Abstract
The hair follicle develops from the primitive embryonic epidermis as a result of complex epithelial-mesenchymal interactions. The full follicle, consisting of epithelial cylinders under control of a proximal lying mesenchymal papilla, grows in cycles giving rise to a new hair shaft during each cycle. The ability to cycle endows the follicle with regenerative properties. The evolution of hair follicle engineering began with the recognition in the early 1960's that hair follicles could be transplanted clinically into a foreign site and still grow a shaft typical of the donor site. Since that time, it has been found that the follicular papilla has hair follicle inducing properties and that the hair follicle houses within it epithelial stem cells that can respond to hair inductive signals. These findings have laid the foundation for isolating hair-forming cells, for expanding the cells in culture, and for forming new follicles in vivo.
Key Words: organogenesis, hair follicle, folliculoneogenesis, organ bioengineering, hair restoration
Introduction
Our purpose is to review recent efforts by clinical and laboratory investigators to restore, or regenerate, new hair follicles. While our emphasis is on hair follicle neogenesis in mice and men with a focus on the clinical problem of male and female pattern balding, we refer, where relevant, to work addressing and sharing similar questions in the greater field of tissue and organ engineering in the adult. Although we attempt to summarize the current understanding of new hair follicle formation, our discussion is limited to the topic of laboratory efforts to understand folliculoneogenesis and to bioengineer this organ; nevertheless, liberal reference is made to literature which should facilitate the inquisitive reader's search into other aspects of hair biology and hair disease.
Because the hair follicle is small, common, and serves a function apparently not essential for life, the study of it has been considered dismissively (at least by research grant committees), and disparagingly, as the study of a very simple structure. Its simplicity is deceptive. Understanding hair shaft formation requires at least some appreciation of stem cell biology, epithelial-mesenchymal interactions, cell adhesion, cytoskeleton and intermediate filament formation, controls of cell lineage formation and differentiation, cell attachments—their formation and breakage, cell motility, cell-cell communication, apoptosis, hormone sensitivity, neuro-humoral-immune interactions, and cell pigmentation. In fact, some of the best current work on epithelial-mesenchymal interactions and new organ formation in the adult is being generated using the hair follicle as a model (e.g., refs. 1–5).
We start with a brief review of hair follicle biology emphasizing its inherent regenerative ability, followed by sections on male pattern balding, and studies of hair follicle growth from transplantation of whole structures, from fragmented structures, and from dissociated cells. Finally, we touch on mechanisms and future challenges.
At the outset, we would like to share our conviction that the understanding of folliculoneogenesis and its translation to the clinic will impact other aspects of regenerative medicine. In other words, the scientific questions and barriers that must be overcome to engineer a hair follicle are very similar to the challenges we face in modern medicine to engineer other tissues and organs. The hair follicle is attractive as a primary target in this regard because it is a readily accessible multicellular organ that shares the biological complexities of other larger organs of greater consequence. In its basic structure the hair follicle has the ability to reform itself in a regular and predictable fashion through its recurring growth cycle over the lifetime of the individual. Its regenerative ability bestows on the hair follicle properties which make it a suitable organ to reform in the laboratory and then transfer back to the clinic. In terms of market demand, the clinical need for new hair follicles arises primarily from patients with male/female pattern balding (alopecia).
Hair Follicle Growth
The elements of hair follicle growth and its control have been (reviewed in refs. 6–8) (Fig. 1 and Fig. 2). Briefly, like the scale, feather, and nail, the hair follicle arises from a primitive epidermis, the multilayered epithelial surface of the vertebrate. In the paleontological record, the hair follicle arose from a primitive reptilian cutaneous structure at the time early mammals separated from the reptiles.9 In the human embryo, the first evidence of follicle formation appears at week 10–1110,11 as an epidermal thickening which is associated with a dermal cellular condensate. That layering of the epidermis appears to be a prerequisite for follicle formation is based in part on a p-63 knockout mouse that contains a single layered epidermis and also lacks hair follicles.12 The epithelial thickening projects down into the dermis to form a finger-like peg, the follicle. The base of the follicle expands to embrace the dermal condensate that forms the follicular, or dermal papilla. The initial epithelial downgrowth occurs by means of extensive molecular interactions between the mesenchyme and the epithelium.11,13,14 The morphogenetic and differentiating molecular signals include members of growth factor families found important to the development and growth of other appendages including the limbs, tooth and feather (e.g., ref. 15, Fig. 2). Although morphological and molecular similarities are recognized between follicular morphogenesis and the adult hair cycle, the mechanisms are probably somewhat different.16 In general, new hair follicles form in the fetus but not in the adult.
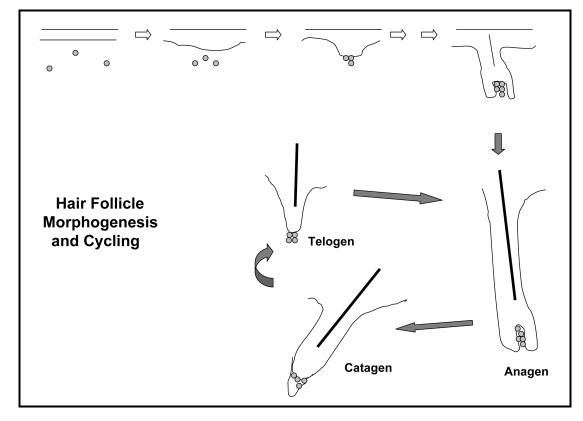
Figure 1.
Schematic representation of hair follicle morphogenesis and cycling. For follicle induction (1) the important early molecular signals involve the Edar and Wnt-Catenin pathways. The first morphological change is placode formation (2) controlled by a balance between inhibitor and activator molecules. Details of molecular events related to early morphogenesis and differentiation (5) are reviewed in Millar (11) and Schmidt-Ullrich and Paus (105). With time a mature growing hair follicle forms. After that, the hair follicle initiates its cyclic growth, experiencing periods of growth and shaft formation, anagen, of regression, the catagen phase mediated by apoptosis, and then a quiescent or resting phase, telogen, before the start of the next anagen. Many of the molecules involved in morphogenesis are also expressed during the growth cycle of the mature hair follicle.
