Abstract
The present case describes a patient who received inappropriate, but potentially life-saving, therapy from her implantable cardioverter defibrillator (ICD) in the setting of acute hyperkalemia (plasma potassium concentration = 8 mM). Hyperkalemia was associated with the development of a slow sinusoidal ventricular tachycardia, at a rate of 100 beats/min to 125 beats/min (610 ms to 480 ms) in a patient who is pacemaker-dependent. There was associated fractionation of the ICD electrogram and T wave oversensing, leading to ventricular oversensing with resultant detection in the ventricular fibrillation rate zone. This was followed by shock therapy, even though the ventricular tachycardia rate was below the programmed detection rate of the ICD. The subsequent emergency treatment of the hyperkalemia normalized the electrogram, corrected the ventricular oversensing and arrhythmia, and restored rate-adaptive single-chamber ventricular pacing.
Keywords: Electrogram fractionation, Hyperkalemia, Inappropriate shock
Abstract
Le présent cas décrit une patiente qui a reçu un traitement inapproprié, mais qui lui a peut-être sauvé la vie, déclenché par son défibrillateur à synchronisation automatique (DSA) en raison d’une hyperkaliémie aiguë (concentration de potassium plasmatique = 8 mM). L’hyperkaliémie s’associait au développement d’une lente tachycardie ventriculaire sinusoïdale, à un taux de 100 battements/minute à 125 battements/minute (610 ms à 480 ms) chez une patiente dépendante d’un stimulateur. Un fractionnement de l’électrogramme du DSA et une surdétection des ondes T en ont découlé, ce qui a provoqué une surdétection ventriculaire à un taux dans la zone de fibrillation ventriculaire. Un traitement de choc s’est ensuivi, même si le taux de tachycardie ventriculaire était inférieur au taux de détection programmé du DSA. Le traitement d’urgence subséquent de l’hyperkaliémie a normalisé l’électrogramme, corrigé la surdétection ventriculaire et l’arythmie et rétabli la stimulation ventriculaire monochambre à fréquence asservie.
A 56-year-old woman with a history of congenital heart disease, type 2 diabetes mellitus and heart failure received inappropriate, but potentially life-saving, therapy from her implantable cardioverter defibrillator (ICD). At five years of age, she underwent surgical repair of a sinus venosus atrial septal defect with redirection of the pulmonary veins. As an adult, an ICD was subsequently implanted for a history of syncope and sustained ventricular tachycardia in the setting of left ventricular dysfunction. A coronary angiogram during her index admission revealed normal coronary artery anatomy. Her clinical course was also complicated by the development of atrial flutter with poorly controlled ventricular response rates, requiring atrioventricular nodal ablation. Thereafter, she was pacemaker-dependent, with no evident escape rhythm. In February 2005, she received seven appropriate device therapies for polymorphic ventricular tachycardia in the setting of hypokalemia (plasma potassium concentration ([K+])=2.1 mM) and a prolonged QT interval. Given this history of hypokalemia and torsade de pointes requiring therapy, she was maintained on oral potassium supplementation.
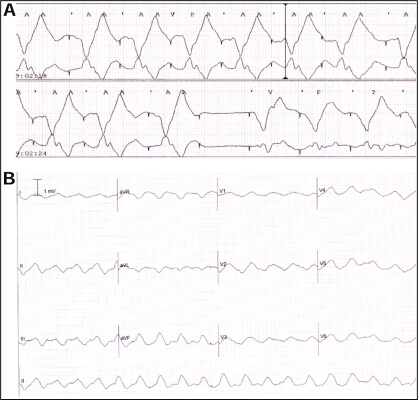
She was readmitted to hospital in April 2006 with pleuritic chest pain, hypotension and leukocytosis. Her baseline 12-lead electrocardiogram (ECG) confirmed a ventricular paced rhythm of 80 beats/min (Figure 1A). Her baseline plasma [K+] was 4.1 mM and magnesium concentration was 0.89 mM on admission. Her medications on admission included furosemide, metolazone, acetylsalicylic acid, warfarin, candesartan, spironolactone, amiodarone, metformin, pantoprazole, and magnesium and potassium supplementation. Over the ensuing 24 h, it became apparent that she was septic. In association with this, she became progressively hypotensive, and her serum creatinine increased from a baseline value of 134 μM to 265 μM, in association with oliguria. Telemetry analysis revealed gradual widening of the paced QRS complex, T wave changes, delay in the pacing artifact to ventricular activation time (Figure 1B) and, ultimately, intermittent loss of ventricular capture (Figure 2A). This was followed by the development of a slow sinusoidal ventricular tachycardia, at a rate of 100 beats/min to 125 beats/min (610 ms to 480 ms) (Figure 2B), which was terminated by the delivery of device therapy. This tachycardia was below the programmed detection rate of the device, which was programmed to deliver defibrillation therapy for rates of or greater than 176 beats/min (340 ms). Interrogation of the ICD logs revealed fractionation of the intracardiac electrogram (Figure 3A), coupled with T wave oversensing (Figure 3B) during the slow ventricular tachycardia, resulting in ventricular oversensing and the delivery of inappropriate, but life-saving, therapy. Interrogation of the ICD revealed a total of six episodes of ventricular arrhythmia detected by the device, which occurred over a 13 min period (Table 1). Three of the detected tachyarrhythmias that resulted in delivered therapy were secondary to fractionated electrograms and/or T wave oversensing in the setting of hyperkalemia, as described above. Two appropriate therapies were delivered for ventricular arrhythmias at true cycle lengths of 220 ms and 320 ms, and one episode was aborted (Table 1). No events were induced by inappropriate shocks.
