Abstract
Wnt proteins are expressed during limb morphogenesis, yet their role and mechanism of action remains unclear during long bone growth. Wnt expression, effects and modulation of signaling events by BMP and transforming growth factor-beta (TGF-β) were evaluated in chick embryonic chondrocytes. Chondrocyte cell cultures underwent spontaneous maturation with increased expression of colX and this was associated with an increase in the expression of multiple Wnts, including Wnts 4, 5a, 8c, and 9a. Both parathyroid hormone related peptide (PTHrP) and TGF-β inhibited colX, but had disparate effects on Wnt expression. While TGF-β strongly inhibited all Wnts, PTHrP did not inhibit either Wnt8c or Wnt9a and had lesser effects on the expression of the other Wnts. BMP-2 induced colX expression, and also markedly increased Wnt8c expression. Overexpression of β-Catenin and/or T cell factor (TCF)-4 also induced the type X collagen promoter. Overexpression of Wnt8c induced maturation, as did overexpression of β-Catenin. The Wnt8c/β-Catenin maturational effects were enhanced by BMP-2 and inhibited by TGF-β. TGF-β also inhibited activation of the Topflash reporter by β-Catenin, suggesting a direct inhibitory effect since the Topflash reporter contains only β-Catenin binding sequences. In turn β-Catenin inhibited activation of the p3TP-Luc reporter by TGF-β, although the effect was partial. Thus, Wnt/β-Catenin signaling is a critical regulator of the rate of chondrocyte differentiation. Moreover, this pathway is modulated by members of the TGF-β family and demonstrates the highly integrated nature of signals controlling endochondral ossification. J. Cell. Biochem.
Keywords: Wnt, Chondrocyte, β-Catenin, TGF-βtype X collagen
Longitudinal bone growth is a highly regulated process in which chondrocytes complete sequential stages of proliferation, maturation, and hypertrophy marked by a 10-fold increase in type X collagen, and expression of alkaline phosphatase [Hunziker, 1994; Johnson and Tabin, 1997]. Local and systemic factors regulate chondrocyte maturation, and act through multiple independent signaling pathways. Several factors are known to influence the rate of chondrocyte differentiation. Transforming growth factor-beta (TGF-β) and parathyroid hormone related peptide (PTHrP) slow the rate of chondrocyte maturation, while bone morphogenetic proteins (BMPs), thyroid hormone, and retinoic acid stimulate terminal differentiation [Lanske et al., 1996, 1999; Serra et al., 1997; Enomoto-Iwamoto et al., 1998; Ballock et al., 1999; Ferguson et al., 2000; Yang et al., 2001; Li et al., 2003].
Growth plate chondrocytes abundantly express TGF-β [Pelton et al., 1990; Matsunaga et al., 1999; Sakou et al., 1999; Serra et al., 1999]. In vitro, TGF-β stimulates proliferation and inhibits differentiation through both Smad and MAP kinase mediated pathways, that involve the transcription factors Smad2, Smad3, and ATF-2 [Ferguson et al., 2000; Ionescu et al., 2003]. In vivo, gain of TGF-β function delays chondrocyte maturation [Ferguson et al., 2004]. In contrast, loss of TGF-β signaling in vivo results in accelerated chondrocyte maturation, and interestingly effects initiate with the post natal period and thus demonstrate a critical role for TGF-β in post-natal growth and development [Serra etal., 1997; Yang et al., 2001]. In contrast BMPs accelerate chondrocyte maturation [Enomoto-Iwamoto et al., 1998; Grimsrud et al., 1999; Li et al., 2003]. In chick caudal sternal chondrocytes BMPs act through the Smad1 and Smad 5 pathway to increase the expression of colX [Li et al., 2003]. While retinoic acid and thyroid hormone accelerate chondrocyte maturation, both have been shown to be dependent on BMP signaling events [Li et al., 2003]. Thus, while TGF-β and BMPs are critical regulators of the rate of chondrocyte maturation their actions are influenced by a complex array of growth factors and signaling molecules.
The Wingless/INT-1-related Wnt proteins are important secreted signaling molecules that are essential during organogenesis and early development [Weidinger and Moon, 2003]. The Wnts have been shown to regulate cell proliferation and differentiation during early stages of chondrogenesis and thus are also important during limb development [Moon et al., 1997; Hartmann and Tabin, 2000; Church et al., 2002]. At least 22 Wnts have been identified [Church et al., 2002; Miller, 2002]. These proteins bind to the Frizzled (FZ) seven transmembrane spanning receptor family [Wang et al., 1996] and activate more than one signaling pathway, the canonical pathway, which targets β-Catenin signaling, and the non-canonical pathway. In the canonical pathway, downstream of Wnt1, Wnt3a, Wnt7a, and Wnt8c, Wnt receptor binding inhibits GSK and results in stabilization of the cytoplasmic protein β-Catenin [Miller et al., 1999; Fischer et al., 2002; Weidinger and Moon, 2003]. Stabilized β-Catenin translocates into the nucleus and associates with its DNA binding partners, T cell factor (TCF) and lymphocyte enhancer factor 1 (LEF-1), and regulates gene transcription [Miller et al., 1999; Weidinger and Moon, 2003]. Interestingly, β-Catenin converts TCF into a transcriptional activator of target genes that may be repressed by TCF alone [Cavallo et al., 1998; Nusse, 1999]. The second Wnt signaling pathway involves intracellular Ca2+ and is activated by Wnt5a and Wnt11 [Miller et al., 1999; Topol et al., 2003; Weidinger and Moon, 2003]. To date, not all Wnts are characterized with regards to their downstream signals and second messengers, and even less is known concerning the interaction of Wnt signals with other signaling pathways [Weidinger and Moon, 2003].
