Abstract
Background
Previous research has revealed that the ratios of the lengths of various pairs of human fingers differ in males and females.
Method
In an attempt to determine whether parallel sex differences also exist in the relative lengths of human metacarpals and metatarsals, the lengths of the metapodials for both hands and both feet were measured in a collection of human skeletons. For each hand and each foot, all of the 10 possible pair-wise ratios for length of the five metapodials were calculated.
Results
For the skeletons of European/Caucasian extraction (Ns = 89 males, 50 females), there were substantial sex differences for several of the metacarpal length ratios, but the pattern was not identical with the pattern previously reported for human fingers. Namely, the largest sex differences were for the three ratios involving metacarpal 5 on the left hand, while the sex difference for the ratio comparing the second and fourth metacarpals (the comparison commonly showing the largest sex difference for fingers) was small and non-significant for both hands in these European-Americans. For the skeletons of African extraction (Ns = 65 males, 55 females), no sex differences were found in any of the 20 metacarpal-length ratios. This outcome was unexpected because past research had shown sex differences in finger-length ratios for people of African extraction. For metatarsals, none of the 20 ratios exhibited a substantial sex difference for either group of skeletons.
Conclusions
A discrepancy apparently exists between the length ratios based on fingers and those based on metacarpals.
Keywords: Human Metapodials, Metapodial-Length Ratios, Sex Differences, Race Differences, 2D:4D Ratio, Finger-Length Ratios, Masculinization, Prenatal Development
Introduction
In humans, there is a sex difference in the relative lengths of the index and ring fingers ([1–4]; for a summary see [5]). In females, the two fingers are about equal in length, making the ratio of their lengths (the 2D:4D finger-length ratio) close to 1.0. In males, however, the index finger is typically slightly shorter than the ring finger, making the 2D:4D ratio smaller than 1.0. Some evidence suggests that this sex difference emerges in the early weeks of prenatal development [6], and it certainly exists by about 2 years of age [5, Figure 1.6], suggesting that the mechanisms responsible for the sex difference operate early in development. The 2D:4D finger-length ratio is not unique; the ratios 2D:5D and 3D:4D also show substantial sex differences for the fingers, and some length ratios for human toes also show modest sex differences [7].
The 2D:4D length ratio has attracted considerable interest in recent years, in part because it has been shown to be correlated with medical conditions such as breast cancer, heart disease, and autism (see [5]), and with certain special populations (e.g., [8–9]. Furthermore, a number of studies have reported differences in the 2D:4D ratio for heterosexuals and homosexuals ([7, 10–13]; but compare [14]). There can be large ethnic and racial differences in the absolute values of the 2D:4D ratio. Of special interest here, the finger-length ratios of groups of people of African extraction generally are smaller than those of groups of people of European extraction. However, substantial sex differences often have been observed in both racial groups despite the large differences in absolute magnitudes of the finger-length ratios in those two racial groups ([5, Figure 1.7; 15–17], for an exception see [14, Table V]). This suggests that developmental processes associated with sexual differentiation play a role in the origin of these sex differences in finger-length ratios. The existence of these ethnic and racial differences in the absolute values of finger-length ratios highlights the fact that this measure must be used with considerable caution and appropriate experimental controls.
Because degree of androgen exposure in prenatal development is responsible for so many other sex differences in body, brain, and behavior (e.g., [18]), it is not unreasonable to presume that it may be a contributing factor to the sex differences in the length ratios of human fingers and toes [19]. There is no definitive evidence about this association, but parsimony argues for the inclusion of prenatal androgen exposure on any short-list of possible explanations for the sex differences in finger-length ratios. The early emergence of these sex differences [6,20] clearly is in accord with explanations that appeal to sex differences in prenatal androgen exposure. The sex differences in finger-length ratios are of general interest, in part because the development of the fingers and toes (and penis) is under the control of the highly conserved homeobox genes Hoxa and Hoxd [21], and mechanisms that can modulate the effects of these genes can be of interest to many sub-disciplines.
In the last few years, sex differences in length ratios for species other than humans have begun to appear in the scientific literature. Roney et al. [22] found sex differences in the length ratios for baboon fingers, Brown et al. [23] and Manning et al. [24] both reported sex differences in certain length ratios for the hindpaws of mice, and Burley and Foster [25] found a sex difference for the foot in zebra finches. Also, some information recently has emerged about the relative sizes of metacarpals and metatarsals in other species based upon measurements obtained from skeleton collections. Namely, substantial sex differences do exist in the length ratios of both metacarpals and metatarsals in baboons [26], gorillas [27], and, to a lesser extent, chimpanzees [27]. Unfortunately, very little is known about the fingers and toes of these species, although Watkins [28] did look at the relationship between metacarpal III and proximal phalanx III in a number of primates and found only marginal sex differences. In all of these studies, the sample sizes were small, so caution is necessary when formulating conclusions and hypotheses.
At this time, then, we have some knowledge about sex differences in the length ratios for metapodials in baboons, gorillas, and chimpanzees, but not for their fingers and toes (except for some baboon fingers—see [22]). And we have knowledge about sex differences in the length ratios for human fingers and toes, but not for human metapodials. Here we extend the study of length ratios to the metacarpals and metatarsals of humans.
One expectation was that there would be sex differences in the length ratios for the metacarpals just as there are for fingers. Another expectation was that the pattern of sex differences seen in human fingers might not be repeated in the metacarpals; that is, the 2Mc:4Mc ratio might not show the largest sex difference just because the 2D:4D ratio does. The basis for this expectation was our previous finding of low correlations between corresponding length ratios calculated for different bones in the same pair of rays [27, Table 5]. Apparently whatever mechanisms are operating to produce the pattern of length differences in the fingers does not operate equally along the entire ray for humans, gorillas, or chimpanzees. A third expectation was that any sex differences seen for the metatarsals would be smaller than those seen for the metacarpals. This expectation stemmed from our previous observation of small sex differences in human toe-length ratios [7]. A fourth expectation evolved when we realized there were specimens of different racial background available for measurement in the collection. Specifically, we expected race differences in metacarpal-length ratios to follow race and ethnic differences previously found in digit ratios [5, Figure 1.7; 14–17]. That is, we expected to see smaller ratios for the African-American sample than for the European-American sample, but to see sex differences in both racial groups. Only some of these expectations were confirmed.
