Abstract
Food intake is controlled by peripheral signals from the gastrointestinal tract and adipocytes, which are integrated within the central nervous system. There is evidence that signals from the GI tract are modulated by long term changes in diet, possibly leading to hyperphagia and increased body weight. We tested the hypothesis that diet-induced obese-prone (DIO-P) and obese-resistant (DIO-R) mice strains differ in the long term adaptive response of the gut-brain pathway to a high fat diet. Immunochemical detection of Fos protein was used as a measure of neuronal activation in the nucleus of the solitary tract (NTS) in response to intragastric administration of lipid in DIO-P (C57Bl6) and DIO-R (129sv) mouse strains maintained on chow or high fat, high energy diets (45% or 60% kcal from fat). Intragastric lipid administration activated neurons in the NTS in both DIO-P and DIO-R mice; the number of activated neurons was significantly greater in DIO-P than in DIO-R mice (P<0.001). However, lipid-induced activation of NTS neurons in DIO-P mice was attenuated by ≈ 30% after maintenance on either 45% or 60% HF diet, for 4 or 8 weeks, compared to chow fed controls (P<0.05). In contrast, in DIO-R mice, maintenance on a HF diet (45% or 60%) had no effect on lipid-induced activation of NTS neurons. These results demonstrate that DIO-P and DIO-R mice strains differ in the adaptation of the pathway to long term ingestion of high fat diets, which may contribute to decrease satiation and increased food intake.
1. Introduction
The gut-brain axis plays an important role in nutrient detection and in control of short term food intake (Schwartz 2000). For example, lipid in the gastrointestinal tract is detected via release of CCK and activation of CCK1 receptors on vagal afferent nerve terminals in close apposition to endocrine cells (Raybould 1999). The CCK1R dependent pathway, together with activation of vagal afferents in response to other gut peptides that can decrease food intake, seems to be important primarily in short-term control of food intake. However, evidence is accumulating to suggest that alteration in the function of this pathway maybe involved in long term control of food intake and body weight. Maintenance of rats on a high fat (HF) diet causes decreased hindbrain sensitivity to lipid (oleate) and to CCK (Covasa and Ritter 1998; Covasa and Ritter 1999); this occurs after ingesting the HF diet for only 2 weeks and even in the absence of obesity, when fed an isocaloric HF diet (Covasa and Ritter 2000; Paulino et al, 2008). It has been suggested that the decrease in vagal afferent sensitivity in response to lipid may, in part, be responsible for increased food intake when rodents, or humans, ingest high fat diets (Savastano and Covasa, 2005).
Different strains of mice have been classified as “obesity prone” or “obesity resistant” (West et al, 1992), dependent on their propensity for weight gain when placed on high fat, high energy diets. However, it is not clear what factors are responsible for these phenotypic differences. Mice maintained on a HF diet can be grouped into two main categories according to their ability to gain weight; mouse strains such as AKR/J, C57BL/6J, DBA/2J, and A/J have been shown to rapidly gain weight on HF diet and have been termed diet-induced obesity prone (DIO-P); in contrast, strains 129sv, SWR/J, I/STN and SJL/J have been termed diet-induced obesity resistant (DIO-R) due to their relative resistance to weight gain when on HF diets (West et al. 1992). Whether there is any difference in the response of the gut-brain axis to lipid in different mice strains, either DIO-P or DIO-R, or adaptation to HF diets, has not been investigated.
We tested the hypothesis that adaptation of the lipid-induced activation of the vagal afferent pathway to long term maintenance on a HF diet differ in DIO-P and DIO-R mice strains. We determined the effect of intragastric gavage of lipid on activation of neurons in the NTS, the region where vagal afferents terminate, in two mouse strains; one classified as DIO-P (C57BL/6) and one classified as DIO-R (129sv) (Almind and Kahn 2004; West et al. 1992). We investigated the effect of long term consumption (4 and 8 weeks) of two diets with increased levels of fat (45% and 60% kcal/fat) which cause obesity in mice (Van Heek et al. 1997).