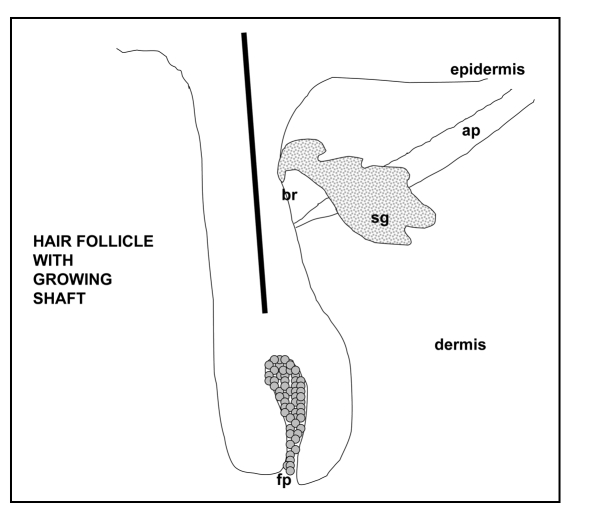
Figure 2.
Sketch of a mature hair follicle. Illustrated is the follicular papilla (Fp), sebaceous gland (sg), arrector pili muscle (ar), and bulge region (br), the stem cell niche of the hair follicle.
As the hair follicle grows down into the primitive dermis, it differentiates stepwise into layers of embedded cylinders of specialized epithelial cells.7 The layers can be grouped into three structures: (1) the central group forms the hair shaft which is made of tightly-packed cells filled with hard keratin,17 (2) the outer group serves to insulate the inner layers from the dermis and (3) the middle group holds and molds the hair shaft on its way from the deep dermis to the skin surface. An intrinsic part of the hair follicle is its sebaceous gland and an attached smooth muscle (arrector pili muscle). Although the muscle of the hair follicle has not been shown to be important to new hair shaft formation, the sebaceous gland has been found to play an important role in shaft-sheath processing.18,19
Once the hair follicle is fully formed it undergoes cyclic periods of regression, rest, regrowth and shaft shedding (Fig. 1). Cycle control is critical to normal hair health. Biologists believe that the hair follicle growth cycle offers the mammal the opportunity to generate, seasonally, a new fur coat. This periodic shedding of hair shafts would provide (1) a means of cleansing the body surface, and (2) an opportunity to change the character of the hair coat (in color, length, curl, or thickness). The actual growth phase of the hair follicle is that portion of the cycle when the follicle is forming a new shaft, the anagen phase. The length of this phase dictates the length of the hair shaft—on the scalp that period can run from 2–6 years. At the end of the growth phase the deep, proximal, portion of the follicle regresses by means of programmed cell death and then undergoes a period of rest (telogen) before a new follicle forms and new shaft production begins. Sometime after new shaft production begins, the shaft is shed by another tightly-controlled process, the exogen phase.20 Although a focus of active investigation,21 the actual molecular signals which drive a hair follicle through the cycle are as yet unknown.
Compelling evidence presented from mouse studies indicates that epithelial stem cells are housed in the skin at two sites: in the bulge of the hair follicle (a portion of the follicle just above the muscle attachment site, (see Fig. 2) and in the basal layer of the epidermis. The slow-cycling cells in both sites have been demonstrated to have stem cell properties22,23). The bulge stem cells normally give rise to the follicle, its shaft and its sebaceous gland24–28 but do not normally contribute to epidermal growth unless the epidermis is injured.29,30 Epidermal stem cells play a role in epidermal homeostasis though under the proper circumstances16) they, like corneal epithelium31 and amnion,22,33 can respond to dermal signals to form a new hair follicle.
Cells with stem cell characteristics are also found in follicle-associated mesenchyme.34–36 As documented in studies described below, dissected follicular papillae, as intact or as collections of cultured cells, have inductive trichogenic properties: they will induce new follicle formation when placed in close proximity to receptive epithelium. Moreover, cells derived from the follicular papilla show the plasticity of mesenchymal cells by differentiating into neurons, glia, smooth muscle cells, adipocytes, bone, cartilage and bone marrow.35–40
One current widely-accepted notion regarding the control of hair follicle cycling, termed the “bulge activation hypothesis”, states that signals from the papilla (the mesenchymal component of the follicle) stimulate the resting epithelial stem cells in the bulge to generate transient amplifying cells which then form the follicle of the next cycle and the next new hair shaft.41 This hypothesis and evidence presented below embrace the concept that these two cell types—the bulge epithelial stem cells and the papilla mesenchymal cells—are necessary and sufficient for the reconstruction of new hair follicles.22
Androgenetic Alopecia: Male Pattern Balding
Androgenetic alopecia, AGA, the most common cause of hair loss, affects 50% of men and 20% to 53% of women by age 50 years.42 It is a patterned form of hair loss in that the resultant baldness occurs in a highly predictable location.43–45 For reasons we do not yet understand, AGA in females is more diffuse and less well patterned than in males.46
Etiologically, AGA is caused by genetic and hormonal factors. While some genetic studies suggest an autosomal dominant inheritance with incomplete penetrance,47 other studies describe a unique polymorphism of the androgen receptor (X-chromosome) in these patients.48,49 Early studies demonstrated that AGA hair loss does not occur in the absence of androgens43 or the androgen receptor;50 moreover, if androgens are administered to genetically predisposed but androgen deficient males, the nonbald androgen recipients will now develop AGA.43
Central to the diagnosis of AGA, is that hair growth on the scalp periphery (temporo-parietal and occipital scalp regions) is resistant to the hair loss process. That peripheral scalp follicles are hormone insensitive, in contrast to those from the vertex scalp, underscores the fact that normal hair, like normal skin, is highly patterned: the follicles of the scalp periphery are always androgen insensitive while follicles of the scalp anterior and vertex are androgen sensitive (e.g., ref. 51). Histological study of scalp skin affected by AGA shows not an absence of hair follicles, or a decreased density of hair follicles but, instead, a diminution of follicle size.52 The follicles found in the bald scalp are very small and superficial, producing very fine (≤30 mm diameter53) non-pigmented hair shafts; this contrasts with the follicles found in a normally haired scalp, which are large (≥60 mm diameter53), deep and pigmented. The therapeutic challenge in treating male pattern balding, then, is to convert, or replace, an abnormally small follicle with a large, normal follicle.