Several Wnt members are expressed in the developing limb with specific spatial and temporal patterns. Wnt7a is expressed in the developing chick limb and ectopic expression of Wnt7a inhibited chondrogenesis in vitro [Rudnicki and Brown, 1997]. Ectopic Wnt5a and Wnt5b are both expressed at the transition from proliferating to hypertrophic chondrocytes, and delay chondrocyte differentiation [Yang et al., 2003]. In contrast, ectopic Wnt4 expression enhanced chondrocyte differentiation [Hartmann and Tabin, 2000]. Overexpression of Wnt14 (now referred to as Wnt9a) in the chick limb resulted in ectopic synovial joint formation [Hartmann and Tabin, 2001].
While independent studies showed that certain Wnt proteins, TGF-β, PTHrP, and BMPs play key roles in cartilage development, very little is known about (i) the expression and regulation of the Wnt factors during chondrogenesis, and (ii) the mechanisms by which these different signals are integrated in the molecular cascade regulating the rate of chondrocyte maturation. Here, we demonstrate that Wnt gene expression is highly regulated during chondrocyte maturation in vitro. β-Catenin and TCF/LEF transcription factors accelerate chondrocyte differentiation and these effects are modulated by signals generated by TGF-β and BMP-2. The findings clearly demonstrate the importance of Wnt signaling and the need to understand effects in the context of its association with the TGF-β superfamily.
MATERIALS AND METHODS
Chondrocyte Isolation and Cell Culture
Chondrocytes were isolated from either the cephalic or caudal portion of the 15-day-old chick embryonic sterna and 19-day-old tibia growth plate by digestion for 4 h at 37°C, 5% CO2 in HBSS containing 0.05% collagenase D (Sigma, St. Louis, MO), and 0.25% trypsin (Sigma) as previously described [Grimsrud et al., 1999]. Chondrocytes were resuspended in Dulbecco’s modified Eagle’s medium containing 10% NuSerum IV (Collaborative Biomedical Products, Bedford, MA) supplemented with (50 U penicillin and streptomycin) and 2 mM of L-glutamine. Cells were then plated in 100 mm plates at a density of 3 × 106 cells/plate. After primary culture for 7 days, chondrocytes were harvested and secondary cultures were placed in 12-well plates at a density of 2 × 105 cells per well for luciferase experiments and 6-well plates at a density of 5 × 105 cells per well for gene expression experiments. For treatment experiments, BMP-2 (50 ng/ml; Peprotech, Inc., Rockyhill, NJ), TGF-β (5 ng/ml; R&D Systems, Minneapolis, MN), or PTHrP (10−7 M/ml; Bachem, Switzerland) were added to the media for 24 h prior to cell harvesting for luciferase assay or RNA extraction.
RNA Extraction and Real-Time RT-PCR
Total RNA was extracted from primary chondrocytes using the RNA easy kit (Qiagen, Valencia, CA) following the manufacturer’s recommendations. One microgram of total RNA was reverse transcribed using Advantage RT-for-PCR kit (Clontech, Palo Alto, CA) following the manufacturer’s protocol. Briefly, RNA was denatured at 72°C for 10 min then 200 U for MMLV reverse transcriptase enzyme were used along with oligo dT primers in a final reaction of 20 μl of cDNA. One microliter of freshly reverse transcribed cDNA was used for real-time PCR. cDNA levels were measured in real-time using the fluorescent dye SYBR Green I (SYBR Green PCR Master Mix, Applied Biosystems, Foster City, CA) using specific primers designed for chicken collagen type X, alkaline phosphatase, Wnt 4, Wnt5a, Wnt7a, Wnt8c, and Wnt9a as shown below.
|
| ||
| Genes | Forward primer | Reverse primer |
|
| ||
| Wnt4 | CTGCTCCGATAACATTGCCTA | TTGAACTCGCACGAGCCT |
| Wnt5a | CGGGAGAGAGTTTACCAGA | TGGCAGCAGCACTATCATATT |
| Wnt7a | CGACATCAGATACGGAATAGG | CCCATGGCATTTGCACTCTAA |
| Wnt8c | GAACGGGCGTGTAGGT | GCACTTGCAGGCTCGCTTCAT |
| Wnt9a | ATGGCCAAAGCGTGCAG | CTTCATGCCCACAAGGTTATT |
| Alk. Phos. | TGGGGGATGTAGTTCTGCTC | AACGGCCCTGGCTATAAGAT |
| Collagen X | AAAATAGTAGACGTTACCTTGACTC | ACATGCATTTACAAATATCGTTAC |
|
| ||
The PCR reaction used the RotorGene real-time DNA amplification system (Corbett Research, Sydney, Australia). The following protocol was used; a 95°C denaturation step for 10 min followed by 45 cycles with denaturation for 20 s at 95°C, annealing for 20 s at 57°C, and extension at 72°C for 30 s. Detection of the fluorescent product was carried out at the end of the extension period. Gene expression was normalized to GAPDH. PCR products were subjected to a melting curve analysis and the data was analyzed and quantified with the RotorGene analysis software.
RCAS-Mediated Cell Infection
Chick embryonic fibroblasts grown in DMEM containing 10% fetal bovine serum, 0.2% fetal chick serum, and 50 U penicillin/streptomycin were transfected with the replication competent avian sarcoma retrovirus in either RCAS BP(A) or RCAS BP(B). RCAS BP(A) vectors included wild-type β-Catenin and Wnt8c (a gift from Dr. Andrew Lassar) was in an RCAS BP(B) vectors. Cells were passed three times and plated in 100 mm dishes. Upon confluence, media was changed to DMEM containing 10% NuSerum IV and 50 U penicillin/streptomycin. Viral supernatants were collected at 24 h intervals for 3 days. At the time of secondary plating, upper sternal chondrocytes were incubated for 48 h with fresh viral supernatant mixed in a 1:1 ratio with plating medium. TGF-β (5 ng/ml) or BMP-2 (50 ng/ml) was added to selected treatment groups in fresh medium and total RNA was extracted from the cells after 3 days of growth factor treatment. Media and growth factors were replenished at 48 h intervals.
