Abstract
Otoacoustic emissions (OAEs) were measured in male and female Suffolk sheep (Ovis aries). Some sheep had been administered androgens or estrogens during prenatal development, some were gonadectomized after birth, and some were allowed to develop normally. As previously reported for spotted hyenas, gonadectomy did not alter the OAEs for either sex; accordingly, the untreated/intact and the untreated/gonadectomized animals were pooled to form the control groups. The click-evoked otoacoustic emissions (CEOAEs) exhibited by the female control group (N = 12) were slightly stronger (effect size = 0.42) than those in the male control group (N = 15), which is the same direction of effect reported for humans and rhesus monkeys. Females administered testosterone prenatally (N = 16) had substantially weaker (masculinized) CEOAEs than control females (effect size = 1.15). Both of these outcomes are in accord with the idea that prenatal exposure to androgens weakens the cochlear mechanisms that underlie the production of OAEs. The CEOAEs of males administered testosterone prenatally (N = 5) were not different from those of control males, an outcome also seen in similarly treated rhesus monkeys. Males administered dihydrotestosterone (DHT) prenatally (N = 3) had slightly stronger (hypo-masculinized) CEOAEs than control males. No spontaneous otoacoustic emissions (SOAEs) were found in any ears, a common finding in non-human species. To our knowledge, this is the first ruminant species measured for OAEs.
Keywords: Sheep, Otoacoustic emissions, Sex Differences, Masculinization, Testosterone, Estradiol, Prenatal Development
Introduction
Otoacoustic emissions (OAEs) are sounds that are produced in the cochlea and propagate out through the middle-ear system into the external ear canal (Kemp, 1978, 1979; Probst et al., 1991; Shera and Guinan, 1999). An interesting feature of OAEs in humans is that they exhibit a large sex difference that exists in newborns (Strickland et al., 1985; Burns et al., 1992, 1994; Morlet et al., 1995, 1996) as well as in adults (Bilger et al., 1990; Talmadge et al., 1993; McFadden, 1993; McFadden et al., 1996; McFadden and Pasanen, 1998, 1999; McFadden and Shubel, 2003). The direction of the sex difference in humans is stronger OAEs in females than in males. Because this sex difference exists at birth, it has been proposed that exposure to high levels of androgens prenatally weakens OAEs by weakening the underlying cochlear mechanisms that contribute to them (commonly called the cochlear amplifiers; see Davis, 1983). This has been called the prenatal-androgen-exposure explanation (e.g., McFadden, 2002, 2008). Because strong OAEs are associated with good hearing sensitivity (e.g., McFadden and Mishra, 1993), the common interpretation is that strong OAEs and sensitive hearing both are results of a common cause—strong cochlear amplifiers.
OAEs of differing types have been measured in a wide array of species (Köppl, 1995; Martin et al., 1985, 1999). The results have revealed both similarities to and differences from the OAEs of humans, but unfortunately sex differences have not commonly been studied in the OAEs of non-humans. One species, rhesus monkeys, did exhibit sex differences similar to those seen in humans; namely, stronger click-evoked OAEs (CEOAEs) in females than in males (McFadden et al., 2006a). In addition, the CEOAEs for male rhesus monkeys showed a seasonal effect; the CEOAEs were weaker in the breeding season (Fall) and stronger in the non-breeding season, when androgen levels are high and low, respectively. Thus, if prenatal androgens are responsible for the sex differences in OAEs, there appear to be both organizational and activational effects of androgens on the CEOAEs of rhesus monkeys (also see McFadden, 2000).
Another type of OAE measured in those same rhesus monkeys (distortion-product OAEs or DPOAEs) exhibited a much smaller sex difference than did the CEOAEs (McFadden et al., 2006a). Examination of the DPOAE data reported by Torre and Fowler (2000) for rhesus monkeys also reveals only a small sex difference favoring the females, and only in the youngest animals (all of which were middle-aged). In humans, the sex difference in DPOAEs also is small (Gaskill and Brown, 1990; Moulin et al., 1993; Cacace et al., 1996; Dhar et al., 1998; Bowman et al., 2000; McFadden, Martin et al., 2008c). Taken together, these facts suggest that CEOAEs are more affected by early developmental mechanisms of sexual differentiation (possibly hormonal mechanisms) than are DPOAEs.
By contrast, both the CEOAEs and DPOAEs were slightly weaker in female spotted hyenas than in males, not stronger (McFadden et al., 2006b). This is an extremely unusual mammalian species because female spotted hyenas are exposed to high levels of androgens during prenatal development and, as a consequence, they are highly masculinized in body, brain, and behavior (Frank et al., 1991; Glickman et al., 1992). The explanation offered for the absence of a human-like sex difference in the OAEs of this species was that the cochlear amplifiers of female spotted hyenas are weakened by the high levels of androgens to which they are naturally exposed prenatally (McFadden et al., 2006b). In accord with this explanation, we observed stronger CEOAEs in spotted hyenas of both sexes that were treated with androgen-blocking agents during prenatal development. An ideal test of the explanation, as yet not done, would be measurements of OAEs in another species of hyena, none of which have highly masculinized females.
Guimaraes et al. (2004) reported sex differences in CBA mice. Females had stronger DPOAEs than males, the same direction of effect as in humans and rhesus monkeys. However, because this sex difference was evident in middle-aged mice, but not in young mice, it may turn out to be an example of a differential, age-related hearing loss rather than an organizational effect of androgens. In chinchillas, S.L. McFadden et al. (1999) found no sex differences in DPOAEs even though females were more sensitive than males at high frequencies according to an evoked-potential measure obtained from a gross electrode implanted in the inferior colliculus. Valero et al. (2008) reported much larger DPOAEs in female than in male marmoset monkeys, a species for which little else is known about the auditory periphery. Only DPOAEs were measured in the Guimaraes et al., the S.L. McFadden et al., and the Valero et al. studies, not CEOAEs.
Here we report OAE measurements made on sheep of the Suffolk variety. To our knowledge, no previous measurements of OAEs in a ruminant species have been published. According to one report (Wollack, 1963), hearing sensitivity in sheep is between 10 and 40 dB worse than in humans over the range of about 0.1 kHz to 5.0 kHz, and it averages about 18 dB worse over the range of 1.0 kHz to 5.0 kHz where we measured CEOAEs; the best hearing is around 10 kHz; and hearing extends to at least 40 kHz. We are not aware of any explanation for why hearing would be so poor in the low frequencies and then extend to such high frequencies in this species, but Masterton et al. (1969) have argued that the audible frequency range in a species may be determined, in part, by its ability to use interaural level differences to localize sound sources in space. We know of no studies on sound localization in sheep.
Based on the known sex differences in the OAEs measured in humans and rhesus monkeys, we predicted that the OAEs of female sheep would be stronger than those of male sheep. Some of the sheep to be tested had been exposed to higher-than-normal levels of androgens or estrogens during the second trimester of prenatal development (see Bormann et al., 2008; Roberts et al., 2008), providing us with additional tests of the effects of prenatal hormones on OAE strength. One prediction was that OAEs would be weakened in sheep treated with androgens prenatally. Some of the sheep tested had been gonadectomized within two weeks of birth and were not receiving supplemental hormones; based on our experience with spotted hyenas (McFadden et al., 2006b), we expected these post-natal manipulations to have little effect on OAE strength.