Methods
Materials
The human skeletons measured for this study reside in the Hamann-Todd Osteological Collection at the Cleveland Museum of Natural History. They were collected during the 1920’s and 1930’s through personal donations from people who wanted to contribute to science, donations by people who could not afford proper burials, and donations of unclaimed cadavers by hospitals and morgues. When available, medical reports were included and catalogued for each specimen. Those records included additional information regarding name, age, sex, ethnicity, and cause of death. This collection was regarded to be representative of the population of Cleveland, Ohio, for that time period. Details of the human specimens have been entered into a computer database and lists of specimens can be generated using various parameters.
A list of the human skeletons suitable for this study was compiled by Mr. Lyman Jellema, the physical anthropologist in charge of this skeleton collection, using his computer database. The list included all human specimens between the ages of 18 and 45 at the time of death for which post-cranial bones existed, and it identified the racial background (“Negro” or “Caucasian”) of each specimen. Heights were provided for most of the specimens.
Because the literature on finger-length ratios suggests that the data can be rather different across ethnicity and race [5, Figure 1.7; 14–15, 17], here we report the data separately for what we will call the African-American and European-American specimens. Note that the available information on these specimens was not adequate to make finer distinctions within those two broad categories. The European-American group consisted of 89 males and 50 females, and the African-American group consisted of 65 males and 55 females. Of the 139 European-American skeletons measured for this study, 128 had complete sets of metacarpals for both hands and 114 had complete sets of metatarsals for both feet. Of the 120 African-American skeletons measured for this study, 113 and 112 had complete sets of metacarpals and metatarsals, respectively.
Procedure
The list of specimens provided was divided by age (18–30 and 31–45) but mixed for sex and race. The general order of measurement was: European-American males and females from the younger age category, then from the older age category, African-American females from both age categories, African-American males from both age categories. Specimens older than 39 at the time of death were excluded from measurement, in part because they had more bone defects than the younger specimens and in part because of uncertainty about the effects of age on metapodial length ratios [29]. For both races, the collection contains many more males than females. In the end, almost all eligible female specimens were measured for each group, and as many male specimens were measured as time allowed. The resulting difference in N’s for the male and female samples, and for the different metapodials, can be attributed in part to the overall male-female population disparity in the collection, and in part to the condition of the specimens (see below).
As is true for many skeleton collections, the individual phalanges of these specimens were not numbered at the time of dissection. Unambiguous reassembly of individual fingers and toes from disarticulated phalanges is notoriously difficult even for experts, but the reassembly of metacarpals and metatarsals is simple and exact by comparison. Accordingly, only metacarpals and metatarsals were measured for this study. All measurements were made by author MSB, who has considerable experience measuring metapodials of different species.
Basically the same standard field-measurement procedures reported previously for gorillas and chimpanzees [27] were employed for the human specimens. The osteometric board had orthogonal grid lines at 1-mm intervals. Each bone was placed on the board with the ventral surface down. The distal end was placed against the top edge of the board, and the shaft of the bone was aligned generally parallel to the vertical grid lines. However, more importance was given to the vertical alignment of the distal and proximal ends of the bone than to the vertical alignment of the shaft. A piece of sheet metal having a 90° bend was placed against the proximal end of the bone and aligned with the grid lines, allowing the measurement of length to be made. All measurements were to the nearest half millimeter. In addition to sex and race, various supplemental facts from the files on the individual specimens were recorded. No second investigator was used to obtain estimates of reliability of measurement because all inter-rater correlations were close to 1.0 in previous studies by this same investigator (author MSB) using the same measurement techniques [26–27].
Specimens having obviously diseased or deformed hands or feet were not measured. When possible, all the metacarpals and metatarsals were measured for each of the other specimens. When one bone of a hand (or a foot) was missing, or had noticeable irregularities or obvious deterioration that could affect the estimate of its length, that bone plus the corresponding bone on the opposite hand (foot) were excluded from measurement, meaning that all length ratios involving those bones were necessarily missing for that specimen. When two or more bones of a hand (or a foot) were missing or had to be excluded, no measurements are reported for either hand (foot) for that specimen. However, the exclusion of hand (foot) measurements for a specimen did not preclude inclusion of the foot (hand) measurements for that specimen.
After the measurements were complete, the data were examined for outlying values; those outliers were either verified or corrected by the second author on subsequent trips to Cleveland or by Mr. Lyman Jellema of the Cleveland Museum of Natural History. One metapodial for each of 11 specimens was identified as an outlier and had to be remeasured, a small fraction of the total number of bones measured.
As noted, our interest was not in the well-known sexual dimorphism in size of the hands and feet, but rather in the relative sizes of pairs of bones in the hands or the feet. Accordingly, little will be said here about the absolute sizes of the bones of interest. The five metacarpals from each hand permit the calculation of 10 length ratios for each hand. These ratios are denoted as 1Mc:2Mc, 1Mc:3Mc, and so on, where the designation 1Mc:2Mc denotes the ratio for the length of the first metacarpal divided by the length of the second metacarpal from the same hand. The corresponding 10 ratios for the metatarsals will be designated 1Mt:2Mt, 1Mt:3Mt, etc.
Because of the large number of pairwise comparisons possible with this data set, and the associated risk of some comparisons achieving statistical significance by chance, the results of statistical tests will be de-emphasized here, and effect sizes will be emphasized instead [30]. Here effect size is defined as the difference between the means for some pairwise comparison divided by the square root of the weighted mean of the variances for those two samples. By convention, effect sizes of 0.2, 0.5, and 0.8 are judged to be small, medium, and large, respectively [30].
Results
Sizes of the Skeletons
The females were invariably shorter than the males in both racial groups. The average heights (SDs) were approximately: 1656 (75) mm, 1598 (88) mm, 1756 (72) mm, and 1707 (78) mm for the African-American females, European-American females, African-American males, and European-American males, respectively. The effect sizes (female minus male) for the sex difference in height were −1.36 and −1.33 for the African-American and European-American specimens, respectively. Unpaired, two-tailed t tests were highly significant for both of those sex differences, with p < 0.0000 for both the African-American and European-American groups.
The two racial groups also differed in height. The African-American specimens were taller than the European-American specimens, and that was true for both the females and the males. The effect sizes for the height differences between ethnic groups (African-American minus European-American) were 0.71 and 0.65 for the females and males, respectively. Unpaired, two-tailed t tests were highly significant for both of those within-sex differences, with p = 0.0005 and p = 0.0002 for the female and male groups, respectively.
