o unos criterios de vigilancia determinados.
ملخص
إن التخلُّص من انتقال الحصبة المتوطنة ەو غاية مجموعة من تدابير المكافحة المتخذة على أي مستوى وطني. وتتطلب الوثائق الخاصة بالتخلص من الحصبة، المقترحة من المكاتب الإقليمية لمنظمة الصحة العالمية، تحقيق أەداف معينة لمؤشرات عملية ترصد المرض. ويعرض الباحثون في ەذە الورقة كيف أن استراليا، برغم عدم تحقيقەا ەذە الأەداف المحددة، قد نجحت في تحقيق معايير متعددة تبرر الإعلان رسمياً عن تخلصەا من الحصبة. وتبين ەذە الدراسة أن عدداً قليلاً من البلدان التي سبق أن أعلنت عن التخلص من الحصبة قد حققت أەداف الترصُّد الحالية التي أعلنتەا منظمة الصحة العالمية. ويرى الباحثون أن متطلبات الاعتراف بالتخلص من الحصبة ينبغي ألا تقصُر البلدان على نمط معين من نُظُم الترصُّد أو على نمط معين من معايير الترصُّد.
Defining elimination
Several WHO regions have set target dates for the elimination of the transmission of endemic measles. The WHO Regional Office for the Western Pacific (WPRO) has nominated the target date of 2012.1 Since measles elimination was first proposed, definitions of elimination have progressed from requiring a reduction to zero in the incidence of infection in a defined geographical area,2 to the absence of endemic measles transmission and the lack of sustained transmission following an importation of measles virus in a large and well populated geographical area, as outlined in guidelines by WPRO.3 The indicators adopted by WPRO to monitor the progress towards measles elimination provide an operational definition of measles elimination.3,4
Papania & Orenstein have argued that elimination can be declared if multiple lines of evidence demonstrate the absence of endemic measles transmission.5 Several countries have declared elimination of endemic measles transmission using criteria that have become more rigorous over time (summarized in Table 1, available at: http://www.who.int/bulletin/volumes/87/1/07-046375/en/index.html), including the criteria we use here to declare elimination in Australia.
Table 1. Criteria used in countries that have declared measles elimination.
| Country and year of declaration | Period of elimination | Population | Criteria |
|---|---|---|---|
| Australia | Since 2005 | In 2005: 21 million6 | High two-dose vaccine coverage; > 95% MCV1 coverage and > 90% MCV2 coverage; geographic homogeneity |
| Low incidence of confirmed cases (0.5–7.3/million; < 1/million since 2005) | |||
| High proportion of cases imported or linked to an imported case | |||
| Containment of outbreaks (without re-establishment of a specific genotype) | |||
| Serological evidence of population immunity > 90% | |||
| Absence of endemic measles genotype | |||
| Estimations of R by several methods = 0.57–0.87 between 1999 and 2003 | |||
| Brazil 20037 | 2000–2001 | In 2000: 169.6 million7 | High two-dose vaccination coverage in routine and supplementary campaigns (95% since 1997) |
| Low incidence of confirmed measles cases (36 cases in 2000, 1 case in 2001) | |||
| Case-based surveillance system with negative weekly reporting and targeted investigation within 48 hours; In 2000: 8 322 suspected measles cases discarded, 92% laboratory tested, discard rate ~4.9/100 000; In 2001: 5 598 suspected measles cases discarded, discard rate 3.3/100 000 | |||
| No endemic measles genotypes identified | |||
| Canada 20048 | Since 1998 | In 2000: 30.7 million6 | High two-dose coverage; > 95% MCV1 reported in all regions; 2nd dose implemented 1996; No data on coverage of MCV2 |
| Low numbers of reported confirmed cases (0.4–6/million from 1998 to 2001) | |||
| High proportion of reported cases imported or linked to an imported case and cluster sizes small (3/49 outbreaks/transmission foci > 15 cases) | |||
| 1998–99: laboratory testing for measles IgM performed at a rate of 17–22/100 000 population annually | |||
| Multiple genotypes detected and no endemic genotype identified since 1998 | |||
| 1998–2001: R = 0.82–0.87 | |||
| Cuba 19989 | Since 1993 | In 1995: 10.9 million6 | High vaccine coverage at age 12 months and periodic “catch-up,” “keep-up” and “follow-up” campaigns; > 98% coverage in targeted age groups |
| Low incidence of laboratory confirmed cases; In 1989–1992, < 20 laboratory-confirmed cases reported annually. Last cases reported July 1993 | |||
| Strengthened surveillance including screening of suspected cases | |||
| Absence of circulating virus | |||
| England & Wales 200310 | 1995–2001 | In 2001: 52 million11 | “High routine vaccination coverage”; MCV1 coverage > 90% until 1998, recent decline to 84%; MCV2 introduced in 1996 |
| “High herd immunity” as seen by low number of reported cases (1995–2001: 0.2–8.8/million population, average 1.8/million population/year) and small number of large clusters (4 clusters with 10–24 cases and 4 clusters with 25+ cases) | |||
| “High” proportion of cases imported/import-linked (23% of sporadic cases and 43% of clusters involving 108 cases) | |||
| Enhanced surveillance including laboratory confirmation of suspected cases; 66% of suspected cases tested (IgM oral fluid sample); ~2600 non-measles suspected cases reported per year (~4.4/100 000/year) | |||
| Wide variety of genotypes detected with absence of previously common genotype; “High” proportion of sporadic cases with distinct genotypes | |||
| R = 0.51–0.7 using variety of methods | |||
| Finland 199412 | Since 1994 | In 1995: 5.1 million6 | High two-dose vaccine coverage (97% in targeted programme) |
| Low incidence of serologically confirmed cases since 1987; 13 serologically confirmed cases in 1993. Decline to “almost zero” incidence; No further information reported | |||
| Mexico 200413 | 1996–2000 | In 2000: 99.7 million6 | High two-dose vaccine coverage; > 95% coverage ages 1–6 years since 1996, 97.6% coverage ages 6–10 years since 1999 |
| Low number of reported cases; Zero cases 1996–1999, 2–12 cases per year since with 30 cases in 2000; “Limited” local spread | |||
| Active case ascertainment, e.g. 3.5 million health centre/hospital charts reviewed during outbreak in 2000 | |||