Transfection and Luciferase Assay
For transfection, chick upper sternal chondrocytes were plated at a density of 105 cells/well in six-well plates. After 12 h, cells were transfected. We used either the full-length ABC-640-Luc promoter or the BMP-responsive b2-640-Luc fragment of chick type X collagen promoter driving the expression of the Renilla luciferase reporter gene (a gift from Dr. Phoebe Leboy) [Volk et al., 1998]. Transient transfection used 100 ng of the type X collagen reporters and 500 ng of either wild-type or mutant β-Catenin cDNA (a gift from Dr. Ben Alman) and/or wild-type TCF-4 (a gift from Dr. Ben Alman) and LEF-1 (a gift from Dr. Jennifer Westendorf). The TGF-β responsive reporter, p3TP-Luc (500 ng; a gift from Dr. Joan Massague) and the Topflash reporter (500 ng; a gift from Dr. Jennifer Westendorf) were also used. Transfection was performed with the transfection reagent superfect (Qiagen). Two hours after transfection, cultures were placed in regular media for 24 h, followed by treatment with BMP-2 (50 ng/ml) or TGF-β (5 ng/ml) for the next 24 h. Cells were lysed using the passive lysis buffer (Promega) and luciferase activity in the cell lysate measured using the dual Luciferase assay system (Promega) with an Optocomp luminometer (MGM Instruments, Hamden, CT). Basic PGL3 vector (10 ng) containing the firefly luciferase gene (Promega) was used to standardize transfection efficiency for the type X collagen reporters. For p3TP-Luc and Topflash, transfection efficiency was determined by co-transfection with pRL vector (Promega) and determining the Renilla uniformis luciferase activity. In all experiments, control vectors were used to keep the total amount of transfected DNA identical.
Statistical Analysis
Each of the experiments was repeated at least three times. Differences in between the various treatments or time points were compared using a two-way analysis of variance. Statistical significance was considered present when the P value was <0.05.
RESULTS
Wnts Expression Is Regulated During Chondrocyte Differentiation
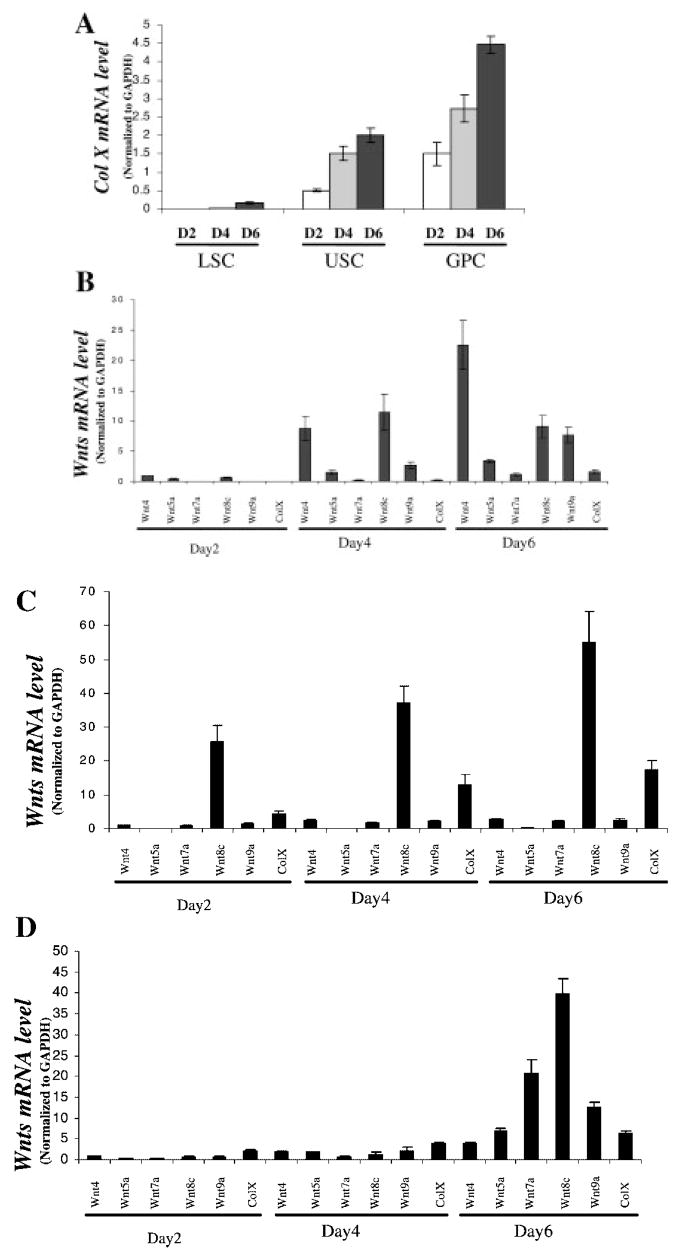
Limited information is available concerning Wnt expression in chondrocytes undergoing endochondral ossification and experiments were conducted examining expression levels over time in three distinct primary chick chondrocyte differentiation models. Initially, we compared the relative levels of colX expression and rate of differentiation in day 14 embryonic chondrocytes obtained from either the cephalic (upper sternal chondrocytes) or caudal (lower sternal chondrocytes) portion of the sternum, and day 18 embryonic growth plate chondrocytes (Fig. 1A). While spontaneous maturation occurred in the three culture models, the basal level of colX expression was highest in growth plate chondrocytes, intermediate in cephalic sternal chondrocytes, and essentially undetectable in caudal sternal chondrocytes. By 6 days, there was a four-fold increase in colX expression in growth plate and upper sternal chondrocytes and detectable levels in lower sternal chondrocyte cultures.
Fig. 1.
Type X collagen and Wnt gene expression in chick chondrocyte cultures. Chondrocytes were isolated from the cephalic (upper) or caudal (lower) sternum or tibia growth plates of day 15 and day 19 chicken embryos as described in Materials and Methods. Total RNA was prepared from cultures at days 2, 4, and 6. Real-time RT-PCR was carried out using primers for chicken type X collagen, Wnt4, Wnt5a, Wnt7a, Wnt8c, and Wnt9a. The relative expression of colX was examined over time in the different cultures (A). Wnt expression was examined over time in lower sternal chondrocytes (B), upper sternal chondrocytes (C), and growth plate chondrocytes (D). Gene expression was normalized to GAPDH. The data shown are representative results of three independent experiments; bars, ±standard error of the mean.