In general, the correlations between body height and metapodial length ratios were low. The majority of the correlations between height and length ratio were between −0.2 and +0.2, with no evident differences between racial groups, sexes, or ages. Accordingly, the differences described below in metapodial length ratios for the European-American and African-American specimens appear not to be attributable primarily to group differences in body size. Previous research also has revealed no strong relationship between height and finger-length ratios in humans [5, p. 7; 31–32]; also see [28] for related conclusions for other primate species.
Lengths of metacarpals
Table 1 (top) provides summary statistics for metacarpal length. Data are shown separately for European-Americans and African-Americans, for females and males, and for left and right hands. Some generalizations can be extracted from this table. As expected, for both ethnic groups, the lengths of the male metacarpals were greater than those for the females. For both sexes, both hands, and both racial groups, the ordering of metacarpals by length was 2>3>4>5>1 from longest to shortest. The same ordering exists in the gorilla and chimpanzee [33, 27]; for other primates, compare [34, Table 5].
Table 1.
Average lengths and variability (in mm) for the metacarpals (top half) and metatarsals (bottom half) for European-American (EA) and African-American (AA) Humans.
| EA Female |
EA Male |
AA Female |
AA Male |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | ||
| Mc1 | Mean | 42.93 | 43.01 | 46.79 | 47.12 | 45.57 | 45.81 | 48.80 | 48.96 |
| SE | 0.340 | 0.362 | 0.328 | 0.335 | 0.381 | 0.360 | 0.376 | 0.385 | |
| N | 48 | 48 | 85 | 85 | 54 | 54 | 64 | 64 | |
| Mc2 | Mean | 64.93 | 65.06 | 69.48 | 69.90 | 68.58 | 68.84 | 73.15 | 73.03 |
| SE | 0.530 | 0.520 | 0.463 | 0.474 | 0.526 | 0.516 | 0.543 | 0.535 | |
| N | 48 | 48 | 86 | 86 | 53 | 53 | 65 | 65 | |
| Mc3 | Mean | 63.31 | 63.10 | 67.79 | 67.85 | 67.63 | 67.63 | 72.35 | 72.04 |
| SE | 0.491 | 0.553 | 0.459 | 0.489 | 0.530 | 0.535 | 0.559 | 0.591 | |
| N | 48 | 48 | 87 | 87 | 54 | 54 | 65 | 65 | |
| Mc4 | Mean | 54.21 | 54.32 | 58.25 | 58.29 | 58.14 | 58.35 | 62.15 | 61.94 |
| SE | 0.456 | 0.455 | 0.401 | 0.404 | 0.466 | 0.456 | 0.518 | 0.521 | |
| N | 47 | 47 | 85 | 85 | 54 | 54 | 65 | 65 | |
| Mc5 | Mean | 49.96 | 50.32 | 54.55 | 54.52 | 53.61 | 53.85 | 57.41 | 57.23 |
| SE | 0.358 | 0.341 | 0.374 | 0.379 | 0.404 | 0.409 | 0.465 | 0.472 | |
| N | 46 | 46 | 86 | 86 | 55 | 55 | 64 | 64 | |
| Mt1 | Mean | 61.78 | 61.72 | 66.00 | 65.97 | 65.19 | 65.26 | 69.02 | 69.31 |
| SE | 0.48 | 0.46 | 0.50 | 0.50 | 0.49 | 0.49 | 0.55 | 0.57 | |
| N | 46 | 46 | 75 | 75 | 54 | 54 | 62 | 62 | |
| Mt2 | Mean | 70.79 | 71.00 | 76.50 | 76.60 | 75.69 | 75.72 | 81.06 | 81.02 |
| SE | 0.59 | 0.54 | 0.56 | 0.56 | 0.63 | 0.64 | 0.61 | 0.63 | |
| N | 46 | 46 | 73 | 73 | 54 | 54 | 62 | 62 | |
| Mt3 | Mean | 66.07 | 66.51 | 71.07 | 71.19 | 71.00 | 70.93 | 76.08 | 75.81 |
| SE | 0.55 | 0.52 | 0.52 | 0.52 | 0.62 | 0.62 | 0.61 | 0.60 | |
| N | 46 | 46 | 75 | 75 | 55 | 55 | 62 | 62 | |
| Mt4 | Mean | 65.28 | 65.77 | 70.16 | 70.37 | 69.60 | 69.62 | 74.13 | 74.37 |
| SE | 0.55 | 0.56 | 0.48 | 0.46 | 0.60 | 0.62 | 0.64 | 0.62 | |
| N | 45 | 45 | 74 | 74 | 55 | 55 | 61 | 61 | |
| Mt5 | Mean | 66.58 | 66.83 | 71.18 | 71.50 | 69.92 | 70.28 | 74.98 | 75.25 |
| SE | 0.60 | 0.65 | 0.56 | 0.57 | 0.58 | 0.59 | 0.64 | 0.63 | |
| N | 46 | 46 | 73 | 73 | 54 | 54 | 61 | 61 | |
Mc = metacarpal
Mt = metatarsal
SE = standard error of the mean
The individual metacarpals in the right hand generally were slightly longer than the corresponding bones in the left hand, but the differences were small and there were numerous exceptions. When compared within-sex, the metacarpals were longer for the African-American specimens than for the European-American specimens (see top of Table 1), and this was true for every ray. As noted above, the African-American specimens also were taller than the European-American specimens. Unlike the small correlations between height and metapodial-length ratios, height was significantly correlated with metacarpal length itself, a bit more strongly for the African-American than for the European-American specimens. Specifically, every correlation between height and metacarpal length for each side of the body was significantly different from zero, each with a p value beyond the 0.01 level. For the females, the average correlations were about 0.69 and 0.50 for the African-American and European-American specimens, respectively, and for the males, the average correlations were about 0.65 and 0.52 for the African-American and European-American specimens, respectively. The correlations for left and right hands were essentially the same within racial group.
Lengths of metatarsals
Table 1 (bottom) provides summary statistics for metatarsal length. For both racial groups, the lengths were greater for males than for females, and the lengths generally were greater for the right foot than for the left. The ordering of metatarsals from longest to shortest in the European-American specimens was 2>5>3>4>1 for both sexes and both feet, and in the African-American specimens it was 2>3>5>4>1 for both sexes and both feet. This race difference in the ordering of length is probably too small to be of any functional difference in locomotion. For comparison, Tague [34] shows metatarsal lengths for several species of primates.