| Sensitive rash illness surveillance system including negative weekly reporting; > 10 000 febrile rash illnesses investigated for measles per year since 1994 (discard rate ~10/100 000) | |||
| Since 1994, > 80% of febrile rash illnesses reported have been investigated within 48 hours of report, with a serum and urine sample collected 6–20 days after rash onset; Since 1996, laboratory specimens have been sent in a timely fashion for > 80% of suspected cases | |||
| Republic of Korea 200614 | 2001–2006 | In 2000: 46.8 million6 | MCV2 coverage 95–99.9%; Uniformly high in all 16 provinces; 2004 seroprevalence study: 91.7–92.9% school children immune |
| Measles incidence of < 1 case per million since 2002 (between 0.12–0.27/million) | |||
| Incidence of suspected cases 2002–2006, 1.2–3.0 per 100 000 | |||
| Surveillance system: Adequate serological specimens collected from ~93% of reported suspected cases; Results from 100% of specimens available within 7 days of receipt to laboratory; 85% of suspected cases investigated within 48 hours | |||
| Virus isolated from all identified chains of measles transmission | |||
| R = 0.81 in 2001, 0.7–0.8 since 2002 | |||
| USA 20045,15 | Since 1997 | In 2000: 284.9 million6 | High population immunity; Vaccine coverage: at least one dose (MCV1+) > 90% 19–35-month-olds, 98% at school entry; Two doses required in 48/50 states; Serological surveillance: 92–93% of population immune; susceptibility 1–4 yrs 14%, 5–9 yrs 8%, 10–19 yrs 5%, > 20 yrs 7% |
| Low incidence of disease; < 1 case per milllion 1997–1999 | |||
| High proportion linked to imported case; 1997–2001, 36% imported, 25% linked to imported case; Duration of outbreaks short (median 18 days); Long periods in which source identified as an imported case (16 periods of at least 4 weeks) | |||
| Adequate measles surveillance system; Validation of separate reporting systems including capture–recapture study, consistent detection of sporadic and imported cases, > 1/100 000 suspected cases investigated per annum | |||
| No genotype has occurred in a repeating pattern that would suggest an endemic strain of measles virus; Isolates closely related to strains currently circulating on other countries | |||
| 1997–1999: R < 0.8. | |||
MCV, measles-containing vaccine; R, reproductive number.
As with other countries that have declared elimination of measles, Australia’s national elimination plan included high two-dose immunization coverage and a disease surveillance system capable of a rapid response to potential measles outbreaks.16 Australia, like many other countries that have declared elimination, would have difficulty meeting the WPRO elimination criteria based on currently available reporting of the investigation of presumptive measles cases (Table 2, available at: http://www.who.int/bulletin/volumes/87/1/07-046375/en/index.html). However we believe multiple lines of evidence conclusively demonstrate the elimination of endemic measles transmission from Australia since 2005 at the latest, when notified confirmed cases were < 1 per million population. In this paper we outline how these criteria have been met, compare them with the WPRO criteria and justify their validity. We argue that a broader range of internationally accepted criteria for measles elimination is warranted.
Table 2. Countries that have declared measles elimination and how they meet the WPRO criteria.
| WPRO criteria on measles elimination4 | Target | Australia | Brazil | Canada | Cuba | England & Wales | Finland | Mexico | Republic of Korea | USA | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Very low incidence | 1. Confirmed measles cases (confirmed by laboratory, epidemiologic linkage or clinically) | < 1/1 million | Noa | Yes | Noa | Yes | Noa | –b | Yes | Yes | Yes |
| High quality surveillance | 2. National reporting of non-measles suspected cases | ≥ 2/ 100 000 | No | 3.3–4.9/ 100 000 | ~17–22/ 100 000c | – | ~4.4/ 100 000 | – | ~10/ 100 000 | Yes | Yesd |
| 3. Percentage of districts reporting ≥ 1/100 000 non-measles suspected cases | ≥ 80% | – | – | – | – | – | – | – | Noe | – | |
| 4. Percentage of suspected cases with adequate investigation within 48 hours of notification | ≥ 80% | – | NFIf | – | – | – | – | Yes | Yes | – | |
| 5. Percentage of suspected cases with adequate blood specimens | ≥ 80% | – | Yes | – | – | – | – | Yes | Yes | – | |
| 6. Percentage of specimens with laboratory results ≤ 7 days after arrival to laboratory | ≥ 80% | – | NFIg | – | – | – | – | NFIh | Yes | – | |
| 7. Transmission chains (outbreaks) with sufficient samples for virus isolation | ≥ 80% | – | – | – | – | No | – | – | Yes | – | |
| High population immunity | 8. National MCV1 and MCV2 coverage | ≥ 95% | Yes | Yes | NFIi | Noj | No | Yes | Yes | Yes | NFIk |
| 9. Percentage of outbreaks or transmission foci with < 10 cases | ≥ 80% | No | Yes | Yes | – | Yes | – | – | – | – | |
| 10. Absence of endemic measles virus | No virus | Yes | Yes | Yes | – | Yes | – | – | Yesl | Yes | |
MCV, measles-containing vaccine; NFI, no further information; USA, United States of America; WPRO, WHO Regional Office for the Western Pacific. a Target achieved for some years only. b Dash indicates criteria is not described in report that declared measles elimination for that country. c Canada: includes confirmed measles cases. d USA: > 90% of sources of data on discarded measles investigations in the USA report a rate > 1/100 000 population. Data sources vary in national completeness. e Republic of Korea: national data only. f Brazil: Policy of targeted investigation within 48 hours, NFI. g Brazil: reporting of % of specimens with results within 4 days: In 2000, 67%, in 2001, 73% of specimens. NFI regarding results at 7 days. h Mexico: reported > 80% of specimens arriving in a “timely fashion.” NFI. i Canada: > 95% coverage for MCV1 achieved. NFI. j Cuba: Vaccination strategy included one dose only. k USA: reporting of at least one dose (MCV1+), 2 states only require 1 dose of MCV. l Republic of Korea: previous endemic strain (H1) resulted in small outbreak in 2006. Likely imported from neighbouring country, China.