Many of our expectations were confirmed, but an unanticipated result also was obtained. Specifically, some sheep yielded strong CEOAEs but only quite weak, or no, DPOAEs. In the past, dissociations between different types of OAE have been observed (e.g., Wier et al., 1988; McFadden and Pasanen, 1994; Whitehead et al., 1996), but only in the context of short-term injury to the cochlea. Here, the presence of strong CEOAEs makes cochlear injury an unlikely explanation. Elsewhere (McFadden et al., 2008b) we discuss this dissociation between and DPOAEs and CEOAEs in sheep and describe some of the tests we made to document its existence. Here we describe only the CEOAE data, which appear to be unaffected by the dissociation from the DPOAE data.
Methods
All experimental procedures were performed in accordance with NIH guidelines and were approved by the Committees on the Use and Care of Animals at both the University of Michigan (UM) and The University of Texas (UT). The sheep lived outdoors in a natural environment at the Reproductive Sciences Program Sheep Research Facility (Ann Arbor, MI @ 42 degs 18 min North latitude). After weaning, lambs had access ad libitum to alfalfa hay and pellets, pasture, and water. Females and same-aged, vasectomized males were housed together. OAE measurements were obtained on three multi-day visits of the UT team to Ann Arbor: in November and December 2006 and in May 2007.
The various groups of treated animals tested for this study were created by the UM team in order to study experimental questions about endocrine function and sex-typical behavior in sheep that arose in part from earlier work by the UM team. The auditory measurements reported here were simply added on to the existing protocol, and were implemented when it was convenient for both the UM and UT teams.
Subjects
The sheep tested (Ovis aries) were of the Suffolk variety. All of the female sheep were born between early March and early April 2005, and all of the male sheep were born between early March and early April 2006. Thus, the females were approximately 20 – 26 months of age and the males approximately 8 – 14 months of age at the time of OAE testing. The nominal gestational period for sheep is 21 weeks, infancy lasts about 2 weeks, and the pre-weaning period lasts about 8 weeks. The pre-pubertal period, as measured by the rising level of luteinizing hormone, lasts about 10 weeks in males and about 30 weeks in females. Measurable testosterone is present at about 4 months of age in males, and estrous cycles are present at about 7 months in females. The nominal lifespan of sheep is 8 – 13 years. Thus, all the sheep tested were young adults. Because some of the sheep had received special treatments during gestation, the data are presented separately for the different groups. The details of the groups are given in what follows, and the Ns for the groups are shown in Table 1.
Table 1.
Number of female and male sheep in the various groups.
| Group | Female | Male |
|---|---|---|
| Untreated | 8 | 9 |
| Untreated/GDX | 4 | 6 |
| Prenatal Testosterone | ||
| Non-Obese | 10 | 5 |
| Obese | 6 | 0 |
| Prenatal Estradiol (E2) | 2 | 0 |
| Prenatal DHT | 0 | 3 |
For this study, the untreated and untreated/GDX sheep were combined within sex to create the two control groups, and the non-obese and obese females were combined to create a single female prenatal-testosterone group.
GDX = gonadectomized
DHT = dihydrotestosterone
Untreated males were vasectomized (VX)
Eight females and nine males were born to mothers who were not treated with hormones during their pregnancies (the untreated animals). At about 14 weeks of age, these males were vasectomized (VX). An additional four females and six males were born without treatment, but then were gonadectomized (GDX) within the first two weeks of life.
Sixteen females were born to mothers who were administered testosterone during their pregnancy. Specifically, during days 30 – 90 of the 147-day gestational period, the mothers received intramuscular injections twice weekly of 100 mg of testosterone propionate (Sigma-Aldrich Corp., St. Louis, MO, USA) in 2 ml of cottonseed-oil vehicle (see Roberts et al., 2008). These testosterone-treated females are born with no vaginal opening, with a scrotal sac that is empty, and with a pseudopenis that is used for urination; no erections ever have been observed. They also exhibit various neuroendocrine and ovarian differences from untreated females (see Padmanabhan et al., 2006). Six of these 16 testosterone-treated females were provided unlimited feed corn and became obese; ten were maintained at normal weight.
Two females were born to mothers who were implanted with one 30-mm silastic implant (i.d. 0.22 cm; o.d. 0.46 cm; sealed with Silastic adhesive Type A, Dow-Corning Corp., Midland, MI) packed with crystalline 17β-estradiol (Sigma, St. Louis, MO) between days 30 – 90 of gestation. Past research revealed that the females receiving a lower dose of prenatal 17β-estradiol (13-mm silastic implant throughout the pregnancy) demonstrated an increase in aggressive displacement behavior as lambs and altered LH-surge properties prepubertally, but had a normal onset of puberty, normal postpubertal LH characteristics, and normal female-typical mating behavior (Malcolm et al., 2006). Because this estradiol-treated group consisted of only two animals, their data will be shown for completeness, but no statistical analyses will be reported. No treated female was ovariectomized.
Female lambs within each treatment group were allowed to interact freely with one another, with male siblings, and with their mothers until 8 weeks of age, when weaning took place. Some of the male siblings were vasectomized (VX) by 16 weeks of age; others were gonadectomized (GDX) prior to 2 weeks of age. After weaning, the mothers and GDX male lambs were removed.
Five males were born to mothers who were administered testosterone from days 30 –90 of gestation; three males were born to mothers who were administered dihydrotestosterone (DHT) over that same time period (Bormann et al., 2008, submitted*). These males were gonadectomized by 10 days of age, and at the time of OAE testing, none were receiving supplemental hormones.
All animals were raised in a mixed-sex flock after weaning. Intact/VX males were present in each flock at a ratio of approximately one intact ram per 20 ewes. The lambs had access ad libitum to alfalfa hay and pellets, pasture, and water. Body weights were obtained weekly until all animals reached 50 kg (about 26 wk of age), a weight typical of an adult sheep. Diet was limited to alfalfa hay and pasture after animals reached adult weight.
Procedure
As noted, OAE measurements were obtained during three sessions: in mid-November and mid-December 2006 (breeding season) and in mid-May 2007. Some sheep were measured on more than one visit, but most only on one visit. In an attempt to better understand the partial dissociation between CEOAEs and DPOAEs, some procedural details were varied across visits. For example, for the early visits, the sheep were in a supine position during OAE testing, but for the May visit, they lay on their sides (in both cases the left ear canal pointed up). Some OAE differences were observed across sessions, but we are unable to know if they were due to body position or season. Most of the data reported here were obtained in Fall 2006. The primary exception was that all four of the untreated/GDX females were tested in May 2007.
Sheep scheduled for testing were housed separately beginning about 48 hours prior to testing, and they were food-deprived during that interval. Test animals initially were anesthetized with ketamine hydrochloride (4 – 6 mg/kg, iv) and diazepam (0.2 – 0.3 mg/kg, iv). Once this anesthetic had taken effect, the animal was intubated and transported to a surgical table where it was secured in a supine position (animals tested in Fall 2006) or on its right side (animals tested in May 2007) on a thick foam pad. The hind-quarters were elevated by several inches above the head to permit drainage from the rumen, throat, and mouth. Anesthesia was maintained with a mixture of halothane gas (approximately 1.5%) in nitrous oxide (1/3) and oxygen (2/3).