Recognizing the preservation of peripheral hair follicles in the patient with severe male pattern balding, Orentreich in a series of seminal clinical studies demonstrated that follicles transplanted from the hormone insensitive peripheral scalp region will continue to grow in a hormone insensitive fashion—donor dominance—when transplanted into a hormone sensitive, bald region.54 This discovery launched the field of cosmetic hair replacement surgery for patients with AGA.
Hair Follicle Growth from Transplantation of Whole Hair Follicles
Because currently there is widespread use of whole hair follicle transplants for the treatment of AGA,55 there is extensive clinical experience placing autologous androgen-insensitive hair follicles from the peripheral (occipital) scalp into autologous androgen-sensitive vertex (frontal and mid-anterior) scalp.
For these procedures a strip of occipital scalp skin is surgically excised and individual hair follicle units are dissected. A scalpel wound is made in the recipient scalp site and one or more dissected follicular units are implanted. In this procedure approximately 90% of the full hair follicle implants grow and produce a shaft. It is generally observed that the transplanted whole anagen follicle will cycle out of anagen through catagen and rest in telogen before a new cycle is initiated;56,57 these transplanted follicles pass through a dystrophic cycle and then re-enter anagen by day 60 after implantation. This transplant procedure is efficient and effective in replacing terminal hair follicles in hair loss areas. Its success clearly shows that whole hair follicles transplanted to another site will grow and that the donor follicle behaves in the recipient site, as if it were still in the donor site. Since the challenge for the patient with severe AGA is that he often runs out of donor follicles before his area of hair loss is covered, he might ask the question: Can the same implant success be achieved using fragments of a donor hair follicle?
Hair Follicle Growth from Transplantation of Follicular Fragments
Two approaches have been taken in attempting to regenerate hair follicles from follicle sections: (1) using fragments of whole follicles, or (2) using carefully dissected follicular papillae.
If new follicles could be generated efficiently by implanting a dissected, trisected or multi-sected mature anagen hair follicle, similar to the regenerative ability of the flatworm, Planaria,58 the bald patient would surely benefit. Unfortunately, although follicle regeneration from fragments of mature whole follicle is reported to occur in the human, its occurrence is irregular and inconsistent. The questions have been how many follicles can be generated from one mature follicle, how small a follicle piece can produce a complete follicle, and which follicular components are requisite for follicle regeneration. In most studies the follicle fragment to be studied is placed within the skin, or under the kidney capsule, of an immunocrippled mouse.
In work with human follicles, Kim et al., for example,59,60 showed that a complete follicle could be regenerated if one-half or two thirds of a full follicle were implanted; they reported that either lower or upper half of the donor follicle could produce a full new follicle but that implanting one-third of the follicle, upper or lower, would not work. When human hair follicles were systematically sectioned, in good numbers, and implanted into nude mouse skin, Tang et al.61 reported that in only one instance did a regenerated follicle result—this was using the upper portion of a follicle which had been transected below the sebaceous gland. Although the claim has been made that two hair follicles can be produced from one (30–70% success rates are anecdotally quoted in the hair transplantation field), the fragmented follicle approach to achieve follicle multiplication has not been critically evaluated or generally practiced.
In contrast to whole follicle fragments, studies with dissected rodent follicular papillae have shown that this structure, when implanted close to a receptive epithelium, will indeed induce a new hair follicle. Early embryonic studies of epithelial mesenchymal interactions in several animal systems suggested to developmental biologists that the rat whisker follicular papilla might be able to induce hair follicles.62 For the feather and hair follicle the important dermal component was found to be the papilla.62,63 These studies revealed that the mesenchyme (dermal tissue) dictates the site and structure of the appendage64–66) and that these signals are highly conserved between species (e.g., human-rabbit, human-rat32; human-mouse67). The power of the papilla was demonstrated in laboratory studies of truncated vibrissal follicles. These studies showed that a truncated hair follicle could reform a hair follicle if no more than the lower half of the follicle were removed;57,63,68 however, if more of the follicle were removed, regrowth could be rescued by placing a freshly dissected papilla within the truncated follicle base.69 More recent studies suggest that the papilla is, in fact, a dynamic structure which is fed by dermal sheath cells lateral to and surrounding the follicular bulb;70,71 moreover, papilla cells can also be cultured and then implanted into the upper skin to generate new follicles.71,72 A recent study using human follicle cells in humans shows that the phenomenon also applies to man.73 In the latter study a fragment of lower dermal sheath arising from a male donor was implanted into the arm skin of a female recipient with resultant new follicle formation. Marker studies indicated that the papilla cells of the new follicle expressed donor characteristics (i.e., Y chromosome). This study was provocative for two reasons: (a) it demonstrated a unique immunological privilege of the donor cells (the recent finding of the immunosuppressive molecule, CD200, expression by follicle stem cells [74] offers a plausible mechanism) and (b) properly placed human dermal cells can induce new follicle formations in living humans. Although it is generally believed that inductive mesenchyme is confined to the lower follicle connective tissue sheath, there may be weak activity in the cells of the upper follicle sheath as well.75
The above studies suggest that folliculoneogenesis requires a receptive epithelium and inductive mesenchyme. One might conclude that hair follicle engineering from dissociated cells is an opportunity whose time has come and that the process would only involve the production and placement of a population of trichogenic inductive dermal cells in close contact with a population of competent trichogenic epidermal cells in order to give the bald patient the relief he is seeking.
Hair Follicle Growth by Implantation of Dissociated Cells
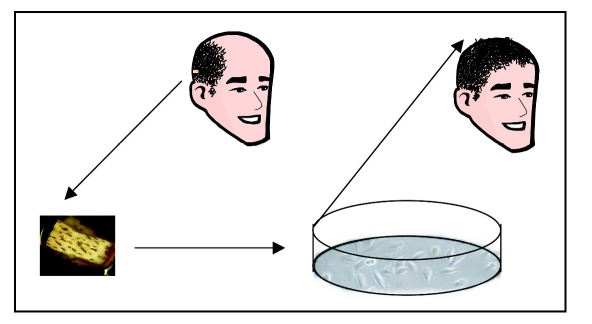
The rationale for attempting to form follicles from dissociated cells is to multiply an organ which usually does not increase in number in the adult. Culturing inductive papilla cells from dissected anagen follicles was first reported in 1984.72 Although these cells grow well in culture, they lose their trichogenic inductive activity within an early passage. Approaches to stabilize that activity has been put forth with some success.76,77 The goal would be to generate the technology to isolate and expand cells arising in a few donor follicles to cover a bald scalp with hair (Fig. 3). Because cells from donor follicles are expanded in vitro, this approach has been also referred to as “hair cloning”.78
Figure 3.