2. Results
Effect of high fat diets on body weight and adiposity
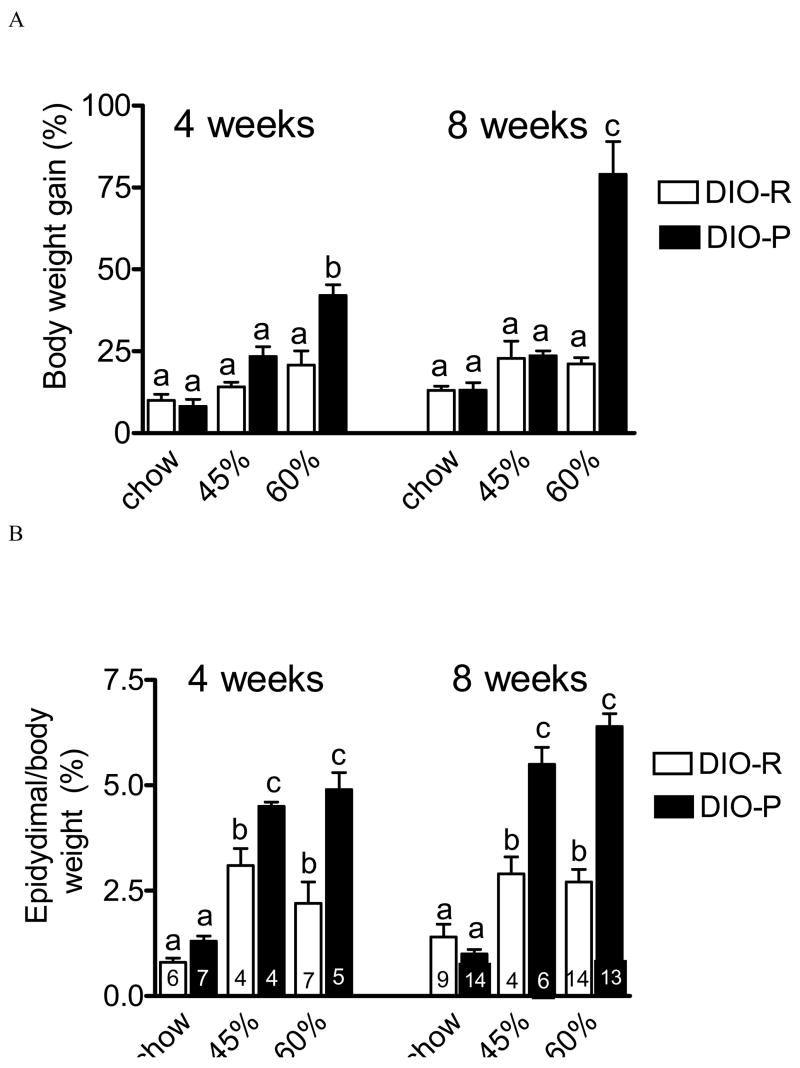
There was no significant difference in body weight gain in DIO-P and DIO-R mice maintained on chow for either 4 or 8 weeks (NS, n=5–14 in each group) (Fig 1 A). Maintenance on a diet containing 45% kcal from fat (45% HF) caused a tendency for increased body weight gain in both strains after either 4 or 8 weeks on the diet, but this did not reach statistical significance (Fig 1 A; NS, n=3–6 in each group). However, maintenance on a 60% HF diet resulted in a significant increase in body weight gain at both 4 and 8 weeks in DIO-P, but not DIO-R mice (Fig 1A). Thus, DIO-P mice gained significantly more weight on the 60% HF diet after 4 weeks and 8 weeks compared to DIO-R mice (4 weeks, p<0.05, 8 weeks 60% p<0.01, n=4–14 in each group).
Figure 1.