Ketamine is commonly used when recording OAEs in non-humans (e.g., Martin et al., 1999; Torre and Fowler, 2000; Hatzopoulos et al., 2002; Jacobson et al., 2003; Valero et al., 2008), and one report concluded that halothane had no effect on CEOAEs in humans (Guven et al., 2006; compare Kettembeil et al., 1995, for birds). No changes in the strength of sheep OAEs were observed when the depth of the gas anesthetic was systematically varied. For three female sheep, CEOAEs were measured both before and after DPOAE measurement, with the time between measurements ranging from 13 to 38 min, and all three pairs of measurements were in very good agreement. The halothane vaporizer equipment used to administer the gas anesthetic was reasonably quiet, and there was no detectable contribution of the anesthetic delivery system to the noise floor measured in the ear canal.
An otoscope was used to examine the ear canal, and cotton swabs and gauze, sometimes dipped in isopropyl alcohol, were used to clean the canal. For the later-tested animals, diluted alcohol was injected into the ear canal about 24 hours prior to the scheduled test session, and this reduced the need for extensive cleaning at the test session. In general, females required less cleaning than males.
The sounds in the ear canal were measured using an Etymotic ER-10B+ microphone (Etymotic Research, Elk Grove Village, IL), and the click stimuli were presented using Etymotic ER-2 earphones. Small silicon sound-delivery tubes attached to the earphones passed through the microphone body and a foam or rubber probe tip that was inserted deeply into the external ear canal. The output of the microphone was amplified by 20 dB and then passed to a custom-made unit that high-pass filtered the waveform at 400 Hz in order to eliminate the contribution of residual body noise to the recordings. The output of the filter was led to a data-acquisition board (PCI-4451, National Instruments, Austin, TX) installed in a PCI-bus-extension chassis connected to a Macintosh laptop computer (Power Macintosh G3, Apple Computer, Cupertino, CA) via a cardbus interface. The computer and extension chassis were located on a table placed several feet from the surgical table. The computer also generated electrical pulses of 100 μsec duration that were delivered to one of the ER-2 earphones to produce the click stimuli for CEOAE measurements. The polarity of the electrical pulse was selected to produce an initial increase in pressure in the ear canal (a condensation click). A 50-kHz sampling rate (16-bit precision) was used both for digitizing the output from the microphone system and for generating the acoustic stimuli for presentation to the ear.
A grounding wire attached to a large syringe needle inserted into the loose skin of the neck was connected to a common ground point for the measurement apparatus. The earphones typically were clipped to the wool on the sheep’s neck to reduce relative movement and noise. Whether the sheep was lying supine or on its side on the surgical table, its head was rotated slightly so that the left ear was pointed upward. The ear canals of sheep have substantial curvature, and considerable practice was required to achieve tight fits of the probe tip. Typically, rubber tips originally made for otoadmittance measurements were fitted over the end of the ER-10B+ probe, but for some especially large canals, special oversized foam tips were used instead. That assembly was inserted tightly into the left ear canal, and the calibration procedure associated with measurement of CEOAEs was begun. Because of time constraints, only left ears were measured for most animals.
All stimulus presentation and data collection was accomplished using custom-written LabView software (National Instruments, Austin, TX) running on the Macintosh laptop computer. The typical test session began with the collection of several 30-sec samples of the sound in the ear canal. This provided information about the possible existence of spontaneous OAEs (SOAEs; none were observed) and about the goodness of the fit of the probe tip in the external canal. If the spectrum of the recorded sound or the spectrum of the click stimulus in the ear canal were not smooth, then the probe tip was refitted in the canal until acceptable measurements were obtained. Typically, the next measurements taken were CEOAEs elicited by the weaker click level, corresponding to approximately 75 dB peak-equivalent sound-pressure level (peSPL re 20 μPa). On a subsequent run, an 81-dB click was used; the latter are the data presented here, but all major outcomes were the same for the 75-dB click. The voltage delivered to the earphone was adjusted in each ear canal to achieve the target level of 75 dB; the amplitude was doubled to achieve the 81-dB click level. Prior to the test runs, click sequences were presented for an initial 20-sec period in order to establish a criterion for rejection of noisy samples. The software then monitored the noise level in the canal for an additional 20 sec. The median and standard deviation of the noise levels obtained during the monitoring period were calculated to establish a criterion for sufficiently quiet conditions for click presentation and data collection. Typically, the noise floor of the system (in 1-Hz bins) was about −15 dB SPL above about 2.0 kHz. For some sheep tested early in November 2006, CEOAE data were collected only for the 75-dB click; for the remainder, both click levels were used.
The presentation and analysis of the clicks were based on the maximum-length-sequence (MLS) procedure described by Hine et al. (2001). We previously had collected CEOAEs from spotted hyenas using both this MLS procedure and our usual procedure; the magnitudes of the CEOAE responses were virtually identical with the two procedures, but data collection was considerably faster with the MLS procedure. The clicks were presented in sets of 16, with successive clicks separated in time by durations that were selected in a pseudorandom manner, and with all 16 clicks always presented within 320 msec. The minimum separation between successive clicks was 10 msec and the maximum was 50 msec. Before beginning the string of 16 clicks, the software examined the noise level in the canal and presented the next scheduled set of clicks only if the noise level was below the criterion value established earlier. Each 16-click sequence yielded a 16-click averaged response of 50 msec duration. The averaged response was immediately extracted, analyzed, and evaluated against the noise criterion. If it was judged to be free of artifacts and excessive noise, it was added to the on-going average, and if not, the entire sequence was discarded. Click presentation and data collection continued until responses to at least 2000 clicks had been obtained for each click level. An individual test session lasted between 30 and 135 mins, depending on ease of inserting the probe tip in the ear canal, interruptions of various sorts, and opportunities to collect additional data.
Our standard procedure for analyzing the averaged CEOAE waveform is to estimate the strength of the CEOAE response by calculating the rms amplitude in a 20-msec segment beginning a few milliseconds after the presentation of the click. The purpose of this short delay is to exclude both the energy of the click itself and the ringing of the middle-ear system in response to the click; by delaying the analysis window, we are including in our measure primarily the echo-like response of the inner ear to the click. Because the echo-like response diminishes with time after the presentation of the click, the longer the delay, the less of the cochlear response that will be included in the 20-msec sample, and with quite long delays, all that is included is the noise in the ear canal. Thus, long delays in the analysis window are expected to yield rms amplitudes that are close to the noise floor of our recording system.
