Abstract
Objective:
To experimentally test whether using pictographs (image matrices), incremental risk formats, and varied risk denominators would influence perceptions and comprehension of side effect risks in an online decision aid about prophylactic use of tamoxifen to prevent primary breast cancers.
Methods:
We recruited 631 women with elevated breast cancer risk from two healthcare organizations. Participants saw tailored estimates of the risks of 5 side effects: endometrial cancer, blood clotting, cataracts, hormonal symptoms, and sexual problems. Presentation format was randomly varied in a three factor design: (A) Risk information was displayed either in pictographs or numeric text; (B) presentations either reported total risks with and without tamoxifen or highlighted the incremental risk most relevant for decision making; and (C) risk estimates used 100 or 1000 person denominators. Primary outcome measures included risk perceptions and gist knowledge.
Results:
Incremental risk formats consistently lowered perceived risk of side effects but resulted in low knowledge when displayed by numeric text only. Adding pictographs, however, produced significantly higher comprehension levels.
Conclusions:
Pictographs make risk statistics easier to interpret, reducing biases associated with incremental risk presentations.
Practice Implications:
Including graphs in risk communications is essential to supporting an informed treatment decision making process.
Keywords: risk communication, decision aids, cognitive biases, patient education
1. Introduction
According to the Cochrane Collaboration, decision aids are interventions designed to help people make specific and deliberative choices by providing information about the options and outcomes relevant to the person's health condition.[1] Their basic purpose is to help patients understand and consider the probable benefits and risks of different medical interventions and to actively participate in making informed decisions about such interventions.[2] In particular, a key aim of many decision aids is to modify patients' unrealistic expectations (e.g., elevated beliefs about the likelihood of a good outcome) by presenting specific probability information regarding both good and bad health outcomes of their decisions and by describing these outcomes in imaginable and identifiable formats.[1] Previous research has demonstrated that the use of decision aids can lead to increased correspondence between patients' medical decisions and their preferences.[3] Decision aids have also been successful in reducing uncertainty and decisional conflict.[4]
In order to provide balance against patient's natural inclination to focus on the benefits of potential medical treatments, a central part of many decision aids is a thorough discussion of the risks associated with interventions. Little consensus exists, however, regarding the optimal formats to use in such presentations. For example, research has compared the use of frequency vs. probability formats,[5, 6] and examined different types of graphical ways to display risk.[7-10] The International Patient Decision Aids Standards (IPDAS) Collaboration does provide some guidance: It concludes that decision aids should use multiple methods to display probabilities (e.g., words, numbers, diagrams).[11] Because comparisons of outcome probabilities can be influenced by variations in the denominator,[12, 13] the IPDAS recommendations also suggest the use of consistent risk denominators.[11] For example, comparing 6 out of 100 to 18 out of 1000 is difficult, whereas comparing 60 out of 1000 to 18 out of 1000 is much simpler. Unfortunately, the IPDAS recommendations do not resolve three important design questions relevant to communications of medication side effects: (1) whether incremental risk presentations (described in greater detail below) are appropriate for communication of side effect risks to patients, (2) which risk denominators lead to better comprehension, and (3) whether the use of pictographs to visually display risks improves knowledge or guards against undesirable biases.
First, in previous survey research we compared the commonly used “total risk” approach to communicating treatment side effect risks, in which patients were shown the total risk of experiencing complications both with and without the medication, to a novel “incremental risk” approach that highlighted the change in risk caused by taking the medication. We showed that survey participants who read hypothetical scenarios describing medication side effects in incremental risk terms perceived side effects as less likely and were significantly less worried than those who received equivalent data presented only as the total risk of experiencing the same conditions.[14] More importantly, incremental risk formats prevented order effects from biasing risk perceptions. [14] However, our prior study used hypothetical decisions and did not assess comprehension of the information presented. Given that incremental risk is a relatively complex concept to communicate, further research in an actual patient sample was clearly warranted.
Second, regardless of whether a decision aid uses total risk or incremental risk formats, the choice of risk denominator can also bias risk perceptions. Reactions to identical risk percentages can change depending on whether it is presented as out of 10, out of 100, or out of 1000, and prior “ratio-bias” research has generally found that the larger the denominator, the larger the perceived risk.[12, 13] Side effect risks often are relatively small (less than 20%) and thus perceptions of such risks may be particularly susceptible to such denominator effects. It was unknown, however, whether actual patients would show ratio-biases when considering risk information directly relevant to their healthcare.