Just as was true for the metacarpals, the absolute lengths of the metatarsals were greater for the African-American specimens than for the European-American specimens, and this was true for both sexes and both feet. Also, every correlation between height and metatarsal length for each side of the body was significantly different from zero, each with a p value beyond the 0.01 level. For the females, the average correlations were about 0.72 and 0.61 for the African-American and European-American specimens, respectively, and for the males, the average correlations were about 0.65 and 0.60 for the African-American and European-American specimens, respectively. The correlations for left and right feet were essentially the same within racial group.
Length ratios for European-American metacarpals
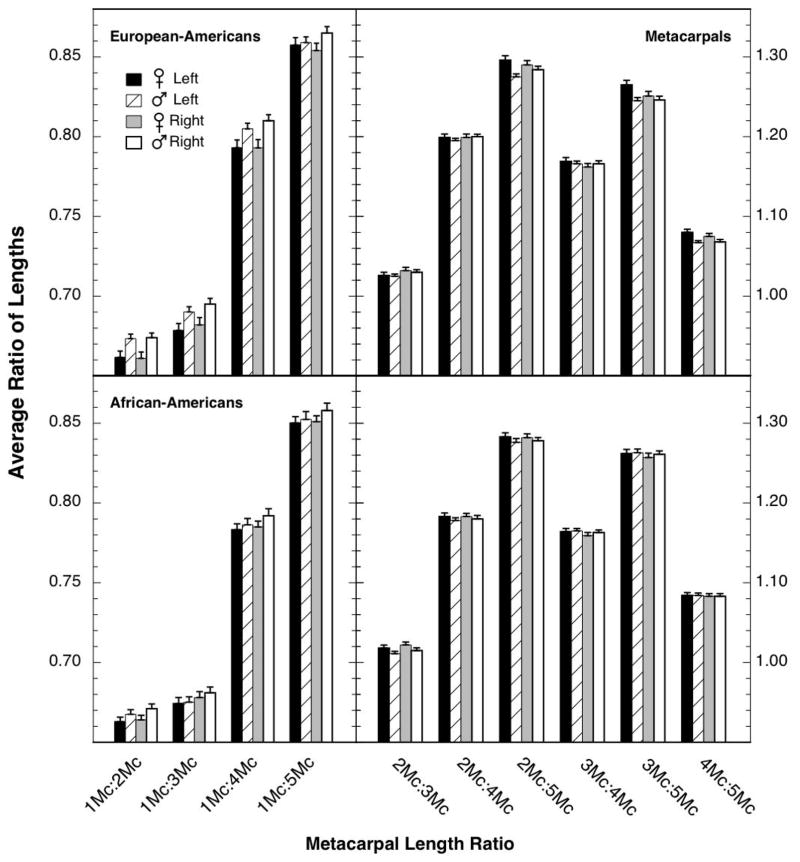
Several length ratios for the metacarpals of the European-American sample exhibited medium-sized sex differences. The average length ratios for European-American metacarpals are shown in the top panel of Figure 1, separately for the two sexes and the two hands. Because all the magnitudes of the four length ratios involving the first metacarpal (the lower-order ratios) were markedly smaller than those for the other six ratios, different ordinate scales were used for the lower- and higher-order ratios (note the left and right ordinate values in Figure 1).
Fig. 1.
Average length ratios for the metacarpals of European-American (top panel) and African-American (bottom panel) specimens. Because the four ratios involving the first metacarpal were substantially smaller than the six ratios not involving it, different ordinate scales were used for plotting the lower- and higher-order ratios (left and right ordinates, respectively). The flags designate standard errors. Ns for the different comparisons can be found in Tables 2 and 3.
As has been found in baboons [26] and gorillas and chimpanzees [27], the sex difference was generally opposite in direction for the lower-order and higher-order ratios. The female ratios were smaller than the male ratios for the lower-order ratios (left side of Figure 1), but were larger than the male ratios for the higher-order ratios (right side of Figure 1). For human fingers also, all the higher-order length ratios of females are larger than those of males (e.g., [7]).
The effect sizes for the sex differences in metacarpal-length ratios (female minus male) are shown in the top portion of Table 2. For both hands of these European-American specimens, many of the lower-order ratios had effect sizes of 0.35 or larger, but for the higher-order ratios, only those involving metacarpal 5 had substantial effect sizes, and then only for the left hand. This absence of any evident sex differences in the higher-order ratios of the right hand is contrary to the pattern seen in human fingers [5, 7], baboon metacarpals [26], and gorilla metacarpals [27]. Note that the ratio of the 2nd to 4th ray (the ratio that commonly shows the largest sex difference for human finger length) exhibited essentially no sex difference for relative metacarpal lengths in either hand. Previously we have shown that the sex differences in relative length in the digits are not highly correlated with the corresponding ratios in the metacarpals [27, Table 5], and this was true for humans, gorillas, and chimpanzees. For all effect sizes greater than 0.30 in the top portion of Table 2, t-tests were calculated, and those achieving significance are designated with asterisks in Table 2. When the significance level of the most significant comparison in the top of Table 2 (2Mc:5Mc, left hand) was Bonferroni-corrected using the 20 comparisons between the sexes shown for the metacarpals [35, p. 249+], the resulting p value was 0.033.
Table 2.
Effect sizes for the sex differences in various ratios of length of the metacarpals (top) and metatarsals (bottom) of European-Americans aged 18–39 at death.