Figure 1).
12-lead electrocardiogram of the ventricular paced rhythm at 80 beats/min (A), followed by a hyperkalemia-induced delay in ventricular activation with QRS and T wave widening (B)
Figure 2).
A Telemetry strip showing QRS widening and increasing delay of the pacing artifact to the onset of the QRS interval, with eventual loss of ventricular capture. B A 12-lead electrocardiogram of the hyperkalemia-induced ventricular tachycardia of sinusoidal morphology
Figure 3).
Two representative examples of electrogram exhibiting fractionation leading to overcounting with an aborted event (episode 5) (A), and T wave oversensing leading to overcounting, followed by an internal defibrillation and a paced rate-adaptive single-chamber ventricular pacing rhythm (episode 2) (B)
TABLE 1.
Outcome of events detected as ‘ventricular fibrillation’ over a 13 min period
| Episode | Cycle length (ms) | Therapy | Duration (s) | Response to therapy |
|---|---|---|---|---|
| 1 | 220 | 1 shock | 17 | I, Ineffective |
| 2 | 280 | 1 shock | 8 | I, E |
| 3 | 180 | 1 shock | 15 | I, E |
| 4 | 220 | 1 shock | 8 | A, E |
| 5 | 240 | None | 10 | Aborted |
| 6 | 320 | 1 shock | 9 | A, E |
A Appropriate; E Effective; I Inappropriate
This markedly slow conduction was secondary to hyperkalemia. The acute renal failure in the setting of angiotensin II and aldosterone receptor blockade, coupled with oral potassium supplementation, had led to a precipitous rise in the patient’s plasma [K+] to 8 mM. The severe hyperkalemia resulted in slowed conduction and fractionated electrograms. The defibrillation, while inappropriate, alerted the medical team to her acute deterioration and resulted in emergency treatment. She was initially treated with intravenous calcium gluconate, sodium bicarbonate and insulin with glucose, and hemodialysis was initiated. Following treatment and correction of the hyperkalemia, her 12-lead ECG reverted to her usual paced morphology, with correction of the pacing-associated latency and exit block.
DISCUSSION
Hyperkalemia-induced oversensing by ICDs has previously been reported (1,2). In both of these cases, hyperkalemia (6.7 mM and 6.1 mM, respectively) resulted in T wave oversensing, observed during paced rhythm in the first patient (1) and sinus rhythm in the second (2), with the latter patient receiving inappropriate therapy. In our patient, the plasma [K+] was measured at 8 mM at the time of device therapy, and the hyperkalemia-induced oversensing occurred in response to marked fractionation of the intracardiac electrogram, coupled with T wave oversensing. This occurred during a slow ventricular tachycardia that led to paradoxically appropriate inappropriate therapy. Fractionation of the ICD and the paced electrogram with inappropriate shock has previously been reported with slowed conduction secondary to structural changes postmyocardial infarction, and following alcohol septal ablation in hypertrophic cardiomyopathy (3). In the present report, we described electrogram fractionation as a consequence of a functional alteration, ie, hyperkalemia-induced conduction delay. A functional block due to hyperkalemia-induced reduction in sodium ion channel function is consistent with experimental findings in mice with knockout of SCN5A, the major cardiac sodium ion channel isoform, which led to fractionation of the electrogram (4).
Potassium is the major intracellular cation; the resting membrane potential is governed primarily by the resting potassium conductance. Hyperkalemia invokes a series of changes to myocardial excitability and conduction, resulting in alterations on the surface ECG and the intracardiac electrogram. Increases in extracellular [K+] lead to a reduction in the electrochemical driving force for the outward movement of potassium ions, resulting in membrane depolarization and inactivation of cardiac voltage-gated sodium channels. Voltage-gated sodium channels are the key mediators of rapid myocardial conduction, and loss of their function produces functional conduction block (5). As such, when the serum potassium rises above 6.5 mM, there is slowing of phase 0 of the cardiac action potential and increased conduction delay, leading to widened QRS complexes and a prolonged PR interval. A sinoventricular rhythm without obvious atrial activity and with marked QRS widening is a recognized ECG manifestation of hyperkalemia, and can mimic ventricular tachycardia. Because our patient had previously been rendered to be pacemaker-dependent by atrioventricular node ablation, the observed rhythm during hyperkalemia must have originated in the ventricle.
REFERENCES
- 1.Arthur W, Kaye GC. Hyperkalemia diagnosed by implantable cardioverter defibrillator T wave sensing. Pacing Clin Electrophysiol. 2001;24:908–9. doi: 10.1046/j.1460-9592.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 2.Koul AK, Keller S, Clancy JF, Lampert R, Batsford WP, Rosenfeld LE. Hyperkalemia induced T wave oversensing leading to loss of biventricular pacing and inappropriate ICD shocks. Pacing Clin Electrophysiol. 2004;27:681–3. doi: 10.1111/j.1540-8159.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 3.Shah P, Harris L, Hill A, Schwartz L, Cameron D. Inappropriate therapy from a defibrillator complicating transcoronary ablation of septal hypertrophy in a patient with hypertrophic obstructive cardiomyopathy. Pacing Clin Electrophysiol. 2004;27:677–80. doi: 10.1111/j.1540-8159.2004.00508.x. [DOI] [PubMed] [Google Scholar]
- 4.Papadatos GA, Wallerstein PM, Head CE, et al. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci USA. 2002;99:6210–5. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George AL., Jr Inherited disorders of voltage-gated sodium channels. J Clin Invest. 2005;115:1990–9. doi: 10.1172/JCI25505. [DOI] [PMC free article] [PubMed] [Google Scholar]