Effect of maintenance on chow (C) or high fat diets (45% or 60% calories from fat) on body weight gain and adiposity in two strains of mice, C57Bl/6J (DIO-P) or 129sv (DIO-R). A. Body weight gain (expressed as % of initial body weight) in DIO-P mice and DIO-R mice maintained on chow, 45% HF or 60% HF diets for 4 weeks or 8 weeks. Maintenance on 60% HF diet for 4 or 8 weeks significantly increased body weight in DIO-P but not DIO-R mice, compared to chow-fed mice (4 weeks, p<0.05, n=; 8 weeks, p<0.001; n=5–14 in each group). B. Adiposity (weight of epididymal fat pad expressed as % of final body weight) following four or eight weeks of maintenance on either the 45% HF or 60% HF diet in DIO-R and DIO-P mice, compared to chow-fed controls (45% p<0.05 and 60% p<0.01, n=5–14). Maintenance on the 45% HF or the 60% HF diet for 8 weeks caused significantly greater adiposity in DIO-P mice compared to DIO-R mice (P<0.001, n=4–8). Columns with different letters are significantly different from each other.
Examination of the epididymal fat pad data revealed differences between diets and phenotype; data is expressed as a % of final body weight (Fig 1B). DIO-R mice maintained on either the 45% HF or 60% HF diet for 4 or 8 weeks had significantly larger epididymal fat pad mass compared to mice maintained on the chow diet; however, there was no significant difference between either the diet or time on the diets. Maintenance of DIO-P mice on either 45% or 60% HF diets for 4 or 8 weeks caused a significant increase in epididymal fat pad mass (p<0.05 vs chow). There was no significant difference between either diet or time point in DIO-P mice. However, there was a significant increase in fat pad weight in DIO-P compared to DIO-R mice at both time points and on both diets (chow versus 45% or 60% HF diets; p<0.05).
Effect of high fat diets on intestinal lipid-induced activation NTS neurons
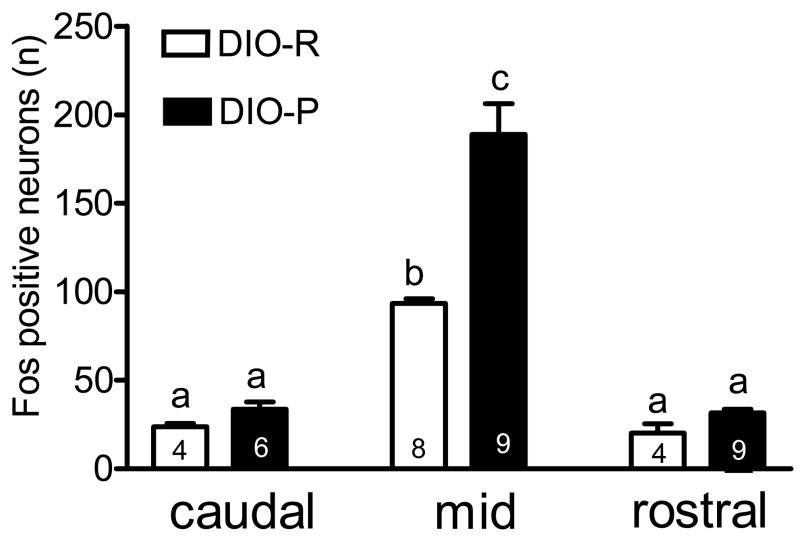
Intragastric gavage with lipid (0.2 ml of 20% lipid; 40 mgs) activated neurons in the NTS of mice as determined by expression of fos protein; in both strains of mice, activation was highest in the mid-NTS as previously described (Whited, Thao et al. 2006) (Figure 2). There was a significantly greater number of fos-positive neurons in mid-NTS in response to lipid gavage in DIO-P mice compared to DIO-R mice (P<0.001, n=8–9 in each group). There was no significant difference between strains in the number of fos-positive cells in the mid-NTS in response to saline as previously described (Whited et al. 2006, 2007) (NS, n=3, data not shown).
Figure 2.
Activation of neurons in the nucleus of the solitary tract (NTS) in DIO-P and DIO-R mice in response to gastrointestinal lipid. Mice were maintained on a chow diet, fasted overnight and gavaged with lipid. Activated neurons were identified by immunoreactivity for fos protein. There was a significantly greater number of fos-positive neurons in the NTS in response to intestinal lipid in DIO-P mice compared to DIO-R mice (p<0.01 and p<0.001, n=4–9 in each group).