Lastly, while many researchers have suggested that the use of visual displays can improve risk communication,[7, 9, 15] a growing literature suggests that pictographs (image matrices) may have particular advantages. Pictographs transform percentages into discrete units,[10] clarify part-whole relationships,[10] appear to be one of the easiest formats for people to process,[8, 16] and have been shown to reduce the degree that side effect risks prevent people from considering beneficial treatments.[9] Our own research has also shown that pictographs limit the biases induced by the presence of powerful anecdotal narratives.[17] Yet again, much of the prior research, including our own, has been based on hypothetical scenarios presented to members of the general public. There remained a need to assess the external validity of these findings for patients who are actively making decisions regarding their own healthcare and to explore potential interactions between pictographs and other design factors.
The “Guide to Decide” project was implemented to provide guidance for decision aid developers and other risk communicators by simultaneously testing all three of these risk communication design issues in an online decision aid for women at elevated risk for developing breast cancer. The “Guide to Decide: Making an Informed Decision About Tamoxifen” program educated women about the risks and benefits of using tamoxifen to reduce the risk of primary breast cancers. By experimentally varying the formats used to present the information about the potential side effects of tamoxifen, we were able to directly assess the influence of each presentation format on women's perceptions and knowledge of these risk statistics.
We had four specific hypotheses, two about risk perceptions (H1 & H2) derived from our previous research[14] and two new hypotheses about risk knowledge (H3a & H3b).
H1: Respondents receiving information about side effects in incremental risk formats will report lower levels of perceived risk than respondents viewing the same information in total risk formats.
H2: Respondents viewing risk information presented using larger risk denominators (e.g., 1000 units) will perceive greater risk of side effects than those who view presentations with smaller risk denominators (e.g., 100 units).
H3a: Because incremental risk is a more complicated concept to grasp than one's total risk, respondents viewing incremental risk formats may show decreased gist knowledge of whether taking tamoxifen increases their risk, as compared to respondents viewing total risk formats.
H3b: Because pictographs visually clarify the relationship between the incremental risk and the total risk, if knowledge deficits are observed per H3a, respondents who are presented with incremental risk information in pictograph form will show smaller knowledge deficits than those who see incremental risk information without a pictograph.
We did not hypothesize any significant effects of pictographs on risk perceptions or risk denominators on risk knowledge.
2. Methods
2.1 Participants
Participants were recruited from two large U.S. healthcare organizations in two different states: the Henry Ford Health System in Michigan, and Group Health in Washington state. Potential participants were identified from health plan records and invited to participate by mail. Interested women were instructed to visit the Guide to Decide website, where they were screened for eligibility, and provided informed consent. Eligible women then completed a baseline survey, viewed a tailored decision aid, and completed a follow-up survey. Women were eligible if they were aged 40-74 and their five year risk of developing breast cancer was ≥ 1.66% as estimated by the Gail model.[18] Tamoxifen chemoprevention is not recommended for women with Gail scores less than 1.66%.[19] Participants were minimally compensated by providing them with the choice of $10 gift cards from one of the three major retail chain stores.
2.2 Design
The online decision aid detailed potential risks and benefits of tamoxifen use. With regards to the possible side effects of tamoxifen, participants saw specific estimates of the risk experiencing endometrial cancer, blood clotting, cataracts, hormonal symptoms, and sexual problems. (Respondents who reported having had a hysterectomy were not shown information about endometrial cancer.) These estimates were tailored based on each woman's age and race/ethnicity to maximize the personal relevance of the information provided. To provide context, the risks for the median participant, a 59 year old Caucasian woman, are itemized in Table 1
Table 1.
Side effect risks seen by the median participant, a 59 year old Caucasian woman.
| Number of Women with Each Side Effect in 5 Years (Out of 1000) | |||
|---|---|---|---|
|
Side Effects |
Baseline Risk Without Tamoxifen |
Total Risk With Tamoxifen |
Incremental Risk Of Tamoxifen |
| Endometrial Cancer | 4 | 16 | 12 |
| Cardiovascular | 11 | 20 | 9 |
| Cataracts | 76 | 86 | 10 |
| Sexual Problems | 98 | 110 | 12 |
| Menopausal Symptoms | 777 | 866 | 89 |
Note: All participants initially viewed the baseline risk, but participants were randomized to next see presentations that emphasized either the total risk or the incremental risk with tamoxifen.