Conceptual representation of scalp hair restoration. Although at present hair restoration is only available clinically using whole hair follicles, it is conceivable that in the near future, a skin biopsy will be taken from a person needing hair replacement, the hair forming cells will be expanded in tissue culture, and then implanted into the skin.
While multiple attempts have been made to grow hair follicles from skin fragments or dissociated cells in vitro,79–81 the greatest successes have been found after injecting trichogenic cells back into syngeneic or immunoincompetent mice32,82–84 or even by a combination of aggregating cells in vitro and placing the aggregates in vivo.76,85 Experimental in vivo tissue systems which have been exploited to grow follicles from dissociated cells include the superficial dermis,71 kidney capsule75,86 and trachea.87 In these studies the origin of the cells, the age of the donor, the tissue culture status, and the ratio of the cells combined are all important parameters affecting the extent of folliculoneogenesis.
A series of highly successful studies have been described using freshly isolated and dissociated mouse newborn epidermal and dermal cells injected into either the silicone subcutaneous chamber83,84 or, by direct injection, into the deep dermis.88
Using the silicone chamber, approach several groups have been able to reform hair follicles starting with completely dissociated, fresh or cultured, most often neonatal, epidermal and dermal cells.32,83,84,89 In these studies a wound is made on the back of an immunoincompetent mouse and a tent like chamber is placed over the wound: the wound bed being the fascial plane below the skin. If both lineages of the implanted cells (epithelial and mesenchymal) are trichogenic, complete regeneration of the mouse skin with formation of a dense population of mouse hair follicles with resultant outgrowing shafts is found. This system allowed for the first time a means of assessing directly (1) if isolated cells were trichogenic and (2) a means of measuring the effect of molecular manipulation on the folliculoneogenesis induced by these cells (e.g., refs. 3,84 and 89). The striking finding in these studies is that complete skin organ regeneration is effected by the implanted trichogenic cells!
Because the chamber method of Lichti83,84 is difficult to set up—it requires one mouse per assay, a large number of cells, 4–6 weeks for development, and is surgically complex—new systems were sought. In an attempt to improve this assay, Zheng et al.88 injected a suspension of trichogenic epidermal and dermal cells directly into mouse skin. Taking this approach it was found88 that mature follicles formed surprisingly rapidly: completely mature follicles are identified within eight days after intradermal injection. Compared to the Lichti/Prouty chamber assay, follicles could be induced using a smaller number of cells, using a smaller number of mice (since more than one assay could be implanted in each mouse) and in a shorter period of time. Follicles generated in this system have the morphology of mouse pelage follicles and their shafts (reflecting the origin of the dermal cells used) showing the same size, shapes, and pigmentation. When a new follicle forms in this system, it behaves like a follicle in that it produces a hair shaft and it shows cyclical growth with essentially the same period as the follicles from which the cells were extracted. While most of the studies were conducted in immune compromised mice, follicle formation from identical cells was also found to occur when dissociated syngeneic cells were implanted into immune competent mice.
To examine how a new follicle develops from dissociated cells, hair follicle formation was differentiated morphologically over time using conventional and specific cell marker stains (Fig. 4). Trichogenic cells were injected into immune crippled mouse skin and the skin harvested at intervals over 60 days. Early in these studies it was found that successful follicle formation depended on placing the cells into a space which confined the cells; if the cells were injected onto the skeletal muscle fascial plane, where the cells were loosely packed, few or no follicles formed. It appeared placing the cells in a packed environment was pivotal for the cell interactions, or aggregations, needed for organogenesis.
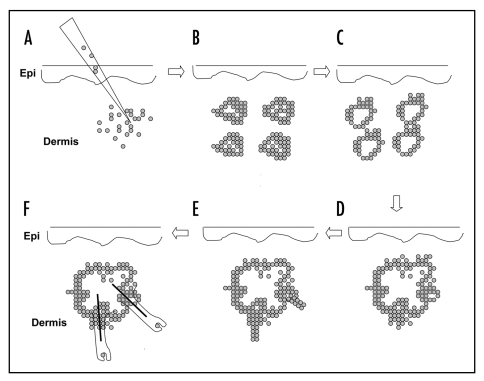
Figure 4.
Schematic representation of hair follicle formation from dissociated cells injected into mouse dermis. After injection of dissociated hair follicle progenitor cells (A), epidermal cell aggregates form (B). The small aggregate fuse to give rise to epidermal cysts (C), some of which fuse to form large cysts (D). Follicular outgrowths with associated follicular papillae initiate from the periphery of the cyst (E). The outgrowths are reminiscent of early follicle morphogenesis in the fetus (Fig. 1). The follicular outgrowths differentiate and eventually form protruding hair shafts (F).
In differentiating the morphogenetic process it was found that within 24 hours after dermal injection, epithelial cells form small aggregates. The surrounding mesenchymal cells did not aggregate but became cytoplasmic-rich with blastemal-like features. Although cell division was scarce among either cell population in the early phases, apoptosis was found to be widespread in the stroma, and focally, in the epithelial aggregates. At 48 hours epithelial clusters had grown in size by cumulative aggregation and some had developed a more cystic appearance with clearing of the aggregate center and lamination of keratinized cells about the cyst cavity; these structures assume the characteristics of the most distal, or infundibular, portion of the hair follicle as they show keratohyalin formation in the inner cell layer of the cystic space wall. At 48 hours, as seen by alkaline phosphatase staining, focal dermal condensations were found at one pole of the epithelial aggregates. At the site of this dermal contact the epithelial cells showed the greatest concentration of cell cycling as detected by Ki67 staining. With time the epithelial clusters continued to fuse with other clusters leading to very large cystic spaces surrounded by finger like peripheral outgrowths suggestive of the early peg state of follicular formation in the embryo.14
By day five, the layers of differentiated follicles were recognizable in these projections and, by day 8, complete mature follicle formations were found with hair shaft formation. These new follicles cycle and return to a telogen (rest) phase follicle in about 20 days. Although a second cycle was observed in this model, eventually all the follicles are destroyed by an inflammatory and foreign body inflammatory cell reaction. Follicular ablation occurs in this model because the shafts produced by these follicles are not shed as they are normally: the newly formed follicles are confined to a hypodermal space, and the naked shafts serve as an irritant in the surrounding dermis. Although the new follicles in this system do not usually produce skin surface hair shafts (because the new follicle growth occurs in the deep dermis), if the trichogenic cells are implanted superficial enough, hair shafts do egress, individually or in tufts.