Evidence of elimination
Low incidence
Notifications of measles cases in Australia were sporadic until the establishment of the National Notifiable Diseases Surveillance System (NNDSS) in 1991.17 Since then, public health legislation in all jurisdictions has included the mandatory reporting of measles cases by laboratories, clinicians and hospitals, to state and territory health departments. Notifications of confirmed cases are forwarded to the NNDSS. Since 2004, all Australian states and territories have adopted a case definition for a confirmed case of measles that requires laboratory evidence from an approved reference laboratory or an epidemiological link to a laboratory-confirmed case in conjunction with clinical evidence.18 These improvements mean that all confirmed measles cases notified to the NNDSS since 2004 are likely to represent true cases (WPRO criterion 1, Table 2).
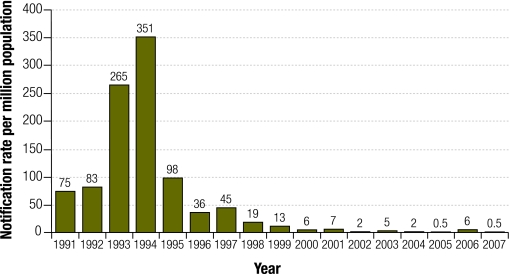
Since a large national outbreak in 1993–4 (Box 1), there has been a progressive downward trend in measles notifications. The 10 confirmed cases of measles in 2005 (0.5 cases per million population) was the lowest annual figure ever reported on the NNDSS (Fig. 1).24 A total of 125 cases were reported in 2006 (6 cases per million).24 However, a large proportion (~54%) was attributable to a nationwide outbreak linked to the tour of a foreign spiritual group. Attendees at tour meetings were disproportionately opposed to vaccination and transmission was predominantly confined to one generation.25,26 In 2007, 11 cases were reported to the NNDSS (0.5 cases per million24). In both 2005 and 2007, Australia met the WPRO target of 1 case per million population but not in 2006, a year in which we believe that endemic measles transmission did not occur in Australia.
Box 1. The evolution of measles elimination in Australia.
Phase 1: pre-vaccine
Pre-vaccine epidemiology: similar to that reported from other comparable regions of the world – major epidemics occurring every 2 years and number of annual cases approximating the birth cohort
1966–1975: 146 deaths from measles were certified19
1962–1971: 1 in every 22 hospitalized cases of measles complicated by encephalitis20
1968: measles control efforts commenced with the licensing of a live attenuated monovalent measles vaccine19
1975: measles vaccine incorporated into the national immunization schedule, as a single dose at 12 months of age
1976–1985: recorded deaths from measles halved to 62 in decade following vaccine introduction, despite slow uptake19
Measles mortality decreases following the introduction of measles immunization, but there is ongoing transmission of a single endemic genotype (often as multiple strains of the same genotype).21,22
Phase 2: measles control
The incidence of measles and strain variation of the endemic genotype decline with expansion of immunization and outbreak control activities,21 but susceptible individuals accumulate and large outbreaks occur after longer inter-epidemic periods.
1993–1994: large epidemic with > 10 000 notified cases
1993: second dose of measles-containing vaccine (measles–mumps–rubella, MMR) for all 10–16 year olds recommended,19 prompted by large epidemic > 10 000 notified cases
Although this led to an overall reduction in measles cases, outbreaks continued in school-aged children and young adults.22
Phase 3: elimination
Progression into the elimination phase facilitated by the Australian Measles Control Campaign, in which 1.33 million children (96% of the target age group) were vaccinated.23
1998: Australian Measles Control Campaign: re–scheduling of the second dose of MMR to 4 years of age; and catch–up vaccination for primary school children aged 5–12 years
Fig. 1.
Measles notification rates per million population, Australia, 1991–200724
Quality surveillance
Local public health authorities are responsible for the active follow-up of all suspected cases of measles (defined as morbilliform rash with fever present at onset of rash and cough)27 to confirm the diagnosis and identify any additional cases. However, information about the investigation of suspected cases found not to be measles is not recorded at the national level, including laboratory performance indicators. Thus, due to reporting mechanisms, Australia is unable to provide data on the WPRO surveillance process criteria 3–7 (Table 2). However, enhanced surveillance from 1998–2003 in the state of Victoria (2005 population estimate = 5 million; approximately 25% of the Australian population)28 showed that 89% of suspected cases could be discarded after laboratory investigation, at a median annual rate of 2.9/100 000.29 This experience is likely to be applicable nationally and meets WPRO criterion 2.
High two-dose vaccine coverage
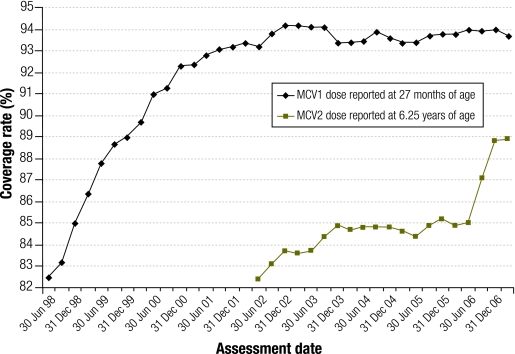
High vaccination coverage (greater than 95% for each new birth cohort) is required for herd immunity against measles and maintenance of measles elimination (WPRO criterion 8, Table 2).30 Since 1998, Australia’s measles elimination strategy has included vaccination coverage targets set to achieve 95% coverage with the first dose of measles-containing vaccine (MCV1) for children by 24 months and 90% two-dose (MCV2) coverage by school entry.31 In 1989, a child health survey indicated coverage with at least one dose of a measles-containing vaccine was 85%.32 Fig. 2 shows how Australia’s elimination strategy has resulted in increased vaccination coverage for both MCV1 and MCV2, as reported to the Australian Childhood Immunization Register (ACIR).