Each presentation occurred over two pages: The first page showed the baseline risk of experiencing each condition if the woman did not take tamoxifen (E.g., “Among 1000 women who did not take tamoxifen, 76 (7.6%) would get cataracts); the second, the risk with tamoxifen. Participants could use easily visible “back” and “forward” buttons to facilitate comparisons. The presentation format of the risk information on both pages was randomly varied in a three factor design:
(1) Half of the participants viewed the risk information in a pictograph format (see Figure 1), while the remaining participants saw the same information (specifically, the exact text of the graph legend) in numeric text form only with the number highlighted (Figure 2).
(2) Half of the participants received presentations that reported the incremental risk of experiencing a side effect (e.g., “among 1000 women your age who did take tamoxifen, 10 more women out of 1000 would now get cataracts”). In the pictograph conditions, this was visually illustrated by using a new, stronger color to highlight the incremental risk units in a pictograph that displayed (but did not enumerate) the total number of women who would experience the condition (Figure 1). The remaining half of our participants received pictograph or numerical text presentations that reported only the total risk of experiencing these conditions both with and without taking tamoxifen. (E.g., Page 1: “Among 1000 women who did not take tamoxifen, 76 (7.6%) would get cataracts.” Page 2: “Among 1000 women who did take tamoxifen, 86 (8.6%) would get cataracts.”; see also Figure 2)
(3) Half of the participants received risk estimates presented using a denominator of 100 people (simplifying interpretation of the risk statistics as percentages; see Figure 2), while the remaining participants saw presentations that used a risk denominator of 1000 people (Figure 1). All risks were presented in both frequency and percentage format.
Figure 1.
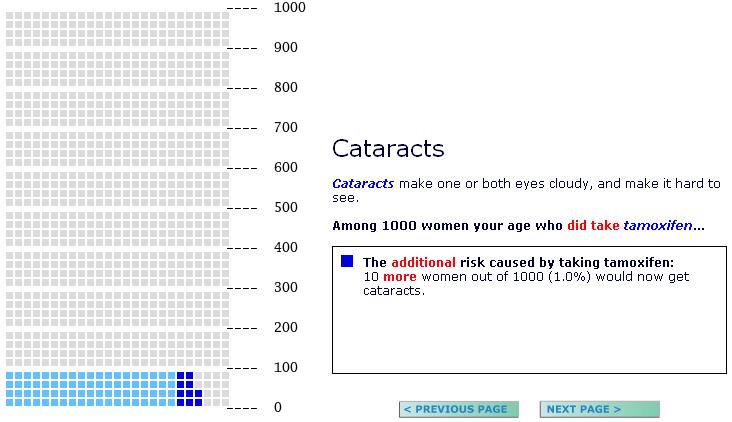
Pictograph display showing a study participant's increased risk of cataracts if she chose to take tamoxifen
Figure 2.
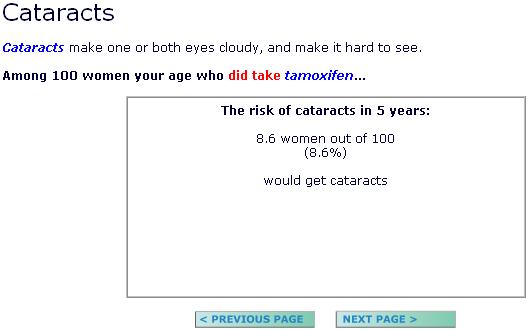
Numeric display showing a study participant's total risk of cataracts if she chose to take tamoxifen
After participants read linearly through all of the sections of the decision aid (and revisited any sections they wished to), they completed a brief set of questions including the main outcome measures discussed below as well as other related measures (e.g., breast cancer anxiety, feelings about the decision aid, intentions to talk to their doctor) not presented here. Participants also completed several individual difference measures. One of particular interest to the present analysis is the Subjective Numeracy Scale (SNS),[20, 21] a validated measure of quantitative ability and of preferences for receiving information in numerical format. The SNS has previously been shown to correlate with the ability to recall and comprehend risk communications.[21]
This design was part of a larger, fractional-factorial design that included two other manipulations of the content of the decision aid not related to how the estimates of side effect risks were presented to participants: (1) the order of content discussion (whether the benefits of tamoxifen were discussed before or after its risks) and (2) the provision of the risks of dying from colon cancer, heart disease, and all causes as statistical context. Controlling for these manipulations in our multivariate analyses had no qualitative impact on any of the results, and hence, for simplicity, we will not include or discuss these factors here. The study was approved by Institutional Review Boards at all sites.