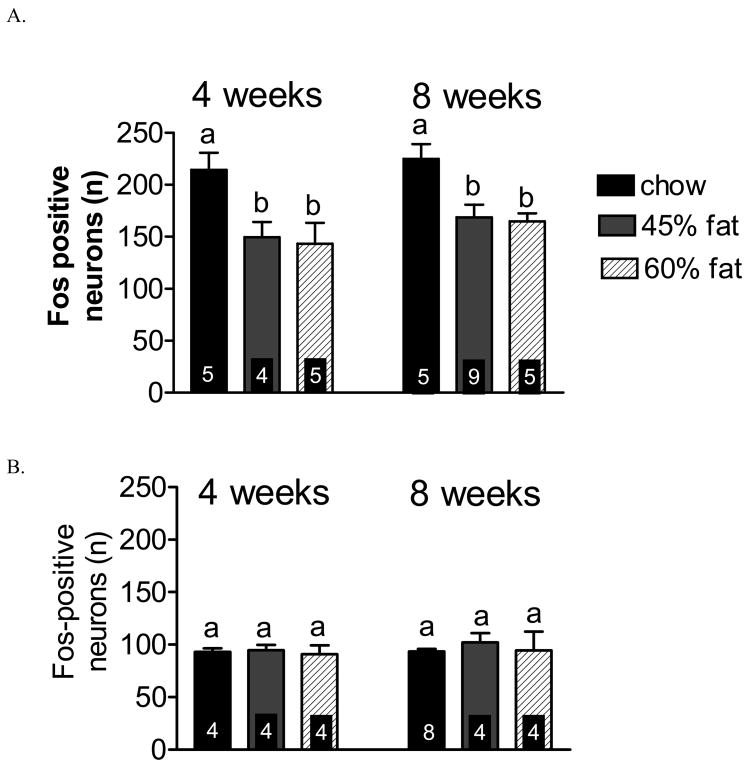
Maintenance on either the 45% or 60% HF diet attenuated lipid-induced hindbrain activation in the mid NTS in DIO-P mice, but not in DIO-R mice (Figure 3). Lipid-induced hindbrain activation in DIO-P mice was attenuated by 30% and 33% after maintenance on HF diet (45% HF or 60% HF, respectively) for 4 weeks compared to chow fed controls (P<0.05, n=4–5), whereas DIO-R mice exhibited no change in lipid-induced hindbrain activation, regardless of diet (NS, n=4–5) (Figure 3). Maintenance on HF diet for 8 weeks similarly attenuated vagal sensitivity to lipid in DIO-P mice (P<0.05, n=5–9) (Figure 3), but not in DIO-R mice (NS, n= 4–8). There was no significant difference in the number of fos-activated neurons on either diet or time point in the DOI-P mice.
Figure 3.
The effect of maintenance on chow, 45% or 60% high fat diets on lipid-induced activation of neurons in the mid region of the nucleus of the solitary tract (−7.76 – 7.32 mm, NTS) in DIO-P and DIO-R mice. A. There was a significant decrease in the number of fos-positive neurons in the NTS in response to intestinal lipid in DIO-P mice following 4 weeks or 8 weeks maintenance on either 45% HF or 60% HF diet compared to chow (P<0.05, n=4–5). B. There was no significant difference in activation of fos-positive neurons in response to lipid in DIO-R mice after maintenance on 45% HF or 60% HF diet for 4 weeks or 8 weeks (NS, n=4–8).