Fig. 2.
Coverage rates of the first and second doses of measles-containing vaccine in Australia by assessment date as reported on the Australian Childhood Immunisation Registera
MCV, measles-containing vaccine.
a Assessment date: MCV1 is scheduled at 12 months of age and assessed at 27 months of age; MCV2 is scheduled at 4 years of age and assessed at 6.25 years of age to allow for delayed notification.
The ACIR is estimated to capture more than 99% of Australian children aged less than 7 years.33,34 Coverage is reported for MCV1 at 2 years and 3 months of age and MCV2 at 6 years and 3 months of age to allow for delayed notification. In 2006, the ACIR recorded that 93.7–94.0% of children aged 2 years (born in 2004) had received at least one dose of MCV and 85.0–88.8% of children aged 6 years (born in 2000) had received both doses (Fig. 2). Substantial geographic homogeneity was demonstrated with coverage of one dose ranging from 92.7–96.2% and coverage of two doses ranging from 85.6–90.2% across all states and territories. These are minimum estimates, with parental recall surveys suggesting that the ACIR underestimates coverage for MCV2 by 5–10%33 and for vaccines scheduled at 12, 18 and 24 months of age by 3–5%.34 When corrected for estimated underreporting, the national and WPRO target of 95% coverage for one dose of measles-containing vaccine is exceeded; coverage with a second dose is likely to be > 90%.
Outbreaks
The available surveillance data for Australia confirms that a high proportion of cases are imported or linked to an imported case, transmission from imported cases is quickly interrupted, and outbreaks following importation of the measles virus are self-limiting and contained. Enhanced surveillance in the state of Victoria identified 58 outbreaks between 1998 and 2006, with a total of 262 cases ranging from 1 to 75 cases.35 Among outbreaks in which the source case could be identified as imported, 33 did not result in transmission, while 22 were associated with secondary cases. Only 3 cases could not be directly linked to importation.35 Satisfying WPRO criterion 9, 91% of measles outbreaks or transmission foci in Victoria between 1998 and 2006 involved < 10 cases. Although such detailed data are not available nationally, the patterns are likely to be similar to those in Victoria. This is based on several lines of evidence. First, results for serosurvey and vaccination coverage are similar between regions. Second, a smaller period of enhanced surveillance in Western Australia between March 1999 and October 2000 identified 28 cases of measles all resulting from nine importations, all of < 10 cases.36
Absence of an endemic genotype
Variability in the nucleotide sequence of the measles virus, of which there are eight clades (A–H) and 23 currently assigned genotypes (A, B1–B3, C1, C2, D1–D10, E, F, G1–G3, H1, H2), can be exploited for molecular epidemiological purposes.37,38 Molecular analysis in routine case and outbreak investigations during the elimination phase of measles control is critical to document the genotype of each new cluster and demonstrate the absence of sustained transmission of one genotype (WPRO criterion 10, Table 2), to identify the source of the measles virus in outbreak situations and to confirm vaccine-associated fever/rash illness (genotype A), which can also assist outbreak investigations.39
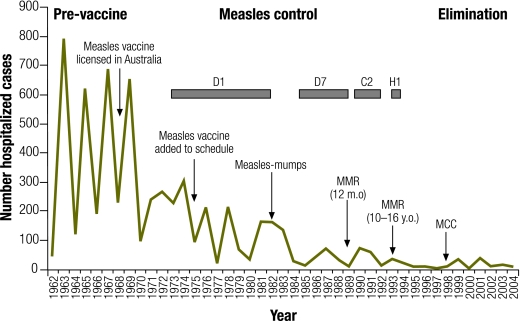
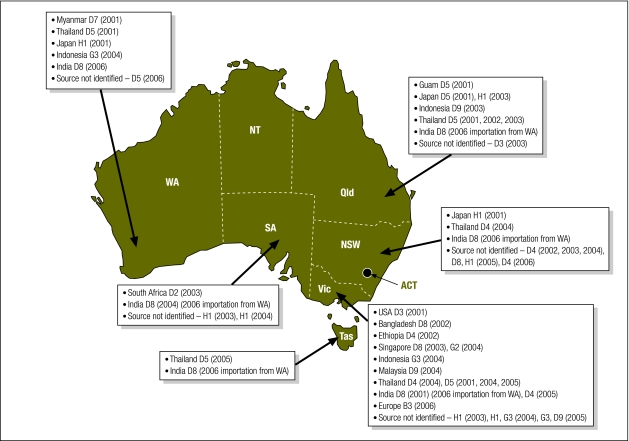
Retrospective molecular analyses of measles cases in Victoria from 1973 to 1998 and prospective molecular analyses nationally from 1999 to 2001 were conducted at the Victorian Infectious Diseases Reference Laboratory.40,41 Fig. 3 shows the steady decline in measles-associated hospitalizations in Victoria since the introduction of a vaccine and the succession of measles genotypes identified during this time, consistent with the WHO phases of measles epidemiology.30 The D1 genotype was identified in the earliest samples available for analysis suggesting that genotype D1 may have been the endemic genotype in Australia in the prevaccine era. By 1985 the D7 genotype appeared to replace D1 as the endemic genotype. In the early 1990s, outbreaks of genotypes C2 and H1 were subsequently identified suggesting Australia had moved to the WHO-defined measles control phase of genotypic replacement. Since this time several genotypes have been identified, but none repeatedly, suggesting there have been no endemic genotypes in circulation since this time. Fig. 4 illustrates the measles virus genotypes identified in outbreaks in Australia between 2001 and 2006. Source countries of measles virus importations could be identified for the majority of clusters and are indicated on the map.