2.3 Outcome Measures
Risk perceptions
We measured participants' perceptions of the risks of tamoxifen using three questions: 1) “Taken all together, how worried would you be about getting any of the above health conditions if you did take tamoxifen,” 2) “Taken all together, how common do you think the above health conditions are for women who take tamoxifen,” and 3) “If you were to choose to take tamoxifen, how likely do you think you would be to experience a side effect?” Participants responded to each question on a 5-point scale, with 1 representing not at all worried / common / likely and 5 representing extremely worried / common / likely. Because these three questions proved to be highly intercorrelated, they were averaged to form a composite measure of risk perceptions. Participants with missing data (N=7) received the average score of the remaining questions. The reliability coefficient (alpha) of the resulting scale was 0.74.
Gist Knowledge
To assess participants' essential knowledge of the side effects discussed in the decision aid, we asked them questions about four of the risks associated with tamoxifen: endometrial cancer, hormonal symptoms, blood clotting, and cataracts. Participants were asked to identify which of the following groups was most likely to experience each of these risks: (1) women who take tamoxifen, (2) women who do not take tamoxifen, (3) both groups are equally likely, or (4) don't know. We scored participants' responses as correct or incorrect and then summed the correct responses, counting unanswered questions as incorrect. We rescaled the scores for participants with hysterectomies (who were not asked the question about endometrial cancer) to match the 0-4 scale of other participants. The reliability coefficient (alpha) of this measure was 0.86.
2.4 Statistical Analysis
We used multivariate analyses of variance (MANOVA) to test for differences in risk perception and knowledge scores across subgroups. These analyses included binary terms for pictographs (vs. numerical text), incremental risk (vs. total risk), and 1000 person denominators (vs. 100), two-way and three-way interaction terms, and participants' numeracy scores (as a continuous covariate). To make the MANOVA results easier to interpret and to illustrate the direction and magnitude of the identified effects, we also calculate predicted risk perception and knowledge scores, varying some factors while holding others at their mean values. All analyses were performed using STATA Version 10.[22]
3. Results
A total of 1,218 women reached the study website and began the eligibility assessment. Of these, 749 met the Gail model criteria. Out of those that met criteria, 663 participants consented to participate, 659 completed the baseline survey, and 631 completed the measures of interest in the posttest survey. Together, the decision aid and the posttest questions took approximately 45 minutes to complete.
Among the 631 women included in the analyses reported here, the average age was 59 (range: 40-74, SD = 7.6), and the majority (94.5%) reported being Caucasian, with 2.2% self-identifying as Black or African-American, 1.1% as Asian, and 2.2% as other, multiracial, or not provided. All of the women in the study were at a high risk for breast cancer, with Gail scores ranging from 1.7 to 17.3 (M = 2.6). Most participants (65.6%) reported having completed a Bachelors' or higher degree. Participants reported relatively high levels of numeracy as well (M=4.5 on the 1-6 scale, SD=0.95), although the correlation between education and numeracy was relatively low (r=0.33), as was expected given the literature on numeracy as an independent construct.[23-26]
3.1 Risk Perceptions
Consistent with both hypothesis H1 and our prior research on the effects of using incremental risk presentations,[14] the MANOVA analyses demonstrated that the use of incremental risk formats has a significant effect on risk perceptions (F = 12.56, p < 0.001). Women perceived side effects to be significantly less common, less likely, and less worrisome when the presentation reported the incremental risk (as opposed to the total risk of experiencing the complication). Contrary to hypothesis H2, however, risk perceptions were not significantly affected by either varying the risk denominator (or the use of pictographs), and no two-way interaction terms were significant. The three-way interaction term was significant (F = 5.30, p = 0.022), indicating that the effect of incremental risk varied somewhat in magnitude when both of the other two factors were adjusted. We also identified a highly significant effect of participants' numeracy scores (F = 8.48, p = 0.004): Higher numeracy scores were correlated with lower perceived risk.