3. Discussion
Perfusion of the gastrointestinal tract with nutrients has been shown to terminate a meal by mechanisms involving both neural and humoral pathways (Schwartz, 2000). There is evidence that activation of the gut-brain pathway transmitting information on the macronutrient content of a meal can be modified by long term changes in the diet. Thus, maintenance of rats on a high fat diet decreases activation of neurons in the NTS in response to intestinal lipid (Covasa et al. 2000) and to exogenous CCK (Covasa and Ritter, 2001). This decrease in lipid-induced activation of NTS neurons is independent of adiposity (Savastano and Covaso, 2005). Recently, we have shown that pair-feeding rats isocaloric normal and high fat diets (40% kcal fat) induced a decrease in activation of the NTS in response to gastrointestinal lipid. After 4 weeks on the diet, the high fat fed rats became hyperphagic and ate longer and larger meals (Paulino et al, 2008). Thus, the decreased activation of the vagal afferent pathway was associated with an altered short-term regulation of meal size and meal termination, components of the meal regulated by GI signals. This inability to regulate meal size and length could result in decreased satiety in response to ingested fat leading to possible overconsumption of food and to body weight gain.
It is well established that different mouse strains can be characterized as diet-induced obesity prone or resistant, depending on the amount of weight gain when maintained on a high fat diet. In the present study, we hypothesized that DIO-R mice would maintain the sensitivity of the gut-brain pathway to lipid when maintained on a high fat diet, thus retaining the ability to accurately track ingested fat at the level of the GI tract. We determined activation of the vagal afferent pathway in response to an intragastric lipid load in C57BL/6 and 129sv mice (DIO-P and DIO-R, respectively) using immunochemical detection of fos protein as a marker of neuronal activation. In both strains of mice maintained on regular mouse chow, intestinal lipid activated neurons in the mid-NTS, the region where vagal afferent terminals from the GI tract terminate. When maintained on a HF diet (either 45% or 60% of calories from fat) for either four or eight weeks, there was a significant reduction in lipid-induced fos activation in the NTS of DIO-P, but not DIO-R mice, compared to chow-fed controls. These results support the hypothesis that there is a decrease in the lipid-induced activation of the vagal afferent pathway in the DIO-P mice; this attenuation of the gastrointestinal lipid-induced activation of neurons in the brainstem maybe associated with decreased lipid-induced satiation, increased food intake and body weight gain.
In the present study, we observed the expected strain difference in body weight gain when maintained on the high fat diets. The DIO-P mice increased body weight when maintained on the 60% fat diet at both four and eight weeks. There was no significant difference in body weight in the DIO-R mice fed either of the HF diets after four or eight weeks. These results agree with data from previous studies which found that the DIO-P mice had an increase in body weight and significantly greater adiposity compared to chow fed controls (West et al, 1992). It is of interest to note that the epididymal fat pad data is more revealing. At four weeks, there was a significant increase in adiposity, as expressed by weight of epidydimal fat pad as a percentage of final body weight, in both DIO-P and DIO-R mice on 45% and 60% HF diets compared to chow fed controls. There was a significantly greater increase in the DIO-P mice fed either HF diet than in DIO-R. However, at eight weeks, there was no further increase in adiposity in the DIO-R mice or in the DIO-P mice maintained on either of the two HF diets. Thus despite the lack of differences in body weight gain at various points, the DIO-P mice had marked and sustained increase in adiposity over the course of the eight weeks. These data suggest that there is an increase in adiposity occurs within the first four weeks and remains at a stable level despite changes in body weight.
We observed that the DIO-P mice had a greater number of activated (fos-positive) neurons in response to intragastric lipid compared to DIO-R mice. This was an interesting strain difference, the significance of which is unclear. Previously, we showed there was a difference in the activation of NTS neurons in these two mouse strains in response to exogenous administration of CCK (Whited et al, 2006, Whited et al, 2007). This difference in the overall number of activated neurons maybe due to differences in the density of the vagal afferent innervation of the intestine, receptor expression, excitability of NTS neurons in the DIO-P mice. Furthermore, the divergence in NTS activation between the two strains could be due to variation in the intestinal processing of fatty acids or triglycerides.