Cellular Mechanisms of New Hair Follicle Formation from Dissociated Trichogenic Cells
The above studies show that hair follicles will form rapidly and efficiently if trichogenic cells are combined in an appropriate environment. The follicles form from an epithelial platform which in this model is cystic in shape. The formation of epithelial aggregates in this study occurred earlier than the dermal aggregates and, in turn, the dermal aggregates appeared to respond to a focal site on the epithelial aggregates suggesting that the first organizing signal in hair follicle formation (from dissociated cells) does not come from the dermal cells but from the epidermal cells—a conclusion that contrasts sharply with the current morphogenetic paradigm.11 The studies show that if a hair follicle forms it will behave like a hair follicle—it will make a sebaceous gland, cycle and make a shaft typical of the shafts formed in the dermal cell donor area.
Although the marker labels used in these organogenesis studies indicate that most of the cells making up the hair follicles arise from the implanted cells, there is evidence that host cells also contribute to the epithelial as well as the dermal lineages (Parimoo unpublished 2005). That the follicles formed in the Zheng et al.88 system do not contact host epithelium (neither skin appendages nor epidermis) suggests that the host contribution to the epithelium comes from surrounding mesenchyme or circulating cells, a process that implies a mesenchymal to epithelial transition.91 Others have also found that either vicinal71 or bone marrow/circulating cells92 will incorporate into the regenerated skin and hair.
Jahoda39 postulates that dermal sheath cells may contribute to dermal repair processes from evidence that dermal sheath cells can incorporate into wounds.38 Studies of regeneration in other organ systems also show promiscuous cross-contribution of host and donor cells (e.g., refs. 93–95). The question raised by stem cell scientists is from where host cells arise and how the cells are incorporated. For the hair follicle we do not know yet from where the host cells arise. There are two schools of thought regarding the integration of host cells into newly formed organs. The first argues that host cells integrate by settling into the new organ structure and assume characteristics of that structure.96–100 A second idea is that host cells integrate by fusing with resident cells in the structure (e.g., ref. 101). These differences in mechanisms may not be mutually exclusive but could reflect different organ/tissue systems (e.g., ref. 102). Understanding how host cells are recruited to a regeneration process could also have important practical implications as pharmaceutical tools may be employed to enhance the organogenesis event. Molecules such as the SDF1 or chemokines systems would be attractive first candidates (e.g., ref. 103). While the preceding studies have focused on the host contribution to regenerating organs, it is not clear if extrafollicular cells also contribute to normal, homeostatic, cycling hair follicles. In one study, however, where that notion was tested, incorporation was not found.104
In the case of the hair follicle we are not yet certain how new hairs form each time dissociated cells are injected into skin—particularly in the skin of male pattern balding scalp where multiple small hair follicles remain. The possibility for new hair follicle formation is two fold: first, the implanted trichogenic cells can lead to new follicle formation by inducing new follicles from receptive epithelium—either follicular or epidermal epithelium; or, second, the implanted cells can incorporate into the resident follicles and direct these follicles to a different morphology. In most studies of the literature it is not possible to determine, when follicular papilla cells alone are injected into the upper dermis, if the cells induce a new follicle from the adjacent epithelium, folliculoneogenesis, or incorporate into a neighboring follicle to produce a bigger follicle, the vellus to terminal switch.8,39,71,73
Summary
The quest for hair regeneration starting as a dream described in the records of antiquity became an achievable mission with the discovery of donor dominance and hair transplantation. Attempts to make this procedure more efficient by using whole follicle fragments has been less productive but the dramatic success with the follicle inductive follicular papilla has lead to attempts to generate follicle organs from dissociated cells expanded in culture. While these studies have been most successful using the laboratory rat and mouse, preliminary work with human cells in human systems is promising.
From dissociated cells we observe that trichogenic cells know how to generate fully mature follicles and that these newly formed follicles behave like follicles in their growth cycle and hair shaft characteristics. Studies in the mouse have demonstrated that the mechanism of new hair formation from cells exploits an epithelial platform from which the follicles grow. That host cells contribute to the mature newly formed organ questions the role and significance of host cells to the regenerative process in general.
Hair regeneration has been accomplished in the laboratory using incisive tools and animal models. Is the opportunity now ready for the clinic? The ease of accessibility of the follicle, its inherent regenerative properties, and the perceived demand suggest it will be in the vanguard for engineering and translating other medically important organ systems to the clinic.
Acknowledgements
Support in formatting this article was given by LaWanda Douglas.