The expression of various Wnt genes was examined over time in the chondrocyte cultures. Wnt4, 5a, 7a, 8c, and 9a all had increased expression over the course of the 6 day culture period in each of the cell culture models (Fig. 1B–D). In lower sternal chondrocytes, the greatest increases were observed in Wnt4, 8c, and 9a (Fig. 1B); in upper sternal chondrocytes in Wnt 8c (Fig. 1C); and in growth plate chondrocytes in Wnt 7a, 8c, and 9a (Fig. 1D). The findings show that the Wnts are regulated during chondrocyte maturation in cell culture. Wnt4 and Wnt8c were highly upregulated in the least differentiated culture model (lower sternal chondrocytes), while Wnt 7a, 8c, and 9a had large increases in the more-mature chondrocyte cultures (upper sternal and growth plate chondrocytes). Wnt8c is highly upregulated at all time points in the upper sternal chondrocytes, which exhibit an intermediate differentiation phenotype, suggesting a role for this factor at different stages of chondrocyte maturation.
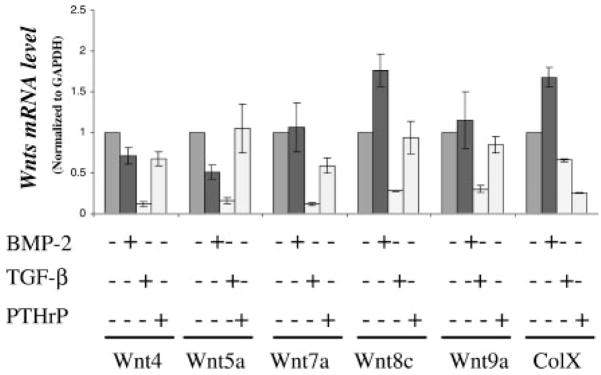
TGF-β, PTHrP, and BMP-2 Differentially Regulate Wnt Expressions in Chondrocytes
Since TGF-β and PTHrP inhibit, and BMP-2 stimulates chondrocyte maturation experiments were performed to determine whether Wnt expression is also regulated by these growth factors. The upper sternal chondrocyte cell culture model was used in these and subsequent experiments because of its intermediate rate of chondrocyte maturation (Fig. 1A). A 24 h treatment with PTHrP or TGF-β inhibited the expression of colX, and the magnitude of the reduction was approximately 70% with both growth factors (Fig. 2). TGF-β caused a reduction in the expression of all of the other Wnts (Wnt4 by 5.2-fold; Wnt5a by five-fold; Wnt7a by six-fold; Wnt8c by three-fold; and Wnt9a by three-fold). In contrast, PTHrP caused a slight reduction in the expression of Wnt7a, and no reduction in the expression of the other Wnts (Fig. 2). To further confirm that Wnt expression is associated with chondrocyte differentiation and differentially regulated by growth factors, upper sternal chondrocyte cultures were treated with BMP-2, which induces colX and other maturational markers in these cultures [Volk et al., 1998]. BMP-2 treatment for 24 h resulted in approximately a two-fold increase in Wnt8c, but caused no significant change in the expression of Wnt 4, Wnt 7a, and Wnt 9a and reduced the expressions of Wnt 5a by at least two-fold.
Fig. 2.
Transforming growth factor-beta (TGF-β), parathyroid hormone related peptide (PTHrP), and BMP-2 regulate Wnt expression in Chondrocytes. Embryonic chick cephalic sternal chondrocyte were cultured for 24 h and were treated with either TGF-β or PTHrP or with BMP-2. After 24 h of treatment, total RNA was extracted from the cultures and gene expressions determined by real-time RT-PCR using chick specific primers. The data shown are representative results of three independent experiments; bars, ±standard error of the mean. The symbol, * represents a significant difference compared to controls with P <0.05.
Thus, while TGF-β and BMP-2 cause opposite effects on maturation, only the expression of Wnt 8c mimicked these effects; Wnt 8c was reduced by TGF-β and increased by BMP-2. Altogether, the experiments suggest that Wnt expressions are associated with the state of chondrocyte differentiation, but also indicate that these growth factors specifically and differentially regulate Wnt expression in chondrocytes.