However, the most interesting finding is the difference in the adaptive response of the gut-brain pathway in signaling gastrointestinal lipid content. Maintenance on a HF diet caused a reduction in the number of activated fos-positive neurons in the NTS of DIO-P mice compared to chow-fed controls. In marked contrast, there was no change in activation of NTS neurons in response to intestinal lipid in DIO-R mice. Despite a significant increase in body weight (but not adiposity) in the DIO-P mice from four to eight weeks, there was no change in the number of activated neurons between these two time points. This suggests that the change in sensitivity of the vagal afferent pathway is more closely associated with adiposity than with increase in body weight. However, in the absence of a pair-fed control, we are unable to make any conclusions as to whether the change in activation of NTS neurons is associated with the changes in body weight, adiposity or food intake. The mechanism by which this decrease activation of NTS neurons in response to intestinal lipid is not known, but may be due to altered receptor expression at the level of vagal afferents. A series of elegant studies has shown that expression of receptors by the nodose ganglion is labile and dependent on the nutritional status of the animal (Burdyga et al, 2004, 2006) and preliminary data from our laboratory suggests changes in receptor expression for orexigenic factors in Sprague Dawley rats when maintained on high fat diets (Paulino and Raybould, unpublished observations).
In conclusion, we have shown that there is a marked decrease in activation of the gut-brain axis in response to the presence of lipid in the gastrointestinal tractwhen DIO-P mice are maintained on high fat diets. The data in the present study is consistent with the hypothesis that there is a difference in the processing of nutrient-derived information coming from the GI tract in the two mice strains, DIO-P and DIO-R. Whether this is contributing to overall increases in food intake, body weight and adiposity is unclear but a deeper understanding of the mechanism may shed light on adaptive response to high fat food and diet-induced obesity in humans.
4. Experimental Procedures
Animals and Diets
All experiments were performed in accordance with protocols approved by the UC Davis Institutional Animal Use and Care Committee. Experiments were performed using male C57BL/6J (diet-induced obesity-prone; DIO-P) (JAX Labs, Sacramento, CA) or 129S6/SvEv (diet-induced obesity-resistant; DIO-R) (Taconic, Oxnard, CA) mice of initial weight 17–27 g (6–8 wk of age) (West et al. 1992). Mice were fed standard Purina rodent chow for the first week and had ad libitum access to water. After one week of acclimatization, mice were randomly assigned to be fed one of two purified diets (45% kcal/fat D12451, 60% kcal/fat D12492, Research Diets; New Brunswick, NJ) or chow. Mice were maintained ad libitum on the diets for 4 weeks or 8 weeks. Body weight was measured biweekly in a subpopulation of mice. There was no significant difference in initial body weight between any of the groups. Mice were fasted overnight before experimental procedures but had ad libitum access to water.
Immunohistochemistry: Fos Protein Expression in the NTS
Fasted mice were gavaged with 0.2 ml of 20% Intralipid (0.04 g lipid, Fresenius Kabi) or 0.9% saline (Whited et al, 2006). After 120 min, mice were anesthetized with pentobarbital sodium (100 mg/kg I.P., 50 mg/ml, Western Medical Supply, Arcadia, CA) and transcardially perfused with 35 ml of heparinized 0.9% saline (0.1 ml heparin/100 ml saline) followed by 35 ml of 4% paraformaldehyde (Sigma-Aldrich, St Louis, MO). The brain stem was removed and post-fixed in 4% paraformaldehyde for 2 h. The epidydimal fat pad was also removed and weighed to within 0.1 gm.
Sections of brainstem were cut at 100 μm using a vibratome. Sections were incubated for 1 h in goat serum-phosphate-buffered saline (PBS) (Chemicon, Temecula, CA, USA) and incubated in primary antibody (1:2000 rabbit FOS antibody; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 3 h. Sections were then incubated with the secondary antibody (1:200 biotinylated goat antirabbit; Vector Laboratories, Burlingame, CA, USA) for 2 h followed by incubation for 3 h in ABC solution (Standard Elite Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, USA). DAB solution (Sigma, St Louis, MO) was added for a 5 min incubation, and then 50 μl H2O2-PBS (0.1 ml 30% H2O2:10 ml PBS) was added to catalyze the DAB reaction; the reaction was stopped with a PBS wash. Tissue was thoroughly washed between each incubation period.
Digital images of the nucleus of the solitary tract (NTS) were captured with a Provis optical imaging system. The NTS and the area postrema (AP) were delineated and all immunopositive nuclei within the NTS were counted using SCION Image computer software (Scion, Bethesda, MD).