In our standard procedure, the 20-msec sample of the averaged response also is filtered between 1.0 kHz and 5.0 kHz. The purpose for removing the frequency components below 1.0 kHz is to eliminate low-frequency ambient noise and body noise from respiration, circulation, and the musculature of the head and neck from our estimate of the echo-like response. The echo-like responses from the very highest frequency regions of the basilar membrane can be emitted with such short latency that the intense mechanical and acoustical “ringing” of the ear canal caused by the click itself prevents the extraction of those weak, high-frequency responses. Thus, our removal of frequency components above 5.0 kHz eliminates energy not likely to have originated from the cochlea, and it provides a slight improvement in signal-to-noise ratio. Past experience suggested that 1.0 and 5.0 kHz were reasonable cutoff frequencies for avoiding these various sources of noise.
Our initial analyses of the sheep CEOAE data did use our standard procedures for delaying the 20-msec analysis window and filtering the average CEOAE waveform from 1.0 – 5.0 kHz. When the analysis window was delayed by 20 or 30 msec, however, the measurements did not fall all the way to the noise floor of our instrumentation. Some additional tests revealed that, for some sheep, the frequency band from 1.0 – 1.5 kHz contained energy not likely to have originated from the cochlea, but from some other source. This source was never unambiguously identified, but by changing our filter bandwidth to 1.5 – 5.5 kHz, the problem was eliminated; accordingly, the latter filter bandwidth was used when extracting all the CEOAE data reported here from the raw waveforms. For analysis, then, a 20-msec segment of the echo-like response, beginning about 4 msec after click onset, first was passed through a gating window having 2-msec (cosine-squared) rise and decay times, and then was passed through a digital bandpass filter having 1.5- and 5.5-kHz cutoff frequencies. The rms of the filter output converted to sound-pressure level (SPL) was taken as the CEOAE response.
The 1.0 – 1.5 kHz band that was anomalous here was examined in data previously collected from rhesus monkeys (McFadden et al., 2006a), spotted hyenas (McFadden et al., 2006b), and humans (Pasanen and McFadden, 2000), and no similar anomalies were found, even though the monkey data had been collected in a test room much like that used for the sheep, with a similar ambient noise level.
Because all of the groups available for study were small, we regard this study as having been exploratory, and as a potential source of ideas for future investigation, rather than as a test of specific hypotheses. Accordingly, we prefer the use of effect size rather than statistical tests for placing the individual comparisons in perspective. Effect sizes were calculated as the difference between the means of the two groups of interest divided by the square root of the weighted mean of the two variances for those two groups. According to Cohen (1992), effect sizes of 0.2, 0.5, and 0.8 correspond to small, medium, and large effects, respectively. The t-tests reported here all were equal-variance, unmatched, and two-tailed.
Issue of Weak DPOAEs
As noted above and detailed elsewhere (McFadden et al., 2008b), some sheep had unmeasurable, or quite weak, DPOAEs, but normal-appearing CEOAEs. Initially, all CEOAE comparisons and analyses reported here were done with the 16 sheep having no DPOAEs excluded. When the comparisons and analyses were repeated with those sheep included, not one outcome or conclusion was changed. Of the seven groups considered (after pooling of the untreated/intact with the untreated/GDX animals—see below), only one group exhibited a larger standard deviation after the sheep with weak DPOAEs were included (the prenatal testosterone-treated males), and that was because the two added sheep had quite strong, not weak, CEOAEs. All six other groups had smaller standard deviations after inclusion of the sheep with weak DPOAEs. Because including the sheep with weak DPOAEs increases the Ns, and thereby strengthens one’s confidence in the conclusions drawn, those sheep have been included for all calculations reported here. The sheep having weak, or no, DPOAEs were: 3 of the 8 untreated/intact females, 4 of the 12 non-obese/testosterone-treated females, 1 of the 6 obese/testosterone-treated females, 4 of the 9 untreated/VX males, 1 of the 6 untreated/GDX males, 2 of the 5 testosterone-treated/GDX males, and 1 of the 4 DHT-treated/GDX males. A few additional sheep had no measurable DPOAEs in one or two frequency regions.
Results
Test/Retest Reliability
Even though successful positioning of the probe tip was more difficult in sheep than in humans, rhesus monkeys, or spotted hyenas, in general, test/retest reliability was quite good. In May 2007, the CEOAEs of three female and one male sheep were measured twice within the same session, with measurements of DPOAEs intervening. For three of those animals, the two CEOAE measures were within 1.25 dB. For the fourth animal, the microphone had to be repositioned in the ear canal, and the second measure was 3.4 dB stronger than the first. One untreated/VX male was tested in the supine condition both in Fall 2006 and Spring 2007. His CEOAEs for the 81-dB click level were 9.9 and 9.1 dB, respectively, which suggests both good reliability of our measures and the absence of a seasonal effect (compare McFadden et al., 2006a); his two sets of DPOAE measurements also were essentially the same. Two other sheep, both untreated/VX males, were tested in both seasons, but positioned supine in the Fall and on the side in the Spring. The CEOAEs were uniformly weaker in the Fall (breeding season) than in the Spring, but we cannot know if this was evidence for a position effect, a seasonal effect, or simple variability of measurement.
One outcome suggests little effect on our measures of either season of the year or of the two body positions used for testing, at least for females. Seven of the ten non-obese females treated prenatally with testosterone were tested in Fall 2006 (supine position) and the remaining three were tested in Spring 2007 (lying on their side). The mean CEOAE calculated for the first seven animals differed from the mean calculated for all ten animals by 0.01 dB.
Establishing Control Groups
The CEOAE data obtained from the untreated/VX males did not differ from the data obtained from the untreated/GDX males, so those groups were pooled to create the male control group. The means (SEs) for the nine untreated/VX males and the six untreated/GDX males were 7.86 (1.17) and 7.19 (1.35) dB, respectively, and an unpaired t-test produced a p value of 0.72 for this comparison. By contrast, the data for the untreated/GDX females were noticeably stronger than those obtained from the untreated/intact females; however, this apparent difference was not significant. The means (SEs) for the eight untreated/intact females and the four GDX females were 7.99 (1.65) and 11.88 (1.44) dB, respectively, and an unpaired t-test produced a p value of 0.16* for this comparison. Accordingly, the data for the eight untreated/intact and four untreated/GDX females were pooled to create the female control group. Spotted hyenas that were castrated or ovariectomized also were indistinguishable from untreated animals (McFadden et al., 2006b), suggesting that, in both species, the changes occurring in body and brain during puberty do not have marked effects on CEOAEs.
The CEOAE data for one of the eight untreated/intact females were anomalously weak. The response to the 81-dB click was more than 2.0 standard deviations weaker than the mean for the group, and it was only about 3 dB stronger than the response to the 75-dB click. By comparison, the typical sheep showed about a 6-dB stronger CEOAE for the stronger click. Fortunately, we had collected a set of CEOAE data for this animal using rarefaction clicks as well as with the standard condensation clicks. The response to the 81-dB rarefaction clicks was within tenths of a decibel of the mean for the other 11 control females (using condensation clicks), and the difference in CEOAE strength for the 75- and 81-dB rarefaction clicks was 6.2 dB. Accordingly, the CEOAE data collected with the 81-dB rarefaction clicks were substituted for the anomalous-appearing data collected from this female using the condensation clicks. Because we delayed our analysis window by 4 msec and filtered between 1.5 and 5.5 kHz (see above), the difference in click polarity ordinarily should have had minimal effect on the strength of the CEOAE.