β-Catenin-TCF/LEF Signaling Pathway Enhances Chondrocyte Maturation
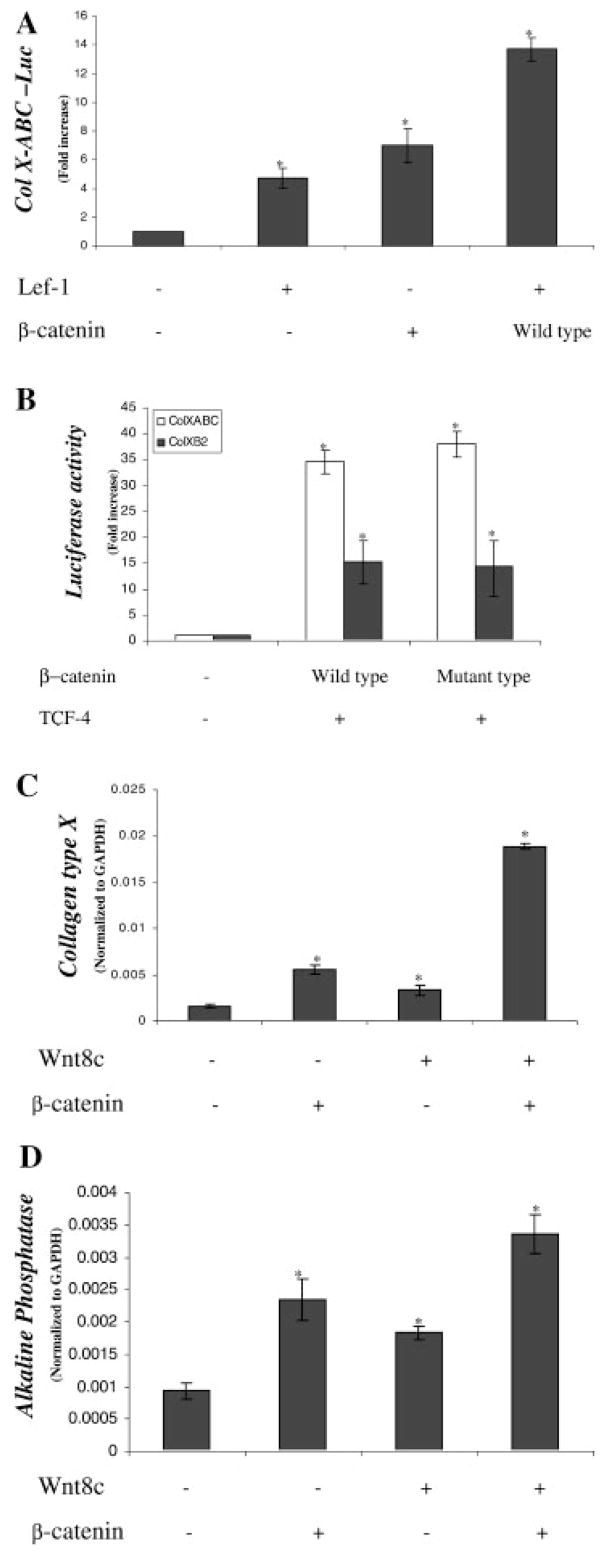
Members of the Wnt family of proteins signal through various second messenger-signaling pathways. The canonical pathway involves downstream signals mediated by β-Catenin and its binding partners, the TCF/LEF family of transcription factors. Alternatively, some Wnts stimulate the generation of calcium transients and activation of phospholipase C. While Wnt5a stimulates calcium transients [Slusarski et al., 1997; Liang et al., 2003], recent evidence supports activation of β-Catenin signaling by Wnt8C [Lewis et al., 2004; Ramel and Lekven, 2004]. In order to determine whether β-Catenin is involved in the acceleration of chondrocyte differentiation, various β-Catenin signaling molecules were co-transfected into upper sternal chondrocyte cultures along with chicken type X collagen promoter fragments.
Co-transfection of either β-Catenin (sevenfold), or LEF-1 (five-fold) increased transactivation of the full-length type X collagen promoter (ABC-640-Luc), and when overexpressed together, the effect was further enhanced (14-fold) (Fig. 3A). We next examined the relative responsiveness of the full-length type X collagen promoter (ABC) and a partial promoter, b2-640-Luc. The b2 fragment is 533 bases and contains the BMP responsive region of the promoter [Volk et al., 1998]. Co-transfection of β-Catenin and TCF-4 induced both promoters, but the effect was greater with the full-length promoter (ABC-640-Luc; 37-fold) than that observed with the truncated promoter (b2-640-Luc; 15-fold). Co-transfection with a mutant β-Catenin, which lacks sequences necessary for degradation and thus remains active, resulted in a similar degree of activation as that observed in the cultures transfected with wild-type β-Catenin (Fig. 3B). Thus, the type X collagen promoter is highly responsive to β-Catenin signaling and subsequent experiments utilized the more responsive full-length promoter.
Fig. 3.
β-Catenin and Wnt8c cooperate to stimulate chondrocyte maturation. Embryonic chick upper sternal chondrocyte were transfected with the full-length type X collagen reporter (ABC-640-Luc; 100 ng) and lymphocyte enhancer factor 1 (LEF-1) (500 ng) and/or β-Catenin (500 ng) or control vectors after 12 h in culture. Two hours after transfection, standard medium was added and the cultures were harvested 48 h later for luciferase assay. A basic PGL3 vector (10 ng) containing the firefly luciferase gene was used to standardize transfection efficiency. Results are represented as relative luciferase activities obtained by dividing the firefly luciferase by renilla luciferase (A). Similarly, chondrocytes were transfected with ABC-640-Luc (100 ng) or b2-640-Luc (100 ng) and/or β-Catenin (500 ng) and/or T cell factor (TCF)-4 (500 ng) and luciferase assay performed after 48 h (B). Embryonic chick upper sternal chondrocyte were infected with either a control RCAS virus, or viruses containing inserts for either Wnt8c in RCAS BP (B) and/or β-Catenin in RCAS BP (A) (C,D) at the time of plating. After a 48 h culture period in medium containing fresh viral supernatant mixed in a 1:1 ratio with plating medium, fresh control medium was added. Total RNA was extracted from the cultures 3 days later and gene expressions determined by real-time RT-PCR using chick specific primers for either colX (C) or alkaline phosphatase (D). The data shown are representative results of three independent experiments; bars, ±standard error of the mean. The symbol, * represents a significant difference compared to controls with P <0.05.
The ability of β-Catenin and the β-Catenin activator, Wnt8c, to stimulate colX and alkaline phosphatase gene expressions were examined in the chondrocyte cultures (Fig. 3C,D). Both β-Catenin and Wnt8c overexpressions induced colX and alkaline phosphatase expressions, but the effect was maximal in chondrocytes co-infected with both viruses, consistent with activation of β-Catenin signaling by Wnt8c.