Sections were chosen to represent three regions of the NTS: rostral (bregma −8.00 to −7.92 mm), mid-NTS (−7.76 to −7.32 mm) and caudal to the area postrema (−7.08 to −6.48 mm). Three sections were chosen for each region for a total of nine sections per mouse. The numbers of labeled neurons per section were summed for each region for each mouse; this value was used in subsequent statistical analyses. In experiments using different diets and time poitns, fos in NTS is given for mid-NTS only.
Statistical Analysis
Significant differences in the number of Fos-positive neurons between treatment groups were calculated using a one-way ANOVA followed by a Bonferroni multiple-comparison test. Data is expressed as mean ± SE. Means were considered statistically different when P < 0.05.
The number of mice in each group is indicated in the text and on the figures. There is considerable difference in the number of animals in each group due to inclusion of controls (chow fed animals) in each experimental run, together with experimental loss due to occasional loss of tissue during processing for immunocytochemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53(12):3274–85. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–15. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–97. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- Covasa M, Grahn J, Ritter RC. Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on high-fat diet. Auton Neurosci. 2000;84(1–2):8–18. doi: 10.1016/S1566-0702(00)00176-4. [DOI] [PubMed] [Google Scholar]
- Covasa M, Marcuson JK, Ritter RC. Diminished satiation in rats exposed to elevated levels of endogenous or exogenous cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2001;280(2):R331–7. doi: 10.1152/ajpregu.2001.280.2.R331. [DOI] [PubMed] [Google Scholar]
- Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides. 1998;19(8):1407–15. doi: 10.1016/s0196-9781(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Covasa M, Ritter RC. Reduced sensitivity to the satiation effect of intestinal oleate in rats adapted to high-fat diet. Am J Physiol. 1999;277(1 Pt 2):R279–85. doi: 10.1152/ajpregu.1999.277.1.R279. [DOI] [PubMed] [Google Scholar]
- Covasa M, Ritter RC. Adaptation to high-fat diet reduces inhibition of gastric emptying by CCK and intestinal oleate. Am J Physiol Regul Integr Comp Physiol. 2000;278(1):R166–70. doi: 10.1152/ajpregu.2000.278.1.R166. [DOI] [PubMed] [Google Scholar]
- Paulino G, Darcel N, Tome D, Raybould H. Adaptation of lipid-induced satiation is not dependent on caloric density in rats. Physiol Behav. 2008;93(45):930–6. doi: 10.1016/j.physbeh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE. Nutrient tasting and signaling mechanisms in the gut. I. Sensing of lipid by the intestinal mucosa. Am J Physiol. 1999;277(4 Pt 1):G751–5. doi: 10.1152/ajpgi.1999.277.4.G751. [DOI] [PubMed] [Google Scholar]
- Savastano DM, Covasa M. Adaptation to a high-fat diet leads to hyperphagia and diminished sensitivity to cholecystokinin in rats. Nutr. 2005;135(8):1953–9. doi: 10.1093/jn/135.8.1953. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition. 2000;16(10):866–73. doi: 10.1016/s0899-9007(00)00464-0. [DOI] [PubMed] [Google Scholar]
- Van Heek M, Compton DS, et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99(3):385–90. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DB, Boozer CN, et al. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262(6 Pt 2):R1025–32. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- Whited KL, Thao D, et al. Targeted disruption of the murine CCK1 receptor gene reduces intestinal lipid-induced feedback inhibition of gastric function. Am J Physiol Gastrointest Liver Physiol. 2006;291(1):G156–62. doi: 10.1152/ajpgi.00569.2005. [DOI] [PubMed] [Google Scholar]
- Whited KL, Tso P, Raybould Involvement of apolipoprotein A-IV and cholecystokinin1 receptors in exogenous peptide YY3 36-induced stimulation of intestinal feedback. HE Endocrinology. 2007;148:4695–703. doi: 10.1210/en.2006-1665. [DOI] [PubMed] [Google Scholar]