| Effect Size (F minus M) | |||
|---|---|---|---|
| Ratio | Number F/M | Left | Right |
| Hands | |||
| 1Mc:2Mc | 48/84 | −0.448* | −0.473** |
| 1Mc:3Mc | 48/85 | −0.384* | −0.387* |
| 1Mc:4Mc | 47/83 | −0.363* | −0.494** |
| 1Mc:5Mc | 46/84 | −0.044 | −0.331 |
| 2Mc:3Mc | 48/86 | 0.032 | 0.050 |
| 2Mc:4Mc | 47/84 | 0.169 | −0.042 |
| 2Mc:5Mc | 46/85 | 0.588** | 0.156 |
| 3Mc:4Mc | 47/85 | 0.091 | −0.123 |
| 3Mc:5Mc | 46/86 | 0.524** | 0.104 |
| 4Mc:5Mc | 45/84 | 0.533** | 0.253 |
| Feet | |||
| 1Mt:2Mt | 46/72 | 0.323 | 0.240 |
| 1Mt:3Mt | 46/74 | 0.195 | 0.057 |
| 1Mt:4Mt | 45/73 | 0.227 | 0.075 |
| 1Mt:5Mt | 46/72 | 0.029 | 0.025 |
| 2Mt:3Mt | 46/72 | −0.078 | −0.263 |
| 2Mt:4Mt | 45/71 | −0.125 | −0.261 |
| 2Mt:5Mt | 46/70 | −0.207 | −0.138 |
| 3Mt:4Mt | 45/73 | −0.179 | −0.086 |
| 3Mt:5Mt | 46/72 | −0.194 | −0.010 |
| 4Mt:5Mt | 45/71 | −0.052 | 0.104 |
Mc = metacarpal
Mt = metatarsal
F = Female
M = Male
Results from unpaired t tests, two-tailed:
= 0.05 > p > 0.01;
= 0.01 > p > 0.001
The effect sizes shown in Table 2 for the lower-order ratios are negative because of a fact already noted in Figure 1; for the lower-order ratios, the European-American females had smaller length ratios than the males (as in [26–27]).
Length ratios for African-American metacarpals
In marked contrast to the data for the European-American sample, the African-American sample exhibited no sizable sex differences in any of the metacarpal-length ratios in either hand. The directionality of effect was generally similar to the results seen for European-Americans, but the magnitudes of the differences were smaller and less consistent. The average ratios for African-Americans are shown in the bottom panel of Figure 1, and the effect sizes are shown in the top portion of Table 3.
Table 3.
Effect sizes for the sex differences in various ratios of length of the metacarpals (top) and metatarsals (bottom) of African-Americans aged 18–39 at death.
| Effect Size (F minus M) | |||
|---|---|---|---|
| Ratio | Number F/M | Left | Right |
| Hands | |||
| 1Mc:2Mc | 52/64 | −0.209 | −0.307 |
| 1Mc:3Mc | 53/64 | −0.034 | −0.110 |
| 1Mc:4Mc | 53/64 | −0.104 | −0.226 |
| 1Mc:5Mc | 54/63 | −0.068 | −0.222 |
| 2Mc:3Mc | 52/65 | 0.271 | 0.275 |
| 2Mc:4Mc | 52/65 | 0.186 | 0.090 |
| 2Mc:5Mc | 53/64 | 0.171 | 0.115 |
| 3Mc:4Mc | 53/65 | −0.033 | −0.169 |
| 3Mc:5Mc | 54/64 | −0.019 | −0.128 |
| 4Mc:5Mc | 54/64 | 0.012 | −0.008 |
| Feet | |||
| 1Mt:2Mt | 53/62 | 0.294 | 0.180 |
| 1Mt:3Mt | 54/62 | 0.305 | 0.161 |
| 1Mt:4Mt | 54/61 | 0.139 | 0.145 |
| 1Mt:5Mt | 53/61 | 0.248 | 0.132 |
| 2Mt:3Mt | 54/62 | 0.042 | −0.026 |
| 2Mt:4Mt | 54/61 | −0.165 | −0.023 |
| 2Mt:5Mt | 53/61 | 0.025 | 0.018 |
| 3Mt:4Mt | 55/61 | −0.281 | 0.001 |
| 3Mt:5Mt | 54/61 | 0.023 | 0.047 |
| 4Mt:5Mt | 54/60 | 0.193 | 0.053 |
Mc = metacarpal
Mt = metatarsal
F = Female
M = Male
Results from unpaired t tests, two-tailed:
= 0.05 > p > 0.01;
= 0.01 > p > 0.001
Careful examination of the absolute magnitudes of the ratios shown in Figure 1 reveals that, for each comparison, the ratios generally were slightly smaller for the African-American skeletons (bottom panel) than for the European-American skeletons (top panel). That is the same direction of effect as seen for finger-length ratios, but, for finger-length ratios, the large effect of race typically does not eliminate the sex difference in African-American hands ([5, Figure 1.7; 15–17]; for an exception see [14, Table V]). So this outcome for African-American metacarpals is puzzling. Note again, however, that the differences seen for finger length in humans are not highly correlated with the differences seen for metacarpals [27]. In summary, the African-American metacarpals were both larger (see above) and more similar in length (smaller ratios) than the European-American metacarpals, and the sex differences were smaller.
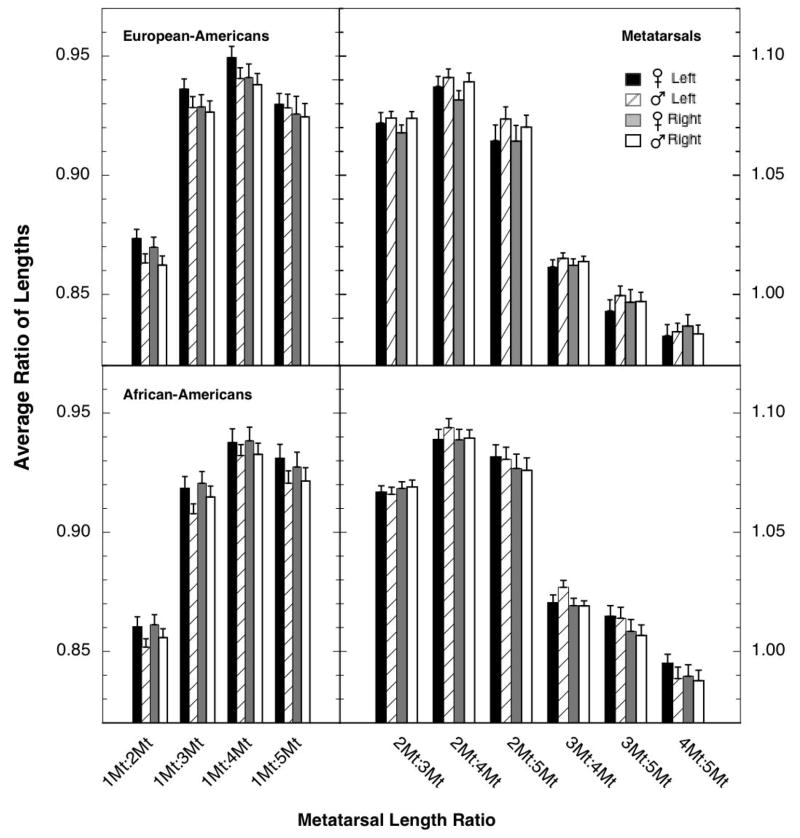
Length ratios for metatarsals
For both the European-American and African-American samples, most of the sex differences in the length ratios for metatarsals were close to zero. The average metatarsal length ratios are shown in Figure 2, and the effect sizes for the sex difference are shown in the bottom portions of Tables 2 and 3. For both racial groups, there was a tendency for the largest sex differences to be for the lower-order ratios and the left foot, but even those effect sizes were small. One study on ratios for human toe length reported small-to-moderate sex differences for the ratios 1D:2D, 2D:4D, and 3D:4D on the right foot [7]. For the lower-order ratios (left side of Figure 2), there was a tendency for the absolute magnitudes of the length ratios to be larger for the European-American than the African-American specimens, whereas for the higher-order ratios (right side of Figure 2), the tendency was the opposite.