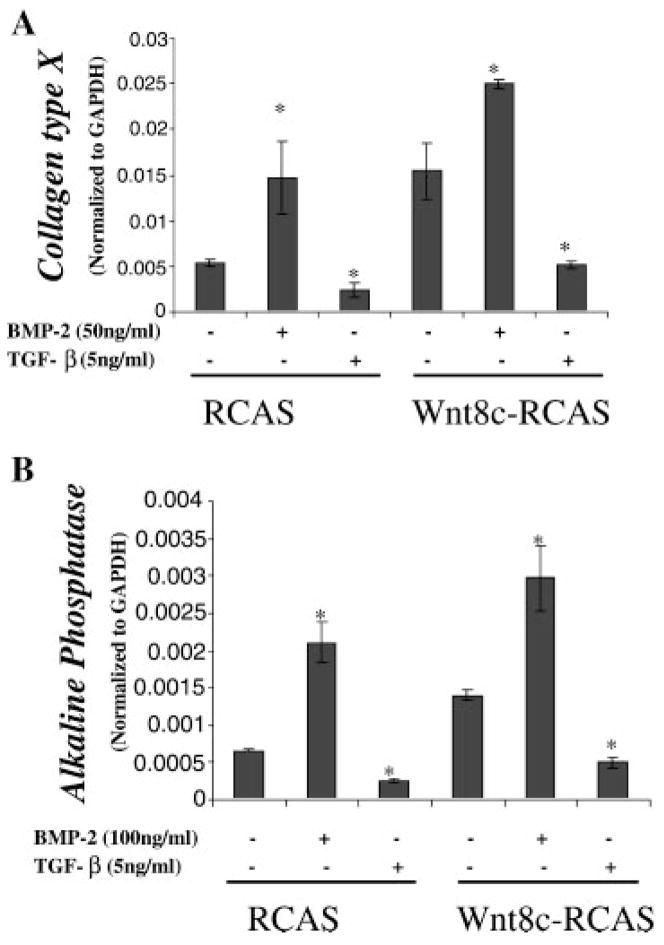
Final experiments were conducted to determine if TGF-β and BMP-2 modulate genes that are regulated by Wnt8C (Fig. 4A,B). Chondrocytes infected with Wnt8C-RCAS had increased expression of both colX and alkaline phosphatase compared to control RCAS infected cultures. Treatment of the cultures with TGF-β resulted in a decrease in basal expression levels and eliminated induction by Wnt8c. In contrast, BMP-2 enhanced gene expression and maximal expressions were observed in Wnt8c-RCAS-infected cultures treated with BMP-2. Thus, members of the TGF-β signaling family modulate responsiveness to Wnt8c generated signals.
Fig. 4.
Stimulation of chondrocyte maturation by Wnt8c is enhanced by BMP-2 and abolished by TGF-β. Embryonic chick upper sternal chondrocyte were infected with either a control RCAS virus, or viruses containing an insert for Wnt8c at the time of plating. After an initial 48 h culture period in medium containing fresh viral supernatant mixed in a 1:1 ratio with plating medium, fresh control medium or medium containing TGF-β (5 ng/ml) or BMP-2 (50 ng/ml) was added. Total RNA was extracted from the cultures 5 days later and gene expressions determined by real-time RT-PCR using chick specific primers for either colX (A) or alkaline phosphatase (B). The data shown are representative results of three independent experiments; bars, ±standard deviation.
BMP-2 Enhances and TGF-β Antagonizes β-Catenin-TCF/LEF Mediated Transcription
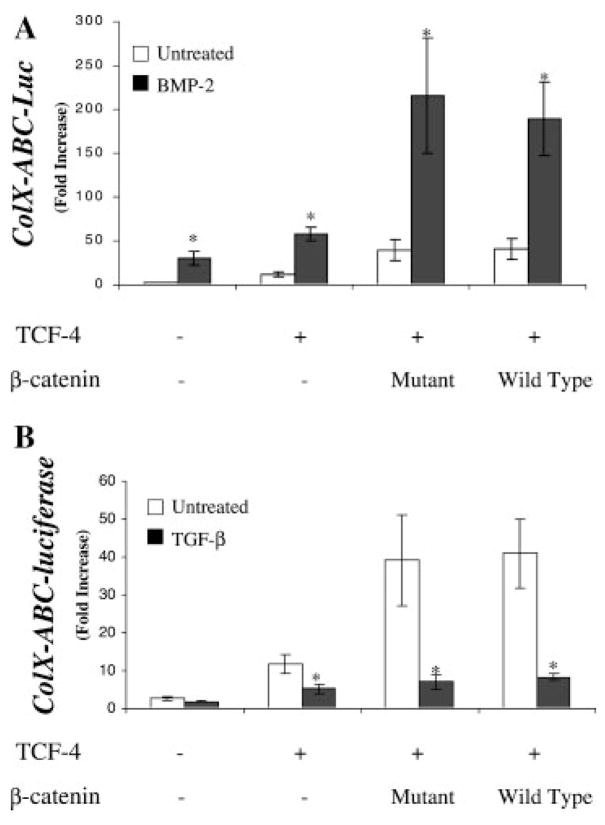
In order to determine whether BMP-2 and TGF-β signaling directly interacts with β-Catenin signal transduction, TCF-4 was transfected alone or in combination with either wild-type or mutant β-Catenin in the presence of BMP-2 (Fig. 5A) or TGF-β(Fig. 5B). TCF-4 alone induced type X collagen promoter activity by approximately 10-fold, while reporter gene expression was dramatically enhanced (40-fold) when co-transfected with either mutant or wild-type β-Catenin (Fig. 5). Addition of BMP-2 alone caused a 25-fold induction over basal levels and the effect was markedly enhanced by TCF-4 alone and TCF-4 with β-Catenin in combination which shows a maximal induction of 200-fold compared to the control (Fig. 5A). In contrast, TGF-β inhibited the type X collagen promoter activity in all cases, both alone and in cells co-transfected with TCF-4 and β-Catenin (Fig. 5B). The level of promoter activation in TCF-4/β-Catenin co-transfected cultures treated with TGF-β remained greater than that observed in control cultures (five-fold induction), but was dramatically reduced compared to the maximally stimulated levels (40-fold) (Fig. 5B).
Fig. 5.