Sex Differences
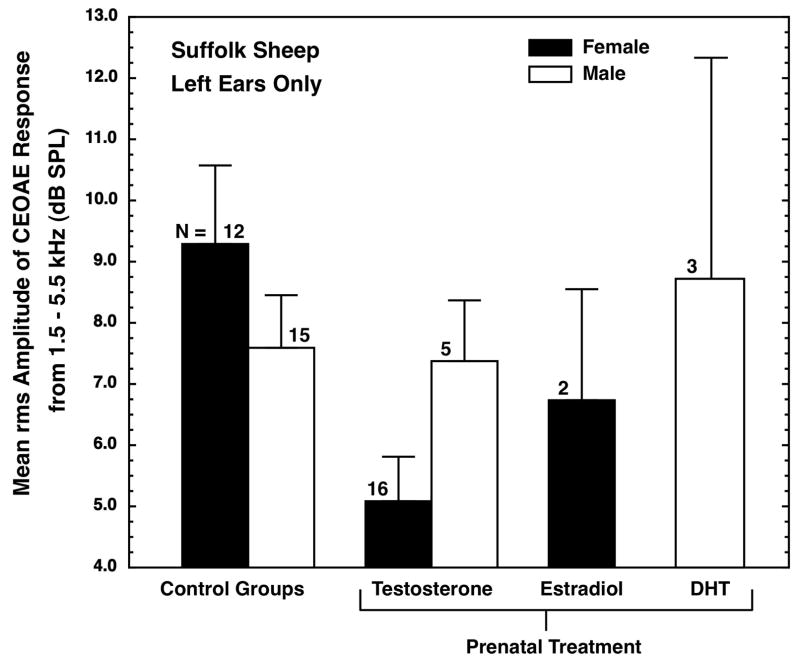
The mean rms amplitudes of the CEOAEs obtained from the various groups of Suffolk sheep using a click level of 81-dB are shown in Fig. 1. Just as in humans (e.g., McFadden et al., 1996) and rhesus monkeys (McFadden et al., 2006a), the CEOAEs of female Suffolk sheep were stronger than those of males, but unlike humans, the difference was small. The means for control female and control male sheep are shown in Fig. 1; the effect size for the difference between the control females and control males (females minus males) was about 0.44 (see comparison 1 in Table 2). The difference was not statistically significant, unpaired t(25) = 1.13, p = 0.27, two tailed. Using somewhat different measurement techniques, we previously have reported sex differences in the CEOAEs of humans and rhesus monkeys that corresponded to effect sizes of about 0.71 (McFadden and Pasanen, 1998) and 1.64 (McFadden et al., 2006a), respectively. Note that this moderate sex difference in the CEOAEs of sheep is not likely to be attributable to the large sex difference in body size that exists in this species because untreated/GDX males are much smaller than untreated/VX males (they are similar in size to control females), yet their CEOAEs were not different from those of the untreated/VX males. The existence of weaker CEOAEs in males than females is in accord with the prenatal-androgen-exposure explanation offered above.
Fig. 1.
Mean rms amplitude of the averaged echo-like response to a click stimulus of 81 dB peSPL for sheep of the Suffolk variety. Results are shown for control females, control males, and various treatment groups. For both sexes, the control groups are a pool of prenatally untreated/intact plus prenatally untreated then neutered (GDX) animals. Analyzed were 20.5-ms samples of the response (filtered between 1.5 and 5.5 kHz) beginning 4 ms after click presentation. Clicks were presented as a maximum-length sequence in groups of 16, with the inter-click interval ranging between 10 and 50 msec. The response was based on a total of 2000 clicks. The flags designate one standard error of the mean. Only left ears were measured. The pattern of results obtained with a 75-dB click was the same as the pattern shown.
Table 2.
Effect sizes for various pair-wise comparisons of groups.
| Comparison | Ns | Effect Size |
|---|---|---|
| 1. Control Females vs. Control Males | 12/15 | 0.44 |
| 2. Control Females vs. Prenatal-T Females | 12/16 | 1.15** |
| 3. Control Males vs. Prenatal-T, GDX Males | 15/5 | 0.07 |
Effect sizes calculated as first group listed minus second group
T = testosterone injections during days 30–90 of gestation
GDX = gonadectomized within 2 weeks of birth
0.01 > p > 0.001; unpaired, two-tailed t-test
Control Females includes Untreated/Intact Group pooled with Untreated/GDX Group
Control Males includes Untreated/VX Group pooled with Untreated/GDX Group
Prenatal-T Females includes Obese Group pooled with Non-Obese Group
Treated Groups
There were two groups of female sheep born to mothers that had been administered testosterone propionate during their pregnancy: those of normal weight and those that had been allowed to become obese. The latter were not diabetic, as revealed by glucose-tolerance and insulin measurements. The CEOAEs of those two groups were not identical, but both had substantially weaker CEOAEs than the control females, which is in accord with the prenatal-androgen-exposure explanation. The means (SEs) for the ten non-obese females and the six obese females were 6.08 (0.78) and 3.43 (1.23) dB, respectively, and an unpaired t-test produced a p value of 0.08 for this difference. Although future research may find the OAEs of these two groups to be different, we had no theoretical reason for keeping the non-obese and obese groups separate, so, for simplicity, those two groups were pooled to create a single female prenatal-T group. The result is shown in Fig. 1. The CEOAEs of the female prenatal-T group were masculinized to the point of being even weaker than those of the control males. The effect size for the difference between the untreated and the testosterone-treated females was about 1.15 (comparison 2 in Table 2), and the unpaired t(26) = 3.03, p = 0.006, two-tailed. The latter p value becomes 0.033 after being corrected for the six t-tests reported here (Darlington, 1990, p. 249+).
Two other females had been exposed to additional estradiol during prenatal development, and their CEOAEs were weaker than those of the control females. Although their data are shown in Fig. 1, no statistical comparisons are reported here because of the small N. Nonetheless, the finding is intriguing and well-deserving of verification in this or other species because the implication is that masculinization of the cochlea ultimately may be accomplished by estradiol that is aromatized from testosterone, not by testosterone itself.
Five males had been administered testosterone propionate during prenatal development, and then also were neutered by 10 days of age (testosterone-treated/GDX males). Their CEOAEs were essentially the same as those of the control males (comparison 3 in Table 2), not noticeably hyper-masculinized (weakened) as the prenatal-androgen-exposure explanation predicts. A parallel outcome was seen for some testosterone-treated male rhesus monkeys (McFadden et al., 2006a).
Three other males had been administered dihydrotestosterone (DHT) during prenatal development, and then also were neutered by 10 days of age. Their CEOAEs were somewhat stronger (hypo-masculinized) than those of the control males (see Fig. 1), but no statistical comparisons are reported here because of the small N. This hypo-masculinization is in accord with the directionality of other measures obtained from these males (see Bormann et al., 2008).
For comparison with the data shown in Figure 1, the male dominance hierarchy among these male sheep was generally: testosterone-treated males at the top, untreated males next, DHT-treated/GDX males next, and untreated/GDX males at the bottom. This hierarchy was based primarily on counts of the number of times each sheep displaced other sheep during monitored feeding sessions.