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/abstract.php?id=3237
References
- 1.Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Austin Texas: RG Landes; 1998. p. 444. [Google Scholar]
- 2.Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal depletion of Rac 1. Science. 2005;309:933–935. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- 3.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biology. 2005;3:1910–1924. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dlougosz AA, Hutchin ME. From hair to eternity: Hedgehog signaling in skin biology and cancer. Progr in Dermatol Dec. 2005;39:1–12. [Google Scholar]
- 5.Yi O, Carroll D, Pasolli HA, Zhang Z, Dietrich FS, Tarakhovsky A, Fuchs E. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nature Genetics. 2006;38:356–362. doi: 10.1038/ng1744. [DOI] [PubMed] [Google Scholar]
- 6.Paus R, Cotsarelis G. The biology of hair follicles. New Eng J Med. 1999;241:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 7.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 8.Stenn KS, Cotsarelis G. Bioengineering the hair follicle:fringe benefits of stem cell technology. Curr Opin Biotech. 2005;16:1–5. doi: 10.1016/j.copbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, Widelitz R, Jiang TX, Chuong CM. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:249–270. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller M, Jasmin JR, Monteil RA, Loubiere R. Embryology of the hair follicle. Early Human Dev. 1991;26:159–166. doi: 10.1016/0378-3782(91)90155-v. [DOI] [PubMed] [Google Scholar]
- 11.Millar S. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 12.Mills AA, Zheng BK, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 13.Widelitz RB, Jiang TX, Noveen Z, Ting-Berreth S, Yin E, Jung HS, Chuong CM. Molecular histology in skin appendage morphogenesis. Microsc Res Tech. 1997;38:452–465. doi: 10.1002/(SICI)1097-0029(19970815)38:4<452::AID-JEMT13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Meckldenburg L, Handjiski B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 15.Botchkarev VA, Paus R. Molecular biology of hair morphogenesis: Development and cycling. J Exp Zool B Mol Dev Evol. 2003;298:164–180. doi: 10.1002/jez.b.33. [DOI] [PubMed] [Google Scholar]
- 16.Inamatsu M, Tochi T, Makabe A, Endo T, Oomizu S, Kabayashi E, Yoshizato K. Embryonic dermal condensation and adult dermal papilla induced hair follicles in adult glabrous epidermis through different mechanisms. Develop Growth Differ. 2006;48:73–86. doi: 10.1111/j.1440-169X.2006.00848.x. [DOI] [PubMed] [Google Scholar]
- 17.Langbein L, Schweizer J. Keratins of the human hair follicle. Int Rev Cytol. 2005;243:1–78. doi: 10.1016/S0074-7696(05)43001-6. [DOI] [PubMed] [Google Scholar]
- 18.Williams DD, Stenn KS. Transection level dictates the pattern of hair follicle sheath growth in vitro. Dev Biol. 1994;165:469–479. doi: 10.1006/dbio.1994.1268. [DOI] [PubMed] [Google Scholar]
- 19.Philpott MP, Sanders DA, Kealey T. Is the sebaceous gland important for inner root sheath breakdown? In: Van Neste DJJ, Randall VA, editors. Hair Research for the Next Millenium. Elsevier Science BV; 1996. pp. 393–395. [Google Scholar]
- 20.Milner Y, Sudnik J, Filippi M, Kizoulis M, Kashgarian M, Stenn K. Exogen, shedding phase of the hair growth cycle: Characterization of a mouse model. J Invest Dermatol. 2002;119:639–644. doi: 10.1046/j.1523-1747.2002.01842.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishimatsu-Tsuji Y, Moro O, Kishimoto J. Expression profiling and cellular localization of genes associated with the hair cycle induced by wax depilation. J Invest Dermatol. 2005;125:410–420. doi: 10.1111/j.0022-202X.2005.23825.x. [DOI] [PubMed] [Google Scholar]
- 22.Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci USA. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghazizadeh S, Taichman LB. Organization of stem cells and their progeny in human epidermis. J Invest Dermatol. 2005;124:367–372. doi: 10.1111/j.0022-202X.2004.23599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 25.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuch E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 27.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nature Biotechnology. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 28.Blanpain C, Lowry WE, Geoghegan A, Polak I, Fuchs E. Self renewal, multipotency and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 30.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Developmental Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Pearton DJ, Ferraris C, Dhouailly D. Transdifferentiation of corneal epithelium: Evidence for a linkage between the segregation of epidermal stem cells and the induction of hair follicles during embryogenesis. J Dev Biol. 2004;48:197–201. [PubMed] [Google Scholar]
- 32.Ferraris C, Bernard BA, Dhouailly D. Adult epidermal keratinocytes are endowed with pilosebaceous forming abilities. Int J Dev Biol. 1997;41:491–498. [PubMed] [Google Scholar]
- 33.Fliniaux I, Viallet JP, Dhouailly D, Jahoda CA. Transformation of amnion epithelium into skin and hair follicles. Differentiation. 2004;72:558–565. doi: 10.1111/j.1432-0436.2004.07209009.x. [DOI] [PubMed] [Google Scholar]
- 34.Jahoda CAB, Whitehouse CJ, Reynolds AJ, Hole N. Hair follicle dermal cells diffferentiate into adipocytes and osteogenic lineages. Exp Dermatol. 2003;12:849–859. doi: 10.1111/j.0906-6705.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 35.Lako M, Armstrong L, Cairns PM, Harris S, Hole N, Jahoda CA. Hair follicle dermal cells repopulate the mouse haematopoietic system. J Cell Sci. 2002;115:3967–3974. doi: 10.1242/jcs.00060. [DOI] [PubMed] [Google Scholar]
- 36.Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nature Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 37.Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadkot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nature Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 38.Gharzi A, Reynolds AJ, Jahoda CAB. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol. 2003;12:126–136. doi: 10.1034/j.1600-0625.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 39.Jahoda CAB. Cell movement in the hair follicle dermis—More than a two-way street? J Invest Dermatol. 2003;121:ix–xi. doi: 10.1111/j.1523-1747.2003.12585.x. [DOI] [PubMed] [Google Scholar]