Fig. 3.
Measles-associated hospitalizations and measles virus genotypes isolated in Victoria (1962–2004) during WHO-defined measles elimination phases20,40,41,a
MCC, measles control campaign; MMR, measles–mumps–rubella vaccine; m.o., months’ old; y.o., years’ old.
a Arrows reflect changes to measles immunization policy. Boxes show genotypes detected retrospectively (pre–1999) from measles virus isolates and clinical samples during the measles control phase. Measles virus isolates prospectively (1999–2004) detected during the elimination phase represent genotypes associated with imported cases or vaccine-related illness (not shown in figure). Prospectively isolated genotypes (number of isolates) include A (4 isolates detected), D3 (1), D4 (2), D5 (4), D7 (1), D8 (4), D9 (2), G2 (2), G3 (2), H1 (2) and H2 (1).
Fig. 4.
Measles virus genotypes detected in Australia, 2001–200641,a
ACT, Australian Capital Territory; NSW, New South Wales; NT, Northern Territory; Qld., Queensland; SA, South Australia; Tas., Tasmania; Vic. Victoria; WA, Western Australia; USA, United States of America.
a Boxes list confirmed country of importation (where available), genotype detected and year of detection in parenthesis for each Australian state as indicated by the arrow. There were no reports of measles disease in the NT or ACT during the period 2001–2006. “Source not identified” refers to individual cases of measles for which the source of importation (index case) could not be identified. Sequence interrogation of the measles virus nucleic acid detected did not substantiate the ongoing circulation of any genotype strain detected.
Population immunity
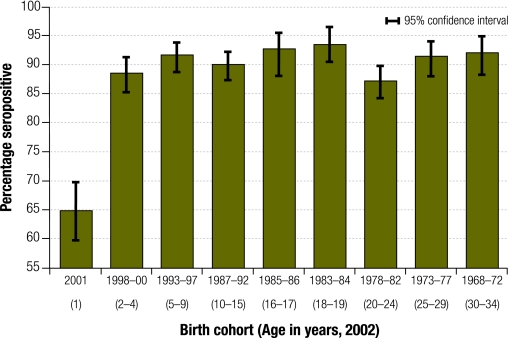
Although serological evidence of population immunity (measuring the proportion of sera samples that are positive for measles IgG antibodies) is not listed as a WPRO criterion, high population immunity is important evidence of elimination. WPRO provides three indirect measures of immunity (criteria 8–10) but population-based serosurveillance is the gold standard for assessing immunity. In Australia, national serosurveillance programmes conducted by the National Centre for Immunization Research and Surveillance of Vaccine Preventable Diseases, have included measles serosurveys during 1996–1998 before the Measles Catch-up Campaign, in 1999 to evaluate the success of the campaign and again in 2002 to evaluate a campaign targeting young adults.23,42 The 2002 serosurvey estimated that 93.9% of the Australian population was immune to measles, with immunity > 90% in all age groups, except 1 year-olds (64.9% positive, 95% CI: 59.7–69.8%), 2–4 year-olds (88.5% positive, 95% CI: 85.3–91.3%) and the 20–24 year age group (87.2% positive, 95% CI: 84.3–89.8%) (Fig. 5).42 More than 97% of people born before 1968, when measles vaccine first became available in Australia, had evidence of measles immunity.43
Fig. 5.
Percentage of sera samples that are positive for measles IgG antibody by birth cohort, Australian national serosurvey, 200242
Evidence from this source is robust as the serosurveys all used the same methods and demonstrated comparable results to prospectively collected, random-cluster sampling of school-age groups.43,44 We therefore believe that Australia’s national serosurveys are an accurate measure of population level measles immunity. In 2002, population immunity was well above 90%.
Estimation of R
In disease modelling, infectious disease elimination is defined as the maintenance of the reproductive number, R below unity (R < 1).45 The reproductive number, R, summarizes the susceptibility of the population, its mixing patterns and the contagiousness of the disease, and represents the average number of secondary cases produced by a typical case.5,45 When R is > 1, the number of cases increase from one generation to the next and an epidemic ensues. When R is < 1, case numbers decrease from one generation to the next. If R is maintained constantly < 1 (the epidemic threshold), endemic transmission is considered to be eliminated. Using this definition, transmission of infective agents can still occur following elimination but endemic transmission is not re-established at a population level.46 Therefore, calculating R is a useful tool for monitoring the progress of elimination efforts. Estimates of R in the Australian setting have been obtained using several different methods with consistent results.35,36,42,47
The most robust method of calculating R is to use serosurveillance data to estimate susceptibility in each age group.45 Using data from the 1996–1998 Australian national serosurvey (before the catch-up campaign), R was estimated as 0.90.47 Since then, estimates of R using serological data have been well below the epidemic threshold; R was estimated as 0.57 from the 1999 serosurvey, and 0.69 in 2002, and modelled to remain below 0.8 until at least 2012.42,47 These estimates from serological data provide evidence that sustained transmission is unlikely to have occurred since 1999.
Besides using serosurveillance data, R can be estimated using enhanced disease notification data. There are three such methods. The first method uses the proportion of all cases that are identified as imported.48 As the recording of whether a measles case is imported is incomplete on the NNDSS database, this method is likely to overestimate R. Using 2001–2006 NNDSS data, 44 measles cases were recorded as imported out of a total of 446 cases and R was calculated as 0.90. Using data from the state of Western Australia, with a more complete follow-up of cases, R was estimated to be 0.62 between March 1999 and October 2000.36 The two other methods of calculating R rely on data for the distribution of the size and duration of outbreaks.46,49 Using outbreak data from enhanced surveillance in Victoria between 1998 and mid-2003, R was calculated to be 0.85–0.87 using the size of outbreak method and 0.73–0.76 using data on the duration of outbreaks.35 The similarity of the estimates of R using serosurveillance data to these estimates from case surveillance data (national and state estimates) supports the reliability of these estimates.