Discussion
Although not a large effect, the finding of stronger CEOAEs in female sheep than male sheep is further evidence that this sex difference may prove typical of mammalian ears. So far, this difference has been found in the CEOAEs of humans (McFadden et al., 1996), rhesus monkeys (McFadden et al., 2006a), and Suffolk sheep, and its absence in spotted hyenas was predicted (McFadden et al., 2006b). To our knowledge, these are the first measurements of OAEs in a ruminant species or a species domesticated for consumption, and neither of those factors prevented females from having slightly stronger CEOAEs than males.
The additional finding of female sheep exposed to testosterone during prenatal development having weaker CEOAEs than untreated females bolsters the idea that the presence of high levels of androgens prenatally somehow weakens the cochlear amplifiers (McFadden, 2002, 2008) that are believed to contribute to OAE production (Davis, 1983; Shera and Guinan, 1999). In support of this implication, the CEOAEs of female rhesus monkeys exposed to additional testosterone during gestation also were weaker than those in untreated female monkeys (McFadden et al., 2006a). The absence of a difference between the neutered and control sheep within either sex further emphasizes that the mechanisms underlying the sex difference in CEOAE strength apparently operate organizationally early in development, possibly during prenatal development, and are not further influenced by post-pubertal hormones.
The absence of hyper-masculinization in the CEOAEs of male sheep administered additional testosterone prenatally runs counter both to prediction and to the results for testosterone-treated females. Accordingly, the eventual understanding of this failure of prediction has the potential to be informative about underlying mechanisms. One possible explanation is that exposure to the exogenous testosterone may have led to a prenatal down-regulation in the normal production of endogenous testosterone in these developing male fetuses. However, these testosterone-treated/GDX males did show hyper-masculinization of their play behavior, and their testosterone-treated/intact siblings were high in the dominance hierarchy, and they were highly successful at locating estrous females and mating with them quickly (unpublished data). So, a systemic down-regulation seems unlikely here. Male rhesus monkeys treated with testosterone early in gestation also showed no hyper-masculinization of their CEOAEs, but males treated late in gestation did (McFadden et al., 2006a), so it may be that the cochleas of male sheep are most sensitive to masculinization late in gestation or even after birth. Apparently female and male fetuses respond differently to the additional testosterone because female sheep, but not male sheep, were markedly masculinized by the same prenatal testosterone treatment.
In humans, developing males are exposed to high levels of androgens during the second trimester of gestation. Then the androgen levels fall, so that at birth the levels are the same in males and females. Beginning soon after birth, and lasting for several months, human males experience a “second surge” of androgen production, during which time additional masculinization and defeminization of body, brain, and behavior are hypothesized to occur (Wallen and Baum, 2002). Although there may be some effects on male OAEs during the second surge, the basic sex difference in OAEs already exists at birth (Strickland et al., 1985; Burns et al., 1992, 1994; Morlet et al., 1995, 1996), so any contributions from the second surge appear to be minor in humans. Male sheep also may experience postnatal surges in androgen production (Yu et al., 1983). Because nothing is known about sex differences in CEOAE strength in newborn sheep, inferences about the relative importance of the prenatal and postnatal exposures are necessarily hazardous. That is, it is logically possible that some of the masculinization of CEOAEs in male sheep, unlike humans, is accomplished during the second surge, not prenatally. However, note that the six males orchiectomized within two weeks of birth had CEOAEs that were indistinguishable from the untreated/VX males.
The weak CEOAEs of the testosterone-treated females do not constitute unambiguous evidence that androgens themselves weaken the cochlear amplifiers because testosterone can be aromatized into estradiol (see Wallen and Baum, 2002, for a review), and the latter may be responsible for the weakened cochlear amplifiers. Indeed, although the sample size was extremely small, the weak CEOAEs in the estradiol-treated females do suggest that the weakening of the cochlear amplifiers ultimately may involve an estrogenic mechanism rather than an androgenic mechanism. Alternatively, the effect eventually may prove to be an indirect effect of androgens (see review by Jordan and DonCarlos, 2008).
The CEOAEs of the small number of males treated with the non-aromatizable androgen DHT appeared to be less masculinized than in control or testosterone-treated males. If this result is confirmed, it would be consistent with some behavioral results obtained from this group. Namely, DHT-treated males are low ranking and poor at identifying potential estrous ewes (unpublished). Also, the testes of these males are quite abnormal (Bormann et al., 2008), suggesting that the prenatal-DHT exposure altered normal HPG function during the 60 days of exposure.
As noted, we observed an unusual partial dissociation between CEOAEs and DPOAEs in these sheep that will be discussed further elsewhere (McFadden et al., 2008b). This colony of sheep does not contain any of the “gay rams” that are reported to prefer to engage in sex behavior with other males rather than with females (e.g., Roselli et al., 2004).
Strength of CEOAEs
The primary interest here was in the relative magnitudes of the CEOAE responses from male and female sheep, and from treated and untreated sheep, not the absolute magnitudes of those CEOAE responses. Nevertheless it is tempting to ask how those absolute magnitudes compare with the absolute magnitudes measured in other species. In order to make the comparison, we re-analyzed some previously collected data so that all estimates involved the 4-msec delay, the 1.5 – 5.5 kHz filter bandwidth, a 75-dB click, and untreated animals only. The result was that the absolute strengths of the CEOAEs from sheep were somewhat weak--about 3 dB weaker than those of rhesus monkeys, about 6 dB weaker than those of humans, and about 16 dB weaker than those of spotted hyenas. The CEOAEs of lemurs were about 4 dB weaker than those of the sheep, but the Ns for lemurs were quite small.
These facts are potentially relevant in the context of the effects of anesthetic on OAEs. The primary anesthetic used with the sheep (halothane) was different from the drugs used with the other species (the humans were not anesthetized, of course). The rhesus monkeys received an initial dose of Telazol which was supplemented with ketamine as needed. The spotted hyenas received an initial dose of ketamine, xylazine, and atropine, which also was supplemented with ketamine as needed. Thus, the weak CEOAEs in sheep compared to other species might be attributable to the halothane anesthetic none of the other species received (but compare Guven et al., 2006). However, any explanation of that sort also needs to explain why the DPOAEs of sheep (McFadden et al., 2008b) were comparably strong as the DPOAEs of these same rhesus monkeys and spotted hyenas. Might DPOAEs also be dissociated from other forms of OAE in the way they respond to anesthetics?
Acknowledgments
This research was supported in part by research grant DC00153 from the National Institute on Deafness and other Communication Disorders (NIDCD) awarded to DM, and in part by research grant HD44232 from the National Institute of Child Health and Human Development (NICHD) awarded to TML as part of a Program Project Grant entitled Prenatal Programming of Reproductive Health and Disease. We thank Douglas D. Doop for expert technical advice and assistance with animal care. In addition, we thank Michael Zakalik and Allie Spencer for assistance with data collection. M.M. Maloney prepared the figures. J.C. Loehlin provided valuable suggestions about the paper. A preliminary report of this work was presented at a meeting of the Acoustical Society of America (McFadden et al., 2008a).