Fig. 2.
Average length ratios for the metatarsals of European-American (top panel) and African-American (bottom panel) specimens. Because the four ratios involving the first metatarsal were substantially smaller than the six ratios not involving it, different ordinate scales were used for plotting the lower- and higher-order ratios (left and right ordinates, respectively). The flags designate standard errors. Ns for the different comparisons can be found in Table 2 and 3.
Effects of age
Harris et al. [29] measured the lengths of the metacarpals of the left hand twice in the same people and found a uniform decrease in length from about age 20 to about age 55 in both sexes. This age-related shortening of the metacarpals was attributed to the cumulative effects of mechanical stress through 30+ years of life. Although we measured no specimens older than 39 years, we partitioned our specimens into young (18–29 years) and old (30–39 years) subgroups for purposes of a cross-sectional test of the Harris et al. longitudinal demonstration. The differences in metacarpal length between these two age groups varied in direction and magnitude in a complex manner across race, sex, and hand. For all the males, the majority of the comparisons were in the direction of longer metacarpals in the older subgroup, contrary to the directionality reported by Harris et al. [29]. For the African-American males, all 10 of the comparisons (5 metacarpals for each of two hands) were longer in the older subgroup, and for the European-American males, 7 of 10 comparisons were. For the females, European-Americans were like the males, but the African-Americans were not. The European-American females also exhibited longer metacarpals in the older subgroup for 8 of the 10 comparisons, while the African-American females did so only for 1 of the 10 comparisons. Thus, it was the African-American females who were most in accord with the findings of Harris et al. [29]. When interpreting these outcomes, it is important to keep in mind the smaller difference in the average ages of our subgroups than in the Harris et al. study. Also, the measures of Harris et al. were longitudinal while ours were cross-sectional, so there is the strong possibility that our two age groups may represent nonequivalent original groups even within race (for example, they did have different longevities). Finally, it is possible that the lives of our specimens put different demands on the bones of the hand than did the lives of the subjects in the Harris et al. [29] study. This was an imperfect attempt at replication of the Harris et al. result, but it did reveal some interesting differences within our dataset.
Discussion
Prior to this study, there was considerable evidence that several finger-length ratios, especially 2D:4D, exhibit sex differences (e.g., [5,7]), and those sex differences exist across a wide array of ethnic and racial groups [5, Figure 1.7; 15–17]. There also was evidence in baboons and gorillas [26–27] that some metacarpal-length ratios exhibit sex differences, although the ratios showing the largest differences were not the ones showing the largest sex differences for human fingers. Were these discrepancies “simply” attributable to species differences, or might different bones in the rays be differentially sensitive to whatever mechanism(s) produce the sex differences in length ratios? Ideally, the answer to this question would come from measurements of both finger-length ratios and metacarpal-length ratios made in the same individual subjects of one of these four species. That has yet to be done. However, as a first step, we provide here some measurements of human metapodial length ratios which can be compared with the finger-length ratios reported for other groups of humans.
Our study of the relative lengths of human metapodials has revealed the following:
The metacarpals of humans do not exhibit the same pattern of sex differences as exist in human finger-length ratios. For the length ratios not involving metacarpal 1, only those involving metacarpal 5 showed substantial sex differences, and those only for the left hand (Table 2, top) and only for the European-American skeletons. So, a discrepancy appears to exist between length ratios based on fingers and those based on metacarpals. We previously had reported poor correlations between corresponding length ratios calculated using different sets of bones of the same rays [27, Table 5], and we interpret the new data as a corollary of that previous finding. That is, it appears that all the bones of each ray are not affected similarly by whatever mechanism(s) produce sex differences in length ratios. Gaining an understanding of this apparently complex pattern of effect is important, but daunting.
For the higher-order metacarpal-length ratios, females had numerically larger ratios than did males, which is the same direction of effect as seen in human finger-length ratios (e.g., [5, 7]). However, for those length ratios involving metacarpal 1 (the lower-order ratios), females had numerically smaller ratios than did males. Similar reversals of direction in the sex difference for metacarpals previously were reported for baboons [26] and gorillas and chimpanzees [27].
The metacarpal-length ratios for the African-American specimens exhibited no substantial sex differences for either the lower-order or higher-order ratios. This is an unexpected outcome because sex differences generally do exist in the finger-length ratios of people of African extraction ([5, Figure 1.7; 15–17], for an exception see [14, Table V]). Thus, it will be important to verify this outcome, preferably using measurements of fingers and metacarpals in the same individuals. If the outcome is verified, any acceptable explanation will have to include why the proffered mechanism for producing a sex difference operates on finger lengths but not on metacarpal lengths in African-Americans, but operates on both in European-Americans. Perhaps melanin is playing some role? Some other facts relevant to this curious outcome are: the absolute lengths of the metacarpals were greater in the African-American skeletons than in the European-American skeletons (see Table 1). Also, the metacarpals were more similar in length in the African-American skeletons, meaning that the overall magnitudes of the metacarpal-length ratios were slightly smaller in African-American skeletons than in European-American skeletons (see Figure 1). That is the same direction of effect as seen in finger-length ratios (e.g., [5, Figure 1.7; 15–17], for an exception see [14]). Body size, as measured by height, was not highly correlated with the metapodial length ratios for either racial group. If this difference between African-Americans and European-Americans is verified, other ethnic and racial groups should be studied to see if possible underlying mechanisms can be identified. Race differences in the hands and wrists of children have been reported previously [36].