To clarify the magnitude of the incremental risk effect, we used the MANOVA model to estimate risk perception scores, varying the incremental risk factor and associated interaction terms but holding all other variables at mean levels. Predicted perceived risk scores were significantly lower when risk statistics were presented in incremental risk form (Estimated score = 3.03 [95% CI: 2.94, 3.12] versus 3.25 [CI: 3.17, 3.34]).
3.2 Gist Knowledge
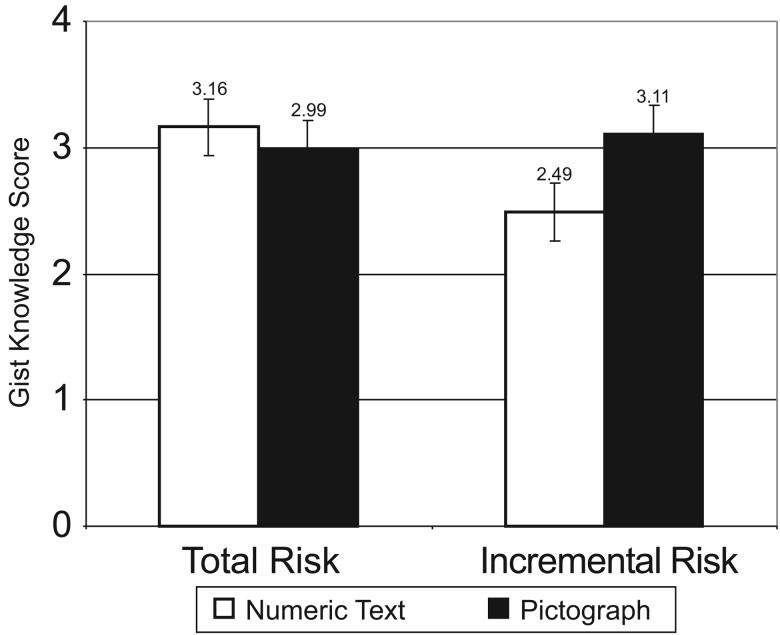
Our MANOVA analysis (Table 2) of women's gist knowledge of the risk information shows a more complicated pattern. To start, we observe an unpredicted mild main effect of pictographs (F = 3.44, p=0.064), with higher knowledge observed for pictograph formats.
Table 2.
MANOVA analysis of participants' gist knowledge scores
| F | p-value | |
|---|---|---|
| Pictograph (vs. Numeric Text) | 3.44 | 0.064 |
| Incremental Risk (vs. Total) | 5.58 | 0.019 |
| 1000 Risk Denominator (vs. 100) | 6.98 | 0.009 |
| Pictograph × Incremental Risk | 11.35 | <0.001 |
| Pictograph × Denominator | 2.52 | 0.113 |
| Incremental Risk × Denominator | 0.58 | 0.447 |
| 3-Way Interaction | 2.02 | 0.155 |
| Numeracy Score (1-6 scale) | 15.32 | <0.001 |
Consistent with Hypothesis H3a, there is also a significant main effect of the use of incremental risk format (F = 5.58, p = 0.019), resulting in lower knowledge scores. Hypothesis H3b is also strongly supported: Given that a main effect of incremental risk was observed, it is fully offset by a highly significant pictograph × incremental risk interaction (F = 11.35, p < 0.001). To clarify these results, Figure 3 shows the predicted gist knowledge scores for each of the types of risk information (total and incremental) in both formats (numeric and pictograph). As the figure shows, according to the model, participants in Guide to Decide who receive total risk presentations, whether text-based or in pictographs, are approximately equally able to correctly identify that tamoxifen increased the risk of the four side effects we asked about. The use of incremental risk formats results in lower knowledge scores among women who received their risk information in numeric text format, but no such decrement exists among women receiving risk information in a pictograph format.
Figure 3.
Predicted gist knowledge scores if pictograph and/or incremental risk formats are used
The MANOVA analysis also shows a significant, unpredicted effect of risk denominator (F =6.98, p = 0.009). Presenting risk statistics using a 1000 person denominator significantly increased knowledge compared to presentations that use a 100 person denominator. The pictograph by denominator interaction, while not significant (F=2.52, p = 0.113), trends in the opposite direction, suggesting that pictographs may also, at least partially, mediate the knowledge deficits resulting from the use of 100 person denominators. Neither the two-way interaction of denominator with incremental risk nor the three-way interaction was significant.