- 40.Amoh Y, Li L, Campill R, Kawahara K, Katsuoka K, Penman S, Hoffman RM. Implanted hair follicle stem cells form schwann cell that support repair of severed peripheral nerves. Proc Natl Acad Sci USA. 2005;49:17734–17738. doi: 10.1073/pnas.0508440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro J. Hair Loss. London: Martin Dunitz Ltd.; 2002. pp. 83–117. [Google Scholar]
- 43.Hamilton JB. Patterned hair loss in man: Types and incidence. Ann NY Acad Sci. 1951;53:708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 44.Norwood OT. Male pattern baldness: Classification and incidence. South Med HJ. 1975;68:1359–1365. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Brit J Dermatol. 1977;97:247–254. doi: 10.1111/j.1365-2133.1977.tb15179.x. [DOI] [PubMed] [Google Scholar]
- 46.Norwood OT, Lehr B. Female androgenetic alopecia: A separate entity. Dermatol Surg. 2000;26:679–682. doi: 10.1046/j.1524-4725.2000.99310.x. [DOI] [PubMed] [Google Scholar]
- 47.Bergfeld WF. Androgenetic alopecia: An autosomal dominant disorder. Amer J Med. 1995;98:95S–98S. doi: 10.1016/s0002-9343(99)80065-5. [DOI] [PubMed] [Google Scholar]
- 48.Ellis JA, Stebbing M, Harrap SB. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J Invest Dermatol. 2001;116:452–455. doi: 10.1046/j.1523-1747.2001.01261.x. [DOI] [PubMed] [Google Scholar]
- 49.Hillmer AM, Hannekin S, Ritzmann S, et al. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Amer J Human Genet. 2005;77:140–148. doi: 10.1086/431425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imperato-McGinley J. 5alpha-reductase-2 deficiency and complete androgen insensitivity: Lessons from nature. Adv Exp Med Biol. 2002;511:121–131. doi: 10.1007/978-1-4615-0621-8_8. [DOI] [PubMed] [Google Scholar]
- 51.Randall VA, Thornton MJ, Messenger AG. Cultured dermal papilla cells from androgen-dependent human hair follicles (e.g., beard) contain more androgen receptors than those from nonbalding scalp. J Endocrinol. 1992;133:141–147. doi: 10.1677/joe.0.1330141. [DOI] [PubMed] [Google Scholar]
- 52.Whiting DA. Possible mechanisms of miniaturization during androgenetic alopecia or pattern hair loss. J Am Acad Dermatol. 2001;45:S81–S86. doi: 10.1067/mjd.2001.117428. [DOI] [PubMed] [Google Scholar]
- 53.Sperling LC. An Atlas of Hair Pathology with Clinical Correlations. Boca Roton Fl: Parthenon Publ Group, CRC Press; 2003. p. 1. [Google Scholar]
- 54.Orentreich N. Autografts in alopecias and other selected dermatological conditions. Ann NY Acad Sci. 1959;83:462. doi: 10.1111/j.1749-6632.1960.tb40920.x. [DOI] [PubMed] [Google Scholar]
- 55.Harbor RS, Stough DB, editors. Hair Transplantation. Philadelphia: Elsevier; 2006. p. 211. [Google Scholar]
- 56.Hashimoto T, Kazama T, Ito M, Urano K, Katakai Y, Yamaguchi N, Ueyama Y. Histologic and cell kinetic studies of hair loss and subsequent recovery process of human scalp hair follicles grafted onto severe combined immunodeficient mice. J Invest Dermatol. 2000;115:200–206. doi: 10.1046/j.1523-1747.2000.00063.x. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto T, Kazama T, Ito M, Urano K, Katakai Y, Yamaguchi N, Ueyama Y. Histologic study of the regeneration process of human hair follicles grafted onto scid mice after bulb amputation. J Invest Dermatol Symp Proc. 2001;6:38–42. doi: 10.1046/j.0022-202x.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- 58.Reddien PW, Sanchez AA. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- 59.Kim JC, Choi YC. Regrowth of grafted human scalp hair after removal of the bulb. Dermatol Surg. 1995;21:312–313. doi: 10.1111/j.1524-4725.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 60.Kim JC, Kim MK, Choi YC. Regeneration of the human scalp hair follicle after horizontal sectioning: Implications for pluripotent stem cells and melanocyte reservoir. In: Van Neste D, Randall VA, editors. Hair Research for the next Millennium. Amsterdam: Elsevier; 1996. pp. 135–139. [Google Scholar]
- 61.Tang L, Madami S, Lui H, Shapiro J. Regeneration of a new hair follicle from the upper half of a human hair follicle in a nude mouse. J Invest Dermatol. 2002;119:983–984. doi: 10.1046/j.1523-1747.2002.00009.x. [DOI] [PubMed] [Google Scholar]
- 62.Cohen J. The transplantation of individual rat and guinea-pig whisker papillae. J Embryol Exp Morphol. 1961;9:117–127. [PubMed] [Google Scholar]
- 63.Oliver RF. Ectopic regeneration of whiskers in the hooded rat from implanted lengths of vibrissa follicle wall. J Embryol Exp Morphol. 1967;17:27–34. [PubMed] [Google Scholar]
- 64.Dhouailly D. Dermo-epidermal interactions during morphogenesis of cutaneous appendages in amniotes. In: Robert L, editor. Frontiers of Matrix. Biology Creteil. 1977. pp. 86–121. (karger S) [Google Scholar]
- 65.Dhouailly D. Regional specification of cutaneous appendages in mammals. Roux Arch Dev Biol. 1977;181:2–10. [Google Scholar]
- 66.Jahoda CA. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: Vibrissa-type fibers are specified. Development. 1992;115:1103–1109. doi: 10.1242/dev.115.4.1103. [DOI] [PubMed] [Google Scholar]
- 67.Jahoda CAB, Oliver RF, Reynolds AJ, Forrester JC, Gillespie JW, Cserhalmi-Friedman PB, Chhristiano Am, Horne KA. Trans-species hair growth induction by human hair follicle dermal papillae. Exp Dermatol. 2001;10:229–237. doi: 10.1034/j.1600-0625.2001.100402.x. [DOI] [PubMed] [Google Scholar]
- 68.Jahoda CAB, Oliver RF, Reynolds AJ, Forrestor JC, Horne KA. Human hair follicle regeneration following amputated and grafted into nude mouse. J Invest Dermatol. 1996;107:804–807. doi: 10.1111/1523-1747.ep12330565. [DOI] [PubMed] [Google Scholar]
- 69.Oliver RF, Jahoda CA. The dermal papilla and maintenance of hair growth. In: Rogers GE, Reis PJ, Ward KA, Marshall RC, editors. The Biology of Wool and Hair. Cambridge Univ Press; 1989. pp. 51–67. [Google Scholar]
- 70.Tobin DJ, Gunin A, Magerl M, Handijski B, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: Implications for hair growth control. J Invest Dermatol. 2003;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. [DOI] [PubMed] [Google Scholar]
- 71.McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121:1267–1275. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- 72.Jahoda CAB, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured demal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- 73.Reynolds AJ, Lawrence C, Cserhalmi-Friedman PB, Christiano AM, Jahoda CAB. Trans-gender induction of hair follicles. Nature. 1999;402:33–34. doi: 10.1038/46938. [DOI] [PubMed] [Google Scholar]