References
- Bilger R, Matthies ML, Hammel DR, Demorest ME. Genetic implications of gender differences in the prevalence of spontaneous otoacoustic emissions. J Speech Hear Res. 1990;33:418–432. doi: 10.1044/jshr.3303.418. [DOI] [PubMed] [Google Scholar]
- Bormann CL, Smith GD, Lee TM. Prenatal testosterone and dihydrotestosterone exposure alters ovine testes development. J Andrology. 2008 doi: 10.1530/REP-10-0210. *submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman DM, Brown DK, Kimberley BP. An examination of gender differences in DPOAE phase delay measurements in normal hearing human adults. Hear Res. 2000;142:1–11. doi: 10.1016/s0378-5955(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Burns EM, Arehart KH, Campbell SL. Prevalence of spontaneous otoacoustic emissions in neonates. J Acoust Soc Am. 1992;91:1571–1575. doi: 10.1121/1.402438. [DOI] [PubMed] [Google Scholar]
- Burns EM, Campbell SL, Arehart KH. Longitudinal measurements of spontaneous otoacoustic emissions in infants. J Acoust Soc Am. 1994;95:385–394. doi: 10.1121/1.408330. [DOI] [PubMed] [Google Scholar]
- Cacace AT, McClelland WA, Weiner J, McFarland DJ. Individual differences and the reliability of 2F1-F2 distortion-product otoacoustic emissions: Effects of time-of-day, stimulus variables, and gender. J Speech Hear Res. 1996;39:1138–1148. doi: 10.1044/jshr.3906.1138. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psych Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Darlington RB. Regression and Linear Models. McGraw-Hill; New York: 1990. [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear Res. 1983;9:79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Dhar S, Long GR, Culpepper NB. The dependence of the distortion product 2f1-f2 on primary levels in non-impaired human ears. J Speech Lang Hear Res. 1998;41:1307–1318. doi: 10.1044/jslhr.4106.1307. [DOI] [PubMed] [Google Scholar]
- Frank LG, Glickman SE, Licht P. Fatal sibling aggression, precocial development, and androgens in neonatal spotted hyenas. Science. 1991;252:702–704. doi: 10.1126/science.2024122. [DOI] [PubMed] [Google Scholar]
- Gaskill SM, Brown AM. The behavior of the acoustic distortion product, 2f1-f2, from the human ear and its relation to auditory sensitivity. J Acoust Soc Am. 1990;88:821–839. doi: 10.1121/1.399732. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Frank LG, Pavgi S, Licht P. Hormonal correlates of “masculinization” in female spotted hyaenas (Crocuta crocuta). 1. Infancy to sexual maturity. J Reprod Fertil. 1992;95:451–462. doi: 10.1530/jrf.0.0950451. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim SH, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear Res. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Guven S, Tas A, Adali MK, Yagiz R, Alagol A, Uzun C, Koten M, Karasalihoglu AR. Influence of anaesthetic agents on transient evoked otoacoustic emissions and stapedius reflex threshold. J Laryngol Otol. 2006;120:10–15. doi: 10.1017/S0022215105004810. [DOI] [PubMed] [Google Scholar]
- Hatzopoulos S, Petruccelli J, Laurell G, Finesso M, Martini A. Evaluation of anesthesia effects in a rat animal model using otoacoustic emission protocols. Hear Res. 2002;170:12–21. doi: 10.1016/s0378-5955(02)00448-3. [DOI] [PubMed] [Google Scholar]
- Hine JE, Ho C, Slaven A, Thornton ARD. Comparison of transient evoked otoacoustic emission thresholds recorded conventionally and using maximum length sequences. Hear Res. 2001;156:104–114. doi: 10.1016/s0378-5955(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Jacobson M, Kim SH, Romney J, Zhu X, Frisina RD. Contralateral suppression of distortion-product otoacoustic emissions declines with age: A comparison of findings in CBA mice with human listeners. Laryngoscope. 2003;113:1707–1713. doi: 10.1097/00005537-200310000-00009. [DOI] [PubMed] [Google Scholar]
- Jordan CL, DonCarlos L. Androgens in health and disease: An overview. Horm Behav. 2008;53:589–595. doi: 10.1016/j.yhbeh.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Evidence of mechanical nonlinearity and frequency selective wave amplification in the cochlea. Ann Otol Rhinol Laryngol. 1979;224:37–45. doi: 10.1007/BF00455222. [DOI] [PubMed] [Google Scholar]
- Kettembeil S, Manley GA, Siegl E. Distortion-product otoacoustic emissions and their anaesthesia sensitivity in the European Starling and the chicken. Hear Res. 1995;86:47–62. doi: 10.1016/0378-5955(95)00053-7. [DOI] [PubMed] [Google Scholar]
- Köppl C. Otoacoustic emissions as an indicator for active cochlear mechanics: a primitive property of vertebrate auditory organs. In: Manley GA, Klump GM, Köppl C, Fastl H, Oeckinghaus H, editors. Advances in Hearing Research. World Scientific; Singapore: 1995. pp. 207–218. [Google Scholar]
- Malcolm KD, Jackson LM, Bergeon C, Lee TM, Padmanabhan V, Foster DL. Long-term exposure of female sheep to physiologic concentrations of estradiol: Effects on the onset and maintenance of reproductive function, pregnancy, and social development in female offspring. Biol Reprod. 2006;75:844–852. doi: 10.1095/biolreprod.106.053264. [DOI] [PubMed] [Google Scholar]
- Martin GK, Lonsbury-Martin BL, Probst R, Coats AC. Spontaneous otoacoustic emissions in the nonhuman primate: a survey. Hear Res. 1985;20:91–95. doi: 10.1016/0378-5955(85)90062-0. [DOI] [PubMed] [Google Scholar]
- Martin GK, Stagner BB, Jassir D, Telischi FF, Lonsbury-Martin BL. Suppression and enhancement of distortion-product otoacoustic emissions by interference tones above f2. I. Basic findings in rabbits. Hear Res. 1999;136:105–123. doi: 10.1016/s0378-5955(99)00119-7. [DOI] [PubMed] [Google Scholar]
- Masterton B, Heffner H, Ravizza R. The evolution of human hearing. J Acoust Soc Amer. 1969;45:966–985. doi: 10.1121/1.1911574. [DOI] [PubMed] [Google Scholar]
- McFadden D. A masculinizing effect on the auditory systems of human females having male co-twins. Proc Natl Acad Sci USA. 1993;90:11900–11904. doi: 10.1073/pnas.90.24.11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D. Masculinizing effects on otoacoustic emissions and auditory evoked potentials in women using oral contraceptives. Hear Res. 2000;142:23–33. doi: 10.1016/s0378-5955(00)00002-2. [DOI] [PubMed] [Google Scholar]
- McFadden D. Masculinization effects in the auditory system. Arch Sex Behav. 2002;31:93–105. doi: 10.1023/a:1014087319682. [DOI] [PubMed] [Google Scholar]
- McFadden D. What do sex, twins, spotted hyenas, ADHD, and sexual orientation have in common? Perspect Psychol Sci. 2008;3:309–323. doi: 10.1111/j.1745-6924.2008.00082.x. [DOI] [PubMed] [Google Scholar]