Lastly, we again observe a highly significant main effect of numeracy (F = 15.32, p < 0.001): Lower numeracy scores strongly predicted worse knowledge of the risks of tamoxifen.
4. Discussion and Conclusion
4.1 Discussion
We presented women with tailored statistical estimates of their likelihood of experiencing side effects if they chose to take tamoxifen. We then tested women's ability to accurately report that the risk of each of four conditions (endometrial cancer, blood clotting problems, cataracts, and menopausal symptoms) is higher for women who take tamoxifen compared to those who do not. Participants did not need to report the magnitude of the risk difference, merely its existence and direction. Such “gist” knowledge is a critical precursor of good decision making,[8, 27, 28] since it underlies the risk-benefit tradeoffs inherent in an informed choice about any medication.
Our results show that women's comprehension of risk statistics was notably low when they received risk statistics presented in text-only format that highlighted the incremental risk. When the same information was presented in pictograph format, however, the knowledge deficit was eliminated. A similar, albeit less significant, interaction was observed when we used 100 unit denominators instead of 1000 units. We speculate that these interactions occurred because the visual nature of a pictograph helps to make statistics more concrete and easier to interpret. The incremental risk of developing a side effect is a complex risk statistic representing the difference between the risks with and without medication. In the absence of a visual aid, patients may have a hard time distinguishing their baseline risk, their total risk post-intervention, and the incremental risk caused by the intervention. Pictographs may resolve this confusion because they visually represent the relationships between all of these statistics simultaneously and implicitly clarify how large or small the incremental risk is when compared to the total risk. By contrast, respondents who saw incremental risks in numerical text format could only derive the total risk with tamoxifen by combining the incremental risk with the previously seen baseline risk. As a result, while incremental risk presentations focus attention on the information most important for informed decision making, we believe that decision aid developers should use incremental risk presentations only when risk data will be shown visually.
Our research also provides three additional pieces of guidance for decision aid developers. First, we conclusively demonstrate that incremental risk formats, which previously have been shown to result in lower perceived risk of medication side effects in a hypothetical scenario study,[14] also reduce the risk perceptions of patients making actual therapy decisions. Although we cannot establish the normatively optimal level of perceived risk, our results are consistent with the hypothesis of our previous research: that total risk presentations may elevate risk perceptions because some women fail to realize that they would still face substantial risk whether or not they take medication. Incremental risk formats, while somewhat complex to understand, directly quantify the risk caused by the medical intervention and have previously been shown to debias unwanted order effects.[14] Although incremental risk formats (in pictograph form) did not show any knowledge benefits versus total risk formats in this study, we support their use in communications of side effects on this basis.
Second, contrary to expectations, risk denominator changes did not affect risk perceptions, and neither did the use of pictographs. It may be that the wide range of risks associated with tamoxifen (see Table 1) helped to diffuse any denominator effect in the overall risk perceptions by emphasizing relative differences between risks. However, use of 100 unit denominators was associated with lower comprehension, perhaps due to the fact that readers had to interpret fractions of a percent.
Third, we add to the growing literature supporting the idea that individual numeracy has a strong predictive relationship with people's responses to risk communications.[21, 23, 24, 29] SNS scores predicted not only gist knowledge but also how worrisome, common, and likely women perceived the side effects of tamoxifen to be, with greater numeracy correlated with lower risk perceptions. We speculate that the more numerate that patients are, the better able they are to place new risk statistics into the larger context of the many risks that they face in everyday life and hence be less alarmed by this new information.
A primary limitation of this research is the lack of demographic diversity in our sample. Despite recruiting from a healthcare organization with a significant African-American patient base, we were still unable to recruit more than a handful of African-American women to participate in this research. In addition, our participants were generally highly educated (though not universally numerate). While this sample is similar to that observed in the P-1 trial of tamoxifen,[30] we note that our results may not fully generalize to other populations.
4.2 Conclusion
We believe that this research identifies several important principles for the communication of medication side effects to patients. We draw particular attention to the fact that the use of visual graphics (in our case, a pictograph) improved our risk communication efforts when certain design factors, which were included because they offer other advantages, might otherwise have led to decreased knowledge. For example, using pictographs improved women's ability to comprehend the incremental risk of side effects caused by tamoxifen, the statistic most relevant to these women's decisions about whether benefits of taking tamoxifen outweigh its risks.