Discussion
The criteria used to justify our declaration of measles elimination in Australia are as follows:
< 1 notified confirmed endemic case per million population since 2005 within an adequate surveillance system since 2004;
consistently high two-dose vaccination coverage: MCV1 > 95% and MCV2 > 90% since 2004;
serological evidence of population immunity > 90% since 2002;
absence of an endemic genotype since 1999;
a high proportion of cases imported or linked to an imported case since 1999;
containment of outbreaks without the re-establishment of a specific genotype since 1999; and
maintenance of an effective reproductive number for measles of < 1 since 1999.
Based on the number of notified cases, the most conservative year for declaration of elimination in Australia is 2005, although multiple lines of evidence suggest interruption of the endemic transmission of measles since 1999. The set of interim criteria defined by WPRO for the documentation of elimination of endemic measles transmission in a region may not be practical in many countries such as Australia, despite the existence of adequate surveillance systems, due to varying capacity in reporting these criteria at a national level. This particularly applies to the extensive documentation on the investigation of suspected cases at national level, which currently includes the discard rate, laboratory performance indicators and obtaining virologic samples from every presumptive chain of transmission (WPRO criteria 2–7, Table 2).
Australia satisfied the WPRO criterion of < 1 case per million population in 2005 and 2007. Although very low incidence is a significant criterion in defining measles elimination, we believe incidence rates > 1 case per million should not exclude declaration of elimination, especially if the cases are acquired outside the country and if other evidence suggests that sustained transmission has not occurred.
Adequate disease surveillance is an important criterion for establishing and monitoring measles elimination. The completeness of reporting, the sensitivity of the surveillance system, the use of laboratory confirmation, adequate epidemiological investigation of suspected measles cases and adequate genotyping of outbreaks are all important surveillance performance indicators.4 Although Australia’s surveillance reporting mechanisms do not currently record the investigation of presumptive measles cases at the national level as required by the WPRO criteria, we believe that surveillance is adequate for investigation of isolated cases of measles. Enhanced surveillance in the state of Victoria between 1998 and 2003 demonstrated reporting of non-measles suspected cases much higher than the WPRO target discard rate.29 However, higher rates of discard (i.e. more clinically suspected cases of measles) were reported during the period directly after notification of a case compared to periods when no measles case had been reported. The discard rate may therefore be a useful criterion at the beginning of the elimination phase but, when measles is rare, other diseases may be more likely to be suspected clinically and measles testing may not be requested by clinicians.
Australia’s national one-dose coverage of a measles-containing vaccine satisfies the WPRO criteria, while Australia’s national two-dose coverage is likely to be > 90%. However, Australia has provided additional data from a series of serosurveys that demonstrate 90% of the Australian population is immune to measles, providing the opportunity to identify population groups in need of targeted programmes. Additionally, modelling seroprevalence and surveillance data provides further evidence of elimination, with the reproductive number being maintained < 1. Finally, comprehensive molecular analyses provide substantial evidence of the absence of an endemic measles virus in Australia.
The declaration of the elimination of endemic measles from a region is not static and requires commitment to maintaining coverage, surveillance and outbreak control. Although England and Wales declared endemic measles eliminated in 2003 (Table 1), sustained transmission has recurred due to a reduction in vaccination rates,50 highlighting the requirement of maintenance as fundamental in declaring the elimination of endemic measles.
Under strict application of the WPRO criteria for case investigation, Australia would find it difficult to demonstrate measles elimination, as would most other countries that have previously declared elimination. However, the Australian criteria for the elimination of the transmission of endemic measles satisfy and extend the other WPRO criteria and lack only the WPRO surveillance process criteria 2–7. We believe the data presented confirm measles elimination in Australia and point to the need to broaden the current criteria for elimination of endemic measles transmission. ■
Acknowledgements
We thank the staff of the laboratories who provided the sera for the national serosurveys and laboratory staff at the Institute of Clinical Pathology and Medical Research for their help in processing and testing these sera. The following individuals and groups have contributed data to this paper: Doris Chibo and colleagues at the Victorian Infectious Diseases Reference Laboratory (genotyping data); Neils Becker from the National Centre for Epidemiology and Population Health, Australian National University and James Wood from NCIRS (estimating R); Brynley Hull from NCIRS (ACIR data); James Fielding, from the Communicable Disease Control Unit, Department of Human Services, Victoria (Victorian measles outbreak data); the Communicable Disease Network Australia (advice and NNDSS data); and the Public Health Laboratory Network and state and territory health departments (notification data).
Footnotes
Funding: CIDM-Public Health is supported by an infrastructure grant from the New South Wales Health Department. The National Centre for Immunization Research and Surveillance of Vaccine Preventable Diseases is supported by the Australian Government Department of Health and Ageing, the New South Wales Health Department and The Children’s Hospital at Westmead. Victorian Infectious Diseases Reference Laboratory acknowledges ongoing support from the Department of Human Services, Victoria. The Western Pacific Measles Regional Reference Laboratory at VIDRL receives support from WHO.
Competing interests: None declared.
References
- 1.Measles elimination, hepatitis B control and poliomyelitis eradication (Resolution WPR/RC56.R8). Manila: WHO Regional Committee for the Western Pacific.;2005.
- 2.Dowdle WR. The principles of disease elimination and eradication. Bull World Health Organ. 1998;76(Suppl 2):22–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Field guidelines for measles elimination Geneva: WHO Regional Office for the Western Pacific.;2004.
- 4.Regional WHO. Office for the Western Pacific. Monitoring measles surveillance and progress towards measles elimination. Measles Bulletin 2007;13:1-6 Available from: http://www.wpro.who.int/sites/epi/documents/MeaslesBulletin.htm [accessed on 24 October 2008].
- 5.Papania MJ, Orenstein WA. Defining and assessing measles elimination goals. J Infect Dis. 2004;189(Suppl 1):S23–6. doi: 10.1086/381556. [DOI] [PubMed] [Google Scholar]
- 6.Department of Economic and Social Affairs. World population prospects: the 2006 revision population database New York: United Nations Population Division; 2007. Available from: http://esa.un.org/unpp/ [accessed on 24 October 2008].