For metatarsals, the majority of the sex differences in length ratios were essentially zero, and this was true for both feet and for both European-American and African-American skeletons.
None of the length ratios comparing a 2nd metapodial with a 4th metapodial showed a substantial sex difference, which is unlike the findings for finger lengths and toe lengths [7] in humans. This and other findings [9] reveal how important it is for investigators to measure all the digits or rays, not just the 2nd and 4th, especially when a new special population or new species is being studied. The failure to observe a group difference using only a 2:4 length ratio is poor information indeed about whether other length ratios might exhibit a difference in the groups being studied. Had we measured only the 2:4 ratios here, we would have missed all of the major sex differences as well as the striking difference between the European-American and African-American groups.
A commonly cited explanation for the sex differences and some other group differences in finger-length ratios is degree of exposure to androgens prenatally. Specifically, it is suggested that the greater the exposure, the smaller the finger-length ratios. Although this explanation is intuitive and is supported by considerable circumstantial evidence, it must remain just an attractive working hypothesis until definitive evidence in mammals, and preferably primates, can be obtained. That said, a reasonable working hypothesis to adopt is that the sex differences reported here for metacarpal-length ratios are attributable, at least in part, to sex differences in exposure to androgens during early development (see [19]). One question this interpretation raises is why the mechanism(s) responsible for these sex differences operate so differently on metacarpal lengths and finger lengths. Second, why do those mechanism(s) operate so differently in European-Americans and African-Americans? Third, why are there differences between the left and right hands of the European-Americans?
If androgen exposure does prove to be relevant to these matters, and if during early development there are marked, short-term fluctuations in androgen production by individual fetuses, it might be that the answer(s) to some of these three questions could involve variations in the timing of those fluctuations in androgen exposure relative to the time course of the development of the various bones of the hands. For example, perhaps the metapodials achieve their essential development prior to the existence of large sex differences in androgen concentrations, but the phalanges develop while a substantial sex difference does exist.
Another possible type of explanation appeals to the prospect that the dimensions of the extremities (and thus the length ratios) may be affected by the uses to which those extremities are put [29]. We are not able to rule out the possibility that our African-American and European-American specimens used their hands in fundamentally different ways during life. Information permitting such analyses was not collected by the original curators of the Hamann-Todd collection. However, it might be possible to find skeleton collections or other samples in which this variable was controlled.
The literature on finger-length ratios suggests a reason to be cautious about some of the results reported here. It has been known for some time that race and ethnicity do affect the absolute values of the finger-length ratios obtained (see [5, Figure 1.7]). Recently, however, it has become evident that even within nominally the same racial/ethnic group (“Caucasians”), there can be differences in the absolute magnitudes of finger-length ratios depending upon such factors as the country of birth (see [15, 37]). The mechanisms underlying these differences are unknown. Also unknown is whether those factors or mechanisms can affect the metapodials. If they can, then attempts to replicate this work may obtain different values for the magnitudes of some of the race differences reported here simply because of differences in the homogeneity of the racial or ethnic populations sampled. On a related point, there is no basis for knowing whether the present samples were more or less homogeneous internally than samples used in recent studies on finger-length ratios.
Acknowledgments
A preliminary report of this work was presented at a meeting of the Society for Behavioral Neuroendocrinology in June 2002 (Horm. Behav. 2002, 41, 479). This work was supported by research grant DC 00153 from the National Institute on Deafness and other Communication Disorders (NIDCD). The gracious assistance of Mr. Lyman Jellema of the Cleveland Museum of Natural History is gratefully acknowledged. M.M. Fisher provided help with the figures. We thank T.S. Simmons and D. Simmons for assistance with data acquisition. J.C. Loehlin, E.G. Pasanen, and E.C. Kirk, kindly critiqued a preliminary version of this paper or provided other assistance and discussions.
Footnotes
Conflict of Interest Statement
We are not aware of any conflicts of interest associated with the publication of this article.
References
- 1.Ecker A. Some remarks about a varying character in the hands of humans. Archiv fur Anthropol. 1875;8:68–74. [Google Scholar]
- 2.George R. Human finger types. Anat Rec. 1930;46:199–204. [Google Scholar]
- 3.Phelps VR. Relative index finger length as a sex-influenced trait in man. Am J Hum Genet. 1952;4:72–89. [PMC free article] [PubMed] [Google Scholar]
- 4.Peters M, Mackenzie K, Bryden P. Finger length and distal finger extent patterns in humans. Am J Phys Anthropol. 2002;117:209–217. doi: 10.1002/ajpa.10029. [DOI] [PubMed] [Google Scholar]
- 5.Manning JT. Digit ratio: a pointer to fertility, behavior, and health. Rutgers University Press; Piscataway, NJ: 2002. [Google Scholar]
- 6.Malas MA, Dogan S, Evcil EH, Desdicioglu K. Fetal development of the hand, digits and digit ratio (2D:4D) Early Hum Dev. 2006;82:469–475. doi: 10.1016/j.earlhumdev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.McFadden D, Shubel E. Relative lengths of fingers and toes in human males and females. Horm Behav. 2002;42:492–500. doi: 10.1006/hbeh.2002.1833. [DOI] [PubMed] [Google Scholar]