4.3 Practice Implications
These findings support the growing consensus among decision aid developers that visual displays of risk are an essential element of decision aids.[11] Their inclusion may help to inoculate patients against potential sources of confusion. Pictographs, in particular, can simultaneously convey both gist impressions of the likelihood that a patient will experience particular conditions as well as specific numerical information. We urge greater consideration of pictographs whenever statistical information is to be provided as part of patient decision aids and other forms of patient-oriented risk communication.
Acknowledgments
Financial support for this study was provided by a Center of Excellence for Cancer Communication Research (CECCR) grant from the National Institutes for Health (P50 CA101451). Dr. Zikmund-Fisher is supported by a career development award from the American Cancer Society (MRSG-06-130-01-CPPB), and Drs. Fagerlin and Smith were supported by Merit Review Entry Program awards from the U.S. Department of Veterans Affairs during this research. The funding agreements ensured the authors' independence in designing the study, interpreting the data, and publishing the report.
Appendix to “Communicating Side Effect Risks in a Tamoxifen Prophylaxis Decision Aid: The Debiasing Influence of Pictographs”
MANOVA analysis of participants risk perception scores
| F | p-value | |
|---|---|---|
| Pictograph (vs. Numeric Text) | 2.86 | 0.091 |
| Incremental Risk (vs. Total) | 12.56 | <0.001 |
| 1000 Risk Denominator (vs. 100) | 1.45 | 0.230 |
| Pictograph × Incremental Risk | 0.01 | 0.944 |
| Pictograph × Denominator | 1.01 | 0.316 |
| Incremental Risk × Denominator | 1.47 | 0.225 |
| 3-Way Interaction | 5.30 | 0.022 |
| Numeracy Score (1-6 scale) | 8.48 | 0.004 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work were presented at the annual meeting of the Society for Medical Decision Making, October 16, 2006 and at the American Psychological Association Science Leadership Conference, December 2, 2006.
References
- 1.O'Connor AM, Stacey D, Entwistle SD, Llewellyn-Thomas H, Rovner D, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2003:CD001431. doi: 10.1002/14651858.CD001431. [DOI] [PubMed] [Google Scholar]
- 2.Fagerlin A, Rovner D, Stableford S, Jentoft C, Wei JT, Holmes-Rovner M. Patient education materials about the treatment of early-stage prostate cancer: A critical review. Annals of Internal Medicine. 2004;140(9):721–8. doi: 10.7326/0003-4819-140-9-200405040-00012. [DOI] [PubMed] [Google Scholar]
- 3.Barry MJ. Health decision aids to facilitate shared decision making in office practice. Ann Intern Med. 2002;136(2):127–35. doi: 10.7326/0003-4819-136-2-200201150-00010. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor AM, Rostom A, Fiset V, Tetroe J, Entwistle V, Llewellyn-Thomas H, et al. Decision aids for patients facing health treatment or screening decisions: Systematic review. Br Med J. 1999;319(7212):731–4. doi: 10.1136/bmj.319.7212.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schapira MM, Davids SL, McAuliffe TL, Nattinger AB. Agreement between scales in the measurement of breast cancer risk perceptions. Risk Anal. 2004;24(3):665–73. doi: 10.1111/j.0272-4332.2004.00466.x. 2004. [DOI] [PubMed] [Google Scholar]
- 6.Schapira MM, Nattinger AB, McHorney CA. Frequency or probability? A qualitative study of risk communication formats used in health care. Medical Decision Making. 2001;21(6):459–67. doi: 10.1177/0272989X0102100604. [DOI] [PubMed] [Google Scholar]
- 7.Lipkus IM, Hollands JG. The visual communication of risk. Journal of the National Cancer Institute Monographs. 1999;(25):149–63. doi: 10.1093/oxfordjournals.jncimonographs.a024191. [DOI] [PubMed] [Google Scholar]
- 8.Feldman-Stewart D, Brundage MD, Zotov V. Further insight into the percetption of quantitative information: Judgments of gist in treatment decisions. Med Decis Making. 2007;27:34–43. doi: 10.1177/0272989X06297101. [DOI] [PubMed] [Google Scholar]
- 9.Waters EA, Weinstein ND, Colditz GA, Emmons KM. Reducing aversion to side effects in preventive medical treatment decisions. J Exp Psychol Appl. 2007;13(1):11–21. doi: 10.1037/1076-898X.13.1.11. [DOI] [PubMed] [Google Scholar]
- 10.Ancker JS, Senathirajah Y, Kikafka R, Starren JB. Design features of graphs in health risk communication: A systematic review. J Am Med Inform Assoc. 2006;13(1):608–18. doi: 10.1197/jamia.M2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collaboration IPDAS IPDAS 2005: Criteria for Judging the Quality of Patient Decision Aids. 2005 cited; Available from: http://www.ipdas.ohri.ca/IPDAS_checklist.pdf.