- 7.Prevots DR, Parise MS, Segatto TC, Siqueira MM, dos Santos ED, Ganter B, et al. Interruption of measles transmission in Brazil, 2000-2001. J Infect Dis. 2003;187(Suppl 1):S111–20. doi: 10.1086/368030. [DOI] [PubMed] [Google Scholar]
- 8.King A, Varughese P, De Serres G, Tipples GA, Waters J.and members of the Working Group on Measles Elimination. Measles elimination in CanadaJ Infect Dis 2004189Suppl 1S236–42. 10.1086/378499 [DOI] [PubMed] [Google Scholar]
- 9.Galindo MA, Santin M, Resik S, Ribas MA, Guzman M, Mas Lago P, et al. Eradication of measles in Cuba. Rev Panam Salud Publica. 1998;4:171–7. doi: 10.1590/S1020-49891998000900004. [erratum appears in Rev Panam Salud Publica 1998 Nov;4(5):345] [in Spanish] [DOI] [PubMed] [Google Scholar]
- 10.Ramsay ME, Jin L, White J, Litton P, Cohen B, Brown D. The elimination of indigenous measles transmission in England and Wales. J Infect Dis. 2003;187(Suppl 1):S198–207. doi: 10.1086/368024. [DOI] [PubMed] [Google Scholar]
- 11.Census 2001: England and Wales Newport: Office for National Statistics UK;2008. Available from: http://www.statistics.gov.uk/census2001/pyramids/pages/727.asp [accessed on 24 October 2008].
- 12.Peltola H, Heinonen OP, Valle M, Paunio M, Virtanen M, Karanko V, et al. The elimination of indigenous measles, mumps, and rubella from Finland by a 12-year, two-dose vaccination program. N Engl J Med. 1994;331:1397–402. doi: 10.1056/NEJM199411243312101. [DOI] [PubMed] [Google Scholar]
- 13.Santos JI, Nakamura MA, Godoy MV, Kuri P, Lucas CA, Conyer RT. Measles in Mexico, 1941-2001: interruption of endemic transmission and lessons learned. J Infect Dis. 2004;189(Suppl 1):S243–50. doi: 10.1086/378520. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Elimination of measles in the Republic of Korea, 2001-2006. Wkly Epidemiol Rec. 2007;82:118–24. [PubMed] [Google Scholar]
- 15.Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16-17 March 2000. J Infect Dis. 2004;189(Suppl 1):S43–7. doi: 10.1086/377696. [DOI] [PubMed] [Google Scholar]
- 16.Heath T, Burgess M, McIntyre P, Catton M. The national measles surveillance strategy. The National Centre for Disease Control Measles Elimination Advisory Committee. Commun Dis Intell. 1999;23:41–50. doi: 10.33321/cdi.1999.23.3. [DOI] [PubMed] [Google Scholar]
- 17.Hall R. Notifiable diseases surveillance, 1917 to 1991. Commun Dis Intell 1993;17:226-36. Available from: http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-pubs-annlrpt-oz_dis19_91.htm/$FILE/ozdis1917_91.pdf [accessed on 24 October 2008].
- 18.Communicable Diseases Network Australia. Interim surveillance case definitions for the Australian National Notifiable Diseases Surveillance System, version 1. Canberra: Department of Health and Ageing; 2004. Available from: http://www.health.gov.au [accessed on 24 October 2008].
- 19.Gidding HF, Burgess MA, Kempe AE. A short history of vaccination in Australia. Med J Aust. 2001;174:37–40. doi: 10.5694/j.1326-5377.2001.tb143144.x. [DOI] [PubMed] [Google Scholar]
- 20.Tobin S, Kelly H. Measles encephalitis in Victoria, 1962-96: down but not out. Aust N Z J Public Health. 1999;23:443. doi: 10.1111/j.1467-842X.1999.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 21.Mulders MN, Truong AT, Muller CP. Monitoring of measles elimination using molecular epidemiology. Vaccine. 2001;19:2245–9. doi: 10.1016/S0264-410X(00)00453-9. [DOI] [PubMed] [Google Scholar]
- 22.Gidding HF. The impact of Australia’s measles control programme over the past decade. Epidemiol Infect. 2005;133:99–105. doi: 10.1017/S0950268804003073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turnbull FM, Burgess MA, McIntyre PB, Lambert SB, Gilbert GL, Gidding HF, et al. The Australian Measles Control Campaign, 1998. Bull World Health Organ. 2001;79:882–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Communicable Diseases Australia. National Notifiable Diseases Surveillance System (NNDSS) data. Canberra: Department of Health and Ageing;2006. Available from: http://www.health.gov.au/cda/source/cda-index.cfm [accessed on 24 October 2008].
- 25.Giele C, Owen R, Sarna M, Van Buynder P. A multi-state measles outbreak associated with a touring spiritual group from India [abstract]. In: Communicable disease control conference: from outbreaks to pandemics in the region - building our capacity to respond, Canberra, 15-16March2007 [Google Scholar]
- 26.Communicable Diseases Network Australia. Vaccine preventable diseases incidence and vaccination status information. In: 30th meeting of Australian Technical Advisory Group on Immunisation, Canberra, 7-8June2006. [Google Scholar]
- 27.Communicable Diseases Network Australia & New Zealand. Guidelines for the control of measles outbreaks in Australia [Technical report series no. 5]. Canberra: Department of Health and Aged Care; 2000. [Google Scholar]
- 28.Australian Bureau of Statistics. Population by age and sex, Australian states and territories, June 2006 [Cat. no. 3201.0]. Canberra: ABS;2006. Available from: http://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/8C8443D8A7E676EECA257242001B232D/$File/32010_jun%202006.pdf [accessed on 24 October 2008].