- 8.Brown WM, Hines M, Fane B, Breedlove SM. Masculinized finger length patterns in human males and females with congenital adrenal hyperplasia. Horm Behav. 2002;42:380–386. doi: 10.1006/hbeh.2002.1830. [DOI] [PubMed] [Google Scholar]
- 9.McFadden D, Westhafer JG, Pasanen EG, Carlson CL, Tucker DM. Physiological evidence of hypermasculinization in boys with the inattentive type of attention-deficit/hyperactivity disorder (ADHD) Clin Neurosci Res. 2005;5:233–245. [Google Scholar]
- 10.Williams TJ, Pepitone ME, Christensen SE, Cooke BM, Huberman AD, Breedlove NJ, Breedlove TJ, Jordan CL, Breedlove SM. Finger-length ratios and sexual orientation. Nature. 2000;404:455–456. doi: 10.1038/35006555. [DOI] [PubMed] [Google Scholar]
- 11.Robinson SJ, Manning JT. The ratio of the 2nd to 4th digit length and male homosexuality. Evol Hum Behav. 2000;21:333–345. doi: 10.1016/s1090-5138(00)00052-0. [DOI] [PubMed] [Google Scholar]
- 12.Brown WM, Finn CJ, Cooke BM, Breedlove SM. Differences in finger length ratios between self-identified “butch” and “femme” lesbians. Arch Sex Behav. 2002;31:123–127. doi: 10.1023/a:1014091420590. [DOI] [PubMed] [Google Scholar]
- 13.Rahman Q, Wilson GD. Sexual orientation and the 2nd to 4th finger length ratio: evidence for organizing effects of sex hormones or developmental instability? Psychoneuroendocrinology. 2003;28:288–302. doi: 10.1016/s0306-4530(02)00022-7. [DOI] [PubMed] [Google Scholar]
- 14.McFadden D, Loehlin JC, Breedlove SM, Lippa RA, Manning JT, Rahman Q. A reanalysis of five studies on sexual orientation and the relative length of the 2nd and 4th fingers (the 2D:4D ratio) Arch Sex Behav. 2005;34:341–356. doi: 10.1007/s10508-005-3123-9. [DOI] [PubMed] [Google Scholar]
- 15.McIntyre MH, Cohn BA, Ellison PT. Sex dimorphism in digital formulae of children. Amer J Phys Anthro. 2006;129:143–150. doi: 10.1002/ajpa.20240. [DOI] [PubMed] [Google Scholar]
- 16.Manning JT, Trivers RL, Thornhill R, Singh D. The 2nd:4th digit ratio and asymmetry of hand performance in Jamaican children. Laterality. 2000;5:121–132. [PubMed] [Google Scholar]
- 17.Manning JT, Churchill AJG, Peters M. The effects of sex, ethnicity, and sexual orientation on self-measured digit ratio (2D:4D) Arch Sex Behav. 2007;36:223–233. doi: 10.1007/s10508-007-9171-6. [DOI] [PubMed] [Google Scholar]
- 18.Nelson RJ. An Introduction to Behavioral Endocrinology. 3. Sunderland, MA: Sinauer; 2005. [Google Scholar]
- 19.McIntyre MH. The use of digit ratios as markers for perinatal androgen action. Reprod Biol Endocrinol. 2006;4:10–18. doi: 10.1186/1477-7827-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garn SM, Burdi AR, Babler WJ, Stinson S. Early prenatal attainment of adult metacarpal-phalangeal rankings and proportions. Am J Phys Anthropol. 1975;43:327–332. doi: 10.1002/ajpa.1330430305. [DOI] [PubMed] [Google Scholar]
- 21.Kondo T, Zakany J, Innis JW, Duboule D. Of fingers, toes and penises. Nature. 1997;390:29. doi: 10.1038/36234. [DOI] [PubMed] [Google Scholar]
- 22.Roney JR, Whitham JC, Leoni M, Bellem A, Wielebnowski N, Maestripieri D. Relative digit lengths and testosterone levels in Guinea baboons. Horm Behav. 2004;45:285–290. doi: 10.1016/j.yhbeh.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Brown WM, Finn CJ, Breedlove SM. Sexual dimorphism in digit- length ratios of laboratory mice. Anat Rec. 2002;267:231–234. doi: 10.1002/ar.10108. [DOI] [PubMed] [Google Scholar]
- 24.Manning JT, Callow M, Bundred E. Finger and toe ratios in humans and mice: implications for the aetiology of diseases influenced by HOX genes. Med Hypotheses. 2003;60:340–343. doi: 10.1016/s0306-9877(02)00400-0. [DOI] [PubMed] [Google Scholar]
- 25.Burley NT, Foster VS. Digit ratio varies with sex, egg order and strength of mate preference in zebra finches. Proc Roy Soc B. 2004;271:239–244. doi: 10.1098/rspb.2003.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFadden D, Bracht M. The relative lengths and weights of metacarpals and metatarsals in baboons (Papio hamadryas) Horm Behav. 2003;43:347–355. doi: 10.1016/s0018-506x(02)00048-x. [DOI] [PubMed] [Google Scholar]
- 27.McFadden D, Bracht M. Sex differences in the relative lengths of metacarpals and metatarsals in gorillas and chimpanzees. Horm Behav. 2005;47:99–111. doi: 10.1016/j.yhbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Watkins BT. Hand bone ratios and their utility in predicting general substrate use in primates. Cour Forsch-Inst Senckenberg. 2003;243:47–59. [Google Scholar]
- 29.Harris EF, Akshanugraha K, Behrents RG. Metacarpophalangeal length changes in humans during adulthood: A longitudinal study. Am J Phys Anthropol. 1992;87:263–275. doi: 10.1002/ajpa.1330870304. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 31.Lippa RA. Are 2D:4D finger-length ratios related to sexual orientation? Yes for men, no for women. J Pers Soc Psychol. 2003;85:179–188. doi: 10.1037/0022-3514.85.1.179. [DOI] [PubMed] [Google Scholar]
- 32.Loehlin JC, McFadden D, Medland SE, Martin NG. Height and 2D:4D within and between ethnic groups. Arch Sex Behav. 2006;35:739–742. doi: 10.1007/s10508-006-9039-1. [DOI] [PubMed] [Google Scholar]
- 33.Susman RL. Comparative and functional morphology of hominoid fingers. Am J Phys Anthropol. 1979;50:215–236. doi: 10.1002/ajpa.1330500211. [DOI] [PubMed] [Google Scholar]
- 34.Tague RG. Variability of metapodials in primates with rudimentary digits: Ateles geoffroyi, Colobus guereza, and Perodicticus potto. Am J Phys Anthropol. 2002;117:195–206. doi: 10.1002/ajpa.10028. [DOI] [PubMed] [Google Scholar]
- 35.Darlington RB. Regression and Linear Models. McGraw-Hill; New York: 1990. [Google Scholar]
- 36.Wingerd J, Peritz E, Sproul A. Race and stature differences in the skeletal maturation of the hand and wrist. Ann Human Biol. 1974;1:201–209. doi: 10.1080/03014467400000211. [DOI] [PubMed] [Google Scholar]
- 37.Loehlin JC, McFadden D, Medland SE, Martin NG. Population differences in finger-length ratios: Ethnicity or latitude? Arch Sex Behav. 2006;35:739–742. doi: 10.1007/s10508-006-9039-1. [DOI] [PubMed] [Google Scholar]