- 12.Denes-Raj V, Epstein S, Cole J. The generality of the ratio-bias phenomenon. Personality and Social Psychology Bulletin. 1995;21(10):1083–92. [Google Scholar]
- 13.Yamagishi K. When a 12.86% mortality is more dangerous than 24.14%: Implications for risk communication. Applied Cognitive Psychology. 1997;11:495–506. [Google Scholar]
- 14.Zikmund-Fisher B, Fagerlin A, Roberts TR, Derry HA, Ubel PA. Alternate methods of framing information about medication side effects: Incremental risk versus total risk occurence. J Health Commun. 2008;13(2):107–24. doi: 10.1080/10810730701854011. [DOI] [PubMed] [Google Scholar]
- 15.Waters EA, Weinstein ND, Colditz GA, Emmons K. Formats for improving risk communication in medical tradeoff decisions. J Health Commun. 2006;11:167–82. doi: 10.1080/10810730500526695. [DOI] [PubMed] [Google Scholar]
- 16.Price M, Cameron R, Butow P. Communicating risk information: The influence of graphical display format on quantitative information perception - accuracy, comprehension and preferences. Patient Educ Couns. 2007;69:121–8. doi: 10.1016/j.pec.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Fagerlin A, Wang C, Ubel PA. Reducing the influence of anectodal reasoning on people's health care decisions: Is a picture worth a thousand statistics? Medical Decision Making. 2005;25(4):398–405. doi: 10.1177/0272989X05278931. [DOI] [PubMed] [Google Scholar]
- 18.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 19.U. S. Preventive Services Task Force . Chemoprevention of breast cancer: recommendations and rationale. Agency for Healthcare Research and Quality; Rockville, MD: 2002. [Google Scholar]
- 20.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry H, Smith DM. Measuring numeracy without a math test: Development of the subjective numeracy scale (SNS) Med Decis Making. 2007;27(5):672–80. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 21.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the subjective numeracy scale (SNS): Effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making. 2007;27(5):663–71. doi: 10.1177/0272989X07303824. [DOI] [PubMed] [Google Scholar]
- 22.Stata Statistical Software. 10 ed. Stata Corporation; College Station, Texas: 2007. [Google Scholar]
- 23.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127(11):966–72. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 25.Peters E, Vastfjall D, Slovic P, Mertz CK, Mazzocco K, Dickert S. Numeracy and decision making. Psychol Sci. 2006;17(5):407–13. doi: 10.1111/j.1467-9280.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 26.Woloshin S, Schwartz LM, Moncur M, Gabriel S, Tosteson AN. Assessing values for health: Numeracy matters. Med Decis Making. 2001;21(5):382–90. doi: 10.1177/0272989X0102100505. [DOI] [PubMed] [Google Scholar]
- 27.Brainerd CJ, Reyna VF. Memory independence and memory interference in cognitive development. Psychol Rev. 1993;100(1):42–67. doi: 10.1037/0033-295x.100.1.42. [DOI] [PubMed] [Google Scholar]
- 28.Brainerd CJ, Gordon LL. Development of verbatim and gist memory for numbers. Dev Psychol. 1994;30(2):163–77. [Google Scholar]
- 29.Fagerlin A, Ubel PA, Smith DM, Zikmund-Fisher BJ. Making numbers matter: Present and future research in risk communication. Am J Health Behav. 2007;31(Suppl 1):S47–S56. doi: 10.5555/ajhb.2007.31.supp.S47. [DOI] [PubMed] [Google Scholar]
- 30.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]