- 29.Wang YH, Andrews RM, Kelly H, Lambert SB. Evaluating measles surveillance using laboratory-discarded notifications of measles-like illness during elimination. Epidemiol Infect. 2007;135:1363–8. doi: 10.1017/S095026880700828X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Guidelines for epidemic preparedness and response to measles outbreaks Geneva: WHO;1999. Available from: http://www.who.int/emc [accessed on 24 October 2008].
- 31.Department of Health and Ageing. Let’s work together to beat measles: a report on Australia’s Measles Control Campaign Canberra: Department of Health and Ageing; 2000. [Google Scholar]
- 32.National Health Survey. Children’s Immunisation, Australia, 1989-1990 Canberra: Australian Bureau of Statistics; 1992. [Google Scholar]
- 33.Lawrence GL, MacIntyre CR, Hull BP, McIntyre PB. Measles vaccination coverage among five-year-old children: implications for disease elimination in Australia. Aust N Z J Public Health. 2003;27:413–8. doi: 10.1111/j.1467-842X.2003.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 34.Hull BP, Lawrence GL, MacIntyre CR, McIntyre PB. Immunisation coverage in Australia corrected for under-reporting to the Australian Childhood Immunisation Register. Aust N Z J Public Health. 2003;27:533–8. doi: 10.1111/j.1467-842X.2003.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 35.Becker NG, Li Z, Hsu E, Andrews RM, Lambert SB. Monitoring measles elimination in Victoria. Aust N Z J Public Health. 2005;29:58–63. doi: 10.1111/j.1467-842X.2005.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 36.Dowse GK, Gill J, Smith DW. Measles elimination in WA: a story of imported cases and nosocomial transmission [abstract 58]. In: Communicable diseases control conference, Canberra, 2-3April2001 [Google Scholar]
- 37.World Health Organization Update of the nomenclature for describing the genetic characteristics of wild-type measles viruses: new genotypes and reference strains. Wkly Epidemiol Rec. 2003;78:229–32. [PubMed] [Google Scholar]
- 38.Muwonge A, Nanyunja M, Rota PA, Bwogi J, Lowe L, Liffick SL, et al. New measles genotype, Uganda. Emerg Infect Dis. 2005;11:1522–6. doi: 10.3201/eid1110.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkin GA, Chibo D, Kelly HA, Lynch PA, Catton MG. What is the cause of a rash after measles-mumps-rubella vaccination? Med J Aust. 1999;171:194–5. doi: 10.5694/j.1326-5377.1999.tb123596.x. [DOI] [PubMed] [Google Scholar]
- 40.Chibo D, Birch CJ, Rota PA, Catton MG. Molecular characterization of measles viruses isolated in Victoria, Australia, between 1973 and 1998. J Gen Virol. 2000;81:2511–8. doi: 10.1099/0022-1317-81-10-2511. [DOI] [PubMed] [Google Scholar]
- 41.Chibo D, Riddell M, Catton M, Lyon M, Lum G, Birch C. Studies of measles viruses circulating in Australia between 1999 and 2001 reveals a new genotype. Virus Res. 2003;91:213–21. doi: 10.1016/S0168-1702(02)00273-3. [DOI] [PubMed] [Google Scholar]
- 42.Gidding HF, Wood J, MacIntyre CR, Kelly H, Lambert SB, Gilbert GL, et al. Sustained measles elimination in Australia and priorities for long term maintenance. Vaccine. 2007;25:3574–80. doi: 10.1016/j.vaccine.2007.01.090. [DOI] [PubMed] [Google Scholar]
- 43.Gidding HF, Gilbert GL. Measles immunity in young Australian adults. Commun Dis Intell. 2001;25:133–6. doi: 10.33321/cdi.2001.25.27. [DOI] [PubMed] [Google Scholar]
- 44.Kelly H, Riddell MA, Gidding HF, Nolan T, Gilbert GL. A random cluster survey and a convenience sample give comparable estimates of immunity to vaccine preventable diseases in children of school age in Victoria, Australia. Vaccine. 2002;20:3130–6. doi: 10.1016/S0264-410X(02)00255-4. [DOI] [PubMed] [Google Scholar]
- 45.De Serres G, Gay NJ, Farrington CP. Epidemiology of transmissible diseases after elimination. Am J Epidemiol. 2000;151:1039–48. doi: 10.1093/oxfordjournals.aje.a010145. [DOI] [PubMed] [Google Scholar]
- 46.Farrington CP, Kanaan MN, Gay NJ. Branching process models for surveillance of infectious diseases controlled by mass vaccination. Biostatistics. 2003;4:279–95. doi: 10.1093/biostatistics/4.2.279. [DOI] [PubMed] [Google Scholar]
- 47.MacIntyre CR, Gay NJ, Gidding HF, Hull BP, Gilbert GL, McIntyre PB. A mathematical model to measure the impact of the Measles Control Campaign on the potential for measles transmission in Australia. Int J Infect Dis. 2002;6:277–82. doi: 10.1016/S1201-9712(02)90161-X. [DOI] [PubMed] [Google Scholar]
- 48.Farrington CP, Whitaker HJ. Estimation of effective reproduction numbers for infectious diseases using serological survey data. Biostatistics. 2003;4:621–32. doi: 10.1093/biostatistics/4.4.621. [DOI] [PubMed] [Google Scholar]
- 49.Gay NJ, De SG, Farrington CP, Redd SB, Papania MJ. Assessment of the status of measles elimination from reported outbreaks: United States, 1997-1999. J Infect Dis. 2004;189(Suppl 1):S36–42. doi: 10.1086/377695. [DOI] [PubMed] [Google Scholar]
- 50.Ashmore J, Addiman S, Cordery R, Maguire H. Measles in north east and north central London, England: a situation report. Eurosurveillance Weekly Release 2007;12(9):E070920.2. Available from: http://www.eurosurveillance.org/ew/2007/070920.asp#2 [accessed on 24 October 2008]. [DOI] [PubMed]