BMP-2 enhances β-Catenin-TCF-4 mediated transcription. Embryonic chick upper sternal chondrocyte were transfected with the full-length type X collagen reporter (ABC-640-Luc; 100 ng) and TCF-4 (500 ng) and/or either mutant (non-degradable) or wild-type β-Catenin (500 ng) after 12 h in culture. Two hours after transfection, standard medium was added with or without BMP-2 (50 ng/ml) (A) or TGF-β (5 ng/ml) (B) and the cultures were harvested 48 h later for luciferase assay. A basic PGL3 vector (10 ng) containing the firefly luciferase gene containing the firefly luciferase gene was used to standardize transfection efficiency. Results are represented as relative luciferase activities obtained by dividing the firefly luciferase by renilla luciferase.
Interdependence of β-Catenin and TGF-β Signaling in Regulating Chondrocyte Maturation
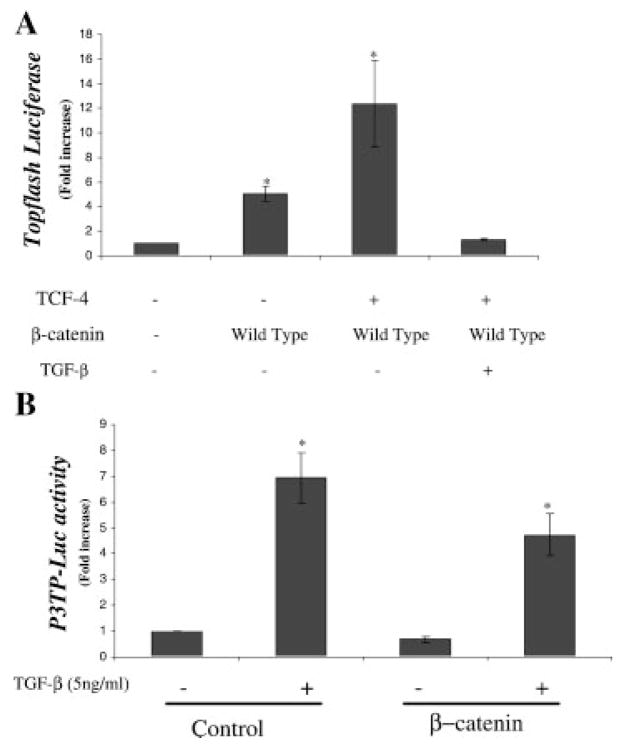
The effect of TGF-β on β-Catenin signaling was further examined. The decrease in promoter activity possibly results from either, or a combination of (i) an independent effect mediated by a region of the promoter distinct from the β-Catenin binding site; or (ii) direct interference with β-Catenin mediated transcriptional activation. To examine the second possibility, chondrocytes were co-transfected with the Topflash reporter along with β-Catenin signaling molecules in the presence and absence of TGF-β (Fig. 6A). The Topflash reporter was induced 13-fold by co-transfection with β-Catenin and TCF-4, but the induction was completely eliminated in cultures treated with TGF-β. Since the Topflash promoter is composed of multiple repeats of the β-Catenin-TCF/LEF consensus binding sequence and does not contain Smad or other binding sites, the data suggests that TGF-β can directly interfere with β-Catenin mediated gene transcription.
Fig. 6.
TGF-β antagonizes β-Catenin-TCF-4 mediated transcription. Similarly, chondrocytes were transfected with the Topflash reporter (500 ng) and β-Catenin (500 ng) alone or with TCF-4 (500 ng) in the presence of TGF-β (5 ng/ml) and luciferase assay performed after 48 h. Transfection efficiency was determined by co-transfection with pRL vector (Promega) and determining the renilla uniformis luciferase activity (A). Embryonic chick upper sternal chondrocyte were transfected with p3TP-Luc (500 ng) and TCF-4 (500 ng) and either mutant wild-type β-Catenin (500 ng) or control vector after 12 h in culture. Two hours after transfection, standard medium was added with or without TGF-β (5 ng/ml) and the cultures were harvested 48 h later for luciferase assay. Transfection efficiency was determined by co-transfection with pRL vector (Promega) and determining the renilla uniformis luciferase activity. Results are represented as relative luciferase activities obtained by dividing the firefly luciferase by renilla luciferase (B).
Chondrocytes were also transfected with the TGF-β responsive reporter, p3TP-Luc and the effect of co-transfection with β-Catenin examined in the presence and absence of TGF-β. Alone, TGF-β caused a seven-fold induction in p3TP-Luc activity, while co-transfection of β-catenin in the absence of TGF-β resulted in approximately a 25% decrease in basal promoter activity. Co-transfection with β-Catenin decreased promoter activation in response to TGF-β to approximately five-fold (Fig. 6B). Thus, although β-Catenin reduces TGF-β activation of the promoter, the response is partial.
DISCUSSION
While Wnts are critical regulators of growth and development, little is known regarding their role during endochondral ossification. All of the Wnts examined including 4, 5a, 7a, 8c, and 9a were increased during chondrocyte differentiation, suggesting a potential role for one or more Wnts in the process of endochondral ossification. Wnt expression was responsive to growth factors and TGF-β decreased the expression of each of the Wnts examined. Wnt5a, which stimulates calcium transients, decreased chondrocyte maturation. TGF-β completely blocked the maturation effects of Wnt8c and β-Catenin, as well as activation of the type X collagen promoter. While these genes and type X collagen promoter contain a complex array of potential transcriptional binding sites, TGF-β also inhibited β-Catenin mediated activation of the Topflash reporter, which is dependent solely on β-Catenin activation.
Previous in vivo studies established that Wnt family members, Wnt3a, Wnt4, Wnt5a, Wnt5b, Wnt7a, Wnt8c, Wnt9a, and Wnt11 have distinct expression patterns in the developing limb and a role for Wnt signaling has been established during chondrogenesis [Moon et al., 1997; Hartmann and Tabin, 2000; Church et al., 2002]. Wnt1, Wnt4, and Wnt7a have been shown to block chondrogenesis [Rudnicki and Brown, 1997; Church et al., 2002; Tufan et al., 2002]. In contrast, overexpression of Wnt3a [Fischer et al., 2002], Wnt5a, and Wnt5b enhance chondrogenesis [Church et al., 2002].