- 74.Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Msison CA, Hopping SB, Brady JN, Udey MC, Vogel JC. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuzaki T, Inamatsu M, Yoshizato K. The upper dermal sheath has a potential to regenerate the hair in the rat follicular epidermis. Differentiation. 1996;60:287–297. doi: 10.1046/j.1432-0436.1996.6050287.x. [DOI] [PubMed] [Google Scholar]
- 76.Inamatsu M, Matsuzaki T, Iwanari H, Yoshizato K. Establishment of rat dermal papilla cell lines that sustain the potential to induce hair follicles from a follicular skin. J Invest Dermatol. 1998;111:767–775. doi: 10.1046/j.1523-1747.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 77.Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes and Development. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- 78.Cooley, author. Follicular cell implantation: An update on “hair follicle cloning”. Facial Plast Surg Clin North Am. 2004;12:219–224. doi: 10.1016/j.fsc.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 79.Hardy MH. The development of mouse hair in vitro with some observations on pigmentation. J Anat. 1949;83:364–384. [PMC free article] [PubMed] [Google Scholar]
- 80.Reynolds AJ, Jahoda CAB. Hair follicle reconstruction in vitro. J Dermatol Sci. 1994;7:S84–S97. doi: 10.1016/0923-1811(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 81.Krugluger W, Rohrbacher W, Laciak K, Moser K, Moser C, Hugeneck J. Reorganization of hair follicles in human skin organ culture induced by cultured human follicle-derived cells. Exp Dermatol. 2005;14:580–585. doi: 10.1111/j.0906-6705.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 82.Rogers G, Martinet N, Steinert P, Wynn P, Roop D, Kilkenny A, Morgan D, Yuspa SH. Cultivation of murine hair follicles as organoids in a collagen matrix. J Invest Dermatol. 1987;89:369–379. doi: 10.1111/1523-1747.ep12471760. [DOI] [PubMed] [Google Scholar]
- 83.Weinberg WC, Goodman LV, Morgan GC, Ledbetter S, Yuspa SH, Lichti U. Reconstitution of hair follicle development in vivo: Determination of follicle formation, hair growth and hair quality by dermal cells. J Invest Dermatol. 1993;100:229–236. doi: 10.1111/1523-1747.ep12468971. [DOI] [PubMed] [Google Scholar]
- 84.Prouty SM, Lawrence L, Stenn KS. Fibroblast dependent induction of murine skin lesion with similarity to human common blue nevus. Amer J Path. 1996;148:1871–1885. [PMC free article] [PubMed] [Google Scholar]
- 85.Takeda A, Matsuhashi S, Nakamura, Shioya N, Uchinuma E, Ihara S. Reconstitution of hair follicles by rotation culture. In: Van Neste DJJ, Randall VA, editors. Hair Research for the Next Millenium. Elsevier Science BV; 1996. pp. 191–193. [Google Scholar]
- 86.Ihara S, Watanabe MN, Nagao E, Dhioya N. Formation of hair follicles from a single-cell suspension of embryonic rat skin by a two-step procedure in vitro. Cell Tissue Res. 1991;266:56–73. doi: 10.1007/BF00678712. [DOI] [PubMed] [Google Scholar]
- 87.Gilmour SK, Teti KA, Wu KQ, Morris RJ. A simple in vivo system for studying epithelialization, hair follicle formation, and invasion using primary epidermal cells from wild-type and transgenic ornithine decarboxylase-overexpressing mouse skin. J Invest Dermatol. 2001;117:1674–1675. doi: 10.1046/j.0022-202x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 88.Zheng Y, Du X, Wang W, Boucher M, Parimo S, Stenn K. Organogenesis from dissociated cells: Generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005;124:867–876. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
- 89.Kamimura J, Lee D, Baden HP, Brissette J, Dotto GP. Primary mouse keratinocyte cultures contain hair follicle progenitor cells with multiple differentiation protential. J Invest Dermatol. 1997;109:534–540. doi: 10.1111/1523-1747.ep12336704. [DOI] [PubMed] [Google Scholar]
- 90.Hardy MH. The secret life of the hair follicle. Trends Genetic. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- 91.Zeisberg M, Hanai JI, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nature Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 92.Kataoka K, Medina RJ, Kageyama T, Miyazaki M, Yoshino T, Makino T, Huh NH. Amer J Path. 2003;163:1227–1231. doi: 10.1016/S0002-9440(10)63482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kashofer K, Bonnet D. Gene therapy progress and prospects: Stem cell plasticity. Gene Therap. 2005;12:1229–1234. doi: 10.1038/sj.gt.3302571. [DOI] [PubMed] [Google Scholar]
- 94.Thiele J, Varus E, Wickenhauser C, Kvasnicka HM, Metz KA, Beelen DW. Regeneration of heart muscle tissue: Quantification of chimeric cardiomyocytes and endothelial cells following transplantation. Histol Histopath. 2004;19:201–209. doi: 10.14670/HH-19.201. [DOI] [PubMed] [Google Scholar]
- 95.Ichinohe T, Teshima T, Matsuoka K, Maruya E, Saji H. Fetal-maternal microchimerism: Impact on hematopoietic stem cell transplantation. Curr Opin Immunol. 2005;17:546–552. doi: 10.1016/j.coi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 96.Borue X, Lee S, Grove J, Herzog EL, Harris R, Diflo T, Glusac E, Hyman K, Theise ND, Krause DS. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol. 2004;165:1767–1772. doi: 10.1016/S0002-9440(10)63431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 98.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 99.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- 100.Brittan M, Braus KM, Reynolds LE, Conti FJ, Reynolds AR, Poulsom R, Alision MR, Wright NA, Hodivala-Dilke KM. Bone marrow cells engraft within the epidermis and proliferate in vivo with no evidence of cell fusion. J Pathol. 2004;205:1–13. doi: 10.1002/path.1682. [DOI] [PubMed] [Google Scholar]
- 101.Brazelton TR, Blau HM. Optimizing techniques for tracking transplanted stem cells in vivo. Stem Cells. 2005;23:1251–1265. doi: 10.1634/stemcells.2005-0149. [DOI] [PubMed] [Google Scholar]
- 102.Brazelton TR, Nystrom M, Blau HM. Significant differences among skeletal muscles in the incorporation of bone marrow-derived cells. Dev Biol. 2003;262:64–74. doi: 10.1016/s0012-1606(03)00357-9. [DOI] [PubMed] [Google Scholar]
- 103.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:3345–3348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 104.Rovo A, Meyer-Monard S, Heim D, Arber C, Passweg JR, Gratwohl A, Tichelli A. No evidence of plasticity in hair follicles of recipients after allogeneic hematopoietic stem cell transplantation. Exp Hematol. 2005;33:909–911. doi: 10.1016/j.exphem.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 105.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005:2247–2261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]