- McFadden D, Loehlin JC, Pasanen EG. Additional findings on heritability and prenatal masculinization of cochlear mechanisms: Click-evoked otoacoustic emissions. Hear Res. 1996;97:102–119. [PubMed] [Google Scholar]
- McFadden D, Martin GK, Stagner BB, Maloney MM. Sex differences in distortion-product and transient-evoked otoacoustic emission compared. J Acoust Soc, Am. 2008c doi: 10.1121/1.3037231. submitted* [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Mishra R. On the relation between hearing sensitivity and otoacoustic emissions. Hear Res. 1993;71:208–213. doi: 10.1016/0378-5955(93)90036-z. [DOI] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Comparison of the auditory systems of heterosexuals and homosexuals: Click-evoked otoacoustic emissions. Proc Natl Acad Sci USA. 1998;95:2709–2713. doi: 10.1073/pnas.95.5.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Spontaneous otoacoustic emissions in heterosexuals, homosexuals, and bisexuals. J Acoust Soc Am. 1999;105:2403–2413. doi: 10.1121/1.426845. [DOI] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Otoacoustic emissions and quinine sulfate. J Acoust Soc Am. 1994;95:3460–3474. doi: 10.1121/1.410022. [DOI] [PubMed] [Google Scholar]
- McFadden D, Shubel E. The relationships between otoacoustic emissions and relative lengths of fingers and toes in humans. Horm Behav. 2003;43:421–429. doi: 10.1016/s0018-506x(03)00014-x. [DOI] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG, Raper J, Lange HS, Wallen K. Sex differences in otoacoustic emissions measured in rhesus monkeys (Macaca mulatta) Horm Behav. 2006a;50:274–284. doi: 10.1016/j.yhbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG, Valero MD, Roberts EK, Lee TM. Otoacoustic emissions in sheep (Ovis aries): Sex differences and prenatal androgen effects. J Acoust Soc Am Abstract. 2008a;123(Pt 2):3855–3856. [Google Scholar]
- McFadden D, Pasanen EG, Valero MD, Roberts EK, Lee TM. Dissociation between distortion-product and click-evoked otoacoustic emissions in sheep (Ovis aries) J Acoust Soc Am. 2008b doi: 10.1121/1.2982402. submitted* [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG, Weldele ML, Glickman SE, Place NJ. Masculinized otoacoustic emissions in female spotted hyenas (Crocuta crocuta) Horm Behav. 2006b;50:285–292. doi: 10.1016/j.yhbeh.2006.03.013. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Henselman LW, Zheng XY. Sex differences in auditory sensitivity of chinchillas before and after exposure to impulse noise. Ear Hear. 1999;20:164–174. doi: 10.1097/00003446-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Morlet T, Lapillone A, Ferber C, Duclaux R, Sann L, Putet G, Salle B, Collet L. Spontaneous otoacoustic emissions in preterm neonates: Prevalence and gender effects. Hear Res. 1995;90:44–54. doi: 10.1016/0378-5955(95)00144-4. [DOI] [PubMed] [Google Scholar]
- Morlet T, Perrin E, Durrant JD, Lapillone A, Ferber C, Duclaux R, Putet G, Collet L. Development of cochlear active mechanisms in humans differs between gender. Neurosci Lett. 1996;220:49–52. doi: 10.1016/s0304-3940(96)13226-2. [DOI] [PubMed] [Google Scholar]
- Moulin A, Collet L, Veuillet E, Morgon A. Interrelations between transiently evoked otoacoustic emissions, spontaneous otoacoustic emissions and acoustic distortion products in normally hearing subjects. Hear Res. 1993;65:216–233. doi: 10.1016/0378-5955(93)90215-m. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol. 2006;246:165–174. doi: 10.1016/j.mce.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Pasanen EG, McFadden D. An automated procedure for identifying spontaneous otoacoustic emissions. J Acoust Soc Am. 2000;108:1105–1116. doi: 10.1121/1.1287026. [DOI] [PubMed] [Google Scholar]
- Probst R, Lonsbury-Martin BL, Martin GK. A review of otoacoustic emissions. J Acoust Soc Am. 1991;89:2027–2067. doi: 10.1121/1.400897. [DOI] [PubMed] [Google Scholar]
- Roberts EK, Padmanabhan V, Lee TM. Differential effects of prenatal testosterone on phenotypic and behavioral masculinization and defeminization of female sheep. Biol Reprod. 2008 doi: 10.1095/biolreprod.107.067074. published online so far. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Larkin K, Schrunk JM, Stormshak F. Sexual partner preference, hypothalamic morphology and aromatase in rams. Physiol Behav. 2004;83:233–245. doi: 10.1016/j.physbeh.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ., Jr Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. J Acoust Soc Am. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Strickland EA, Burns EM, Tubis A. Incidence of spontaneous otoacoustic emissions in children and infants. J Acoust Soc Am. 1985;78:931–935. doi: 10.1121/1.392924. [DOI] [PubMed] [Google Scholar]
- Talmadge CL, Long GR, Murphy WJ, Tubis A. New off-line method for detecting spontaneous otoacoustic emissions in human subjects. Hear Res. 1993;71:170–182. doi: 10.1016/0378-5955(93)90032-v. [DOI] [PubMed] [Google Scholar]
- Torre P, Fowler CG. Age-related changes in auditory function of rhesus monkeys (Macaca mulatta) Hear Res. 2000;142:131–140. doi: 10.1016/s0378-5955(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Valero MD, Pasanen EG, McFadden D, Ratnam R. Distortion product otoacoustic emissions in the common marmoset (Callithrix jacchus): Parameter optimization. Hear Res. 2008 doi: 10.1016/j.heares.2008.05.006. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: Comparative aspects of steroid hormone action. In: Pfaff D, Arnold A, Etgen A, Farhbach S, Rubin R, editors. Hormones, Brain and Behavior. Academic Press; New York: 2002. pp. 385–423. [Google Scholar]
- Whitehead ML, Lonsbury-Martin BL, Martin GK, McCoy MJ. Otoacoustic emissions: Animal models and clinical observations. In: van de Water TR, Popper AN, Fay RR, editors. Clinical Aspects of Hearing, Springer Handbook of Auditory Research. Vol. 7. Springer-Verlag; New York: 1996. pp. 199–257. [Google Scholar]
- Wier CC, Pasanen EG, McFadden D. Partial dissociation of spontaneous otoacoustic emissions and distortion products during aspirin use in humans. J Acoust Soc Am. 1988;84:230–237. doi: 10.1121/1.396970. [DOI] [PubMed] [Google Scholar]
- Wollack CH. The auditory acuity of the sheep (Ovis aries) J Aud Res. 1963;3:121–132. [Google Scholar]
- Yu HK, Cabalum G, Jansen CA, Buster JE, Nathanielsz PW. Androstenedione, testosterone, and estradiol concentrations in fetal and maternal plasma in late pregnancy in the sheep. Endocrinology. 1983;113:2216–2220. doi: 10.1210/endo-113-6-2216. [DOI] [PubMed] [Google Scholar]