However less is known concerning Wnt expression in the growth plate. In the chick embryonic limb, Wnt4 is expressed in cells of the joint region, Wnt5a in the perichondrium, and Wnt 5b in prehypertrophic chondrocytes and in the outermost layer of perichondrium [Hartmann and Tabin, 2000]. Church et al., 2002 also found Wnt4 expression in hypertrophic chondrocytes, and Wnt11 was expressed in a pattern similar to Wnt5b. Similarly the Frzb, a secreted decoy receptor and antagonist of Wnt signaling has expression limited to prehypertrophic chondrocytes [Enomoto-Iwamoto et al., 2002]. In the current study, we established the expression of multiple Wnts including Wnt 4, 5a, 7a, 8c, and 9a in chondrocytes undergoing differentiation. In lower sternal chondrocytes, Wnt4 had the highest level of expression while Wnt8c expression was highest in upper sternal chondrocytes and growth plate chondrocyte cultures. Interestingly, Wnt9a was also highly expressed in the various cultures. Altogether, the findings suggest that these and other Wnts are important regulators of chondrocyte maturation during endochondral ossification.
Previous work established a potential role for Wnts and β-Catenin as a regulator of the rate of chondrocyte maturation. Similar with our in vitro findings, in the developing chick limb bud, Wnt5a misexpression delays the maturation of chondrocytes and the onset of bone collar formation, while Wnt4 and Wnt8c misexpression accelerate chondrocyte maturation [Hartmann and Tabin, 2000; Church et al., 2002; Enomoto-Iwamoto et al., 2002]. β-Catenin and its binding partner LEF-1 also accelerated maturation in developing chick limbs [Hartmann and Tabin, 2000; Enomoto-Iwamoto et al., 2002]. Loss of β-Catenin function in a murine model has recently been shown to delay chondrocyte hypertrophic differentiation and result in a dwarf phenotype [Akiyama et al., 2004]. Similarly, inhibition of β-Catenin through forced overexpression of two dominant negative Wnt receptors, Chfz-1 and Chfz-7, both of which signal through β-Catenin, also delays chondrocyte maturation in vivo [Hartmann and Tabin, 2000]. Finally, the soluble receptor antagonist, Frzb is expressed in the developing chick limb bud [Ladher et al., 2000]. Overexpression of Frzb also delays chondrocyte maturation, suggesting that endogenous Wnt signals are essential for a normal rate of chondrocyte differentiation and endochondral bone formation [Enomoto-Iwamoto et al., 2002].
Wnt8c and Wnt9a were both highly expressed as chondrocytes underwent maturation in vitro. Although Wnt8c overexpression was previously shown to accelerate chondrocyte maturation in vivo [Enomoto-Iwamoto et al., 2002], the expression of Wnt8c in cartilage cells has not been previously investigated. Wnt9a has been reported to be an important regulator of joint formation [Hartmann and Tabin, 2001]. Interestingly Wnt4, which is also expressed in the developing joint, also stimulates chondrocyte maturation, and is highly expressed in hypertrophic chondrocytes in vivo and in vitro in our cell cultures [Church et al., 2002]. In contrast, the relative expression of Wnt5a, which inhibits differentiation was much lower in all of the cell culture systems.
Previous work in our laboratory and by others has established that despite their closely related signaling systems and pathways, TGF-β and BMPs have antagonistic effects, with TGF-β inhibiting and BMP stimulating chondrocyte maturation. While all of the Wnts examined were reduced by TGF-β, only Wnt8c expression was increased by BMP-2. Coupled with its relatively high level of expression in chondrocytes, these finding suggest that Wnt8c might be a particularly important regulator in cartilage. In general, there is less known about Wnt regulation compared to other growth factors involved in endochondral ossification. Previous studies in other models have established that BMPs induce Wnt expression in xenopus mesoderm, while retinoic acid induces its expression in the developing rostral neural plate in the mouse [Bouillet et al., 1996; Hoppler and Moon, 1998].
Our studies confirm a role for Wnt/β-Catenin regulation of chondrocyte maturation, and further establish a critical role for TGF-β as a modulator of β-Catenin effects. In the absence of TGF-β signaling, β-Catenin is a potent enhancer of colX and alkaline phosphatase gene expression. However, in the presence of TGF-β, the maturational effects of β-Catenin are abolished. These findings are consistent with previous work that has established TGF-β as a potent inhibitor of chondrocyte differentiation. Interestingly, loss of TGF-β function in vivo results in articular chondrocyte maturation [Serra et al., 1997; Yang et al., 2001]. Since these cells normally remain immature, these findings demonstrate that TGF-β inhibits factors that in its absence permit differentiation. β-Catenin appears to be a strong candidate as one of these factors. Recent data has shown that β-Catenin is highly expressed in osteoarthritic cartilage, suggesting that this signaling molecule may be involved in the pathogenesis of osteoarthritis [Kim et al., 2002].
Our data support a multi-factorial mechanism involved in TGF antagonism of β-Catenin effects in chondrocytes. We observed an inhibition of β-Catenin signaling even in the presence of forced overexpression of β-Catenin and with the target being a β-Catenin specific binding sequence on the Topflash reporter. Previous work in other models has established interactive effects between TGF-β and β-Catenin. TGF-β and β-Catenin signaling synergistically stimulate chondrogenesis from undifferentiated human marrow mesenchymal cells [Zhou et al., 2004]. In pellet cultures of human mesenchymal precursor cells, TGF-β stimulates Wnt7a expression results in an increase in basal Topflash signaling, and enhances chondrogenesis [Tuli et al., 2003]. Direct interactions between Smad signaling molecules and β-Catenin have also been demonstrated. Treatment of C3H10T1/2 cells with BMP-2 results in association and co-immunoprecipitation of Smad4 and β-Catenin [Fischer et al., 2002]. In Xenopus embryos, β-Catenin/LEF-1/TCF complexes with Smad4 with direct effects on gene expression [Nishita et al., 2000]. Labbe et al., 2000 have shown that that Smad3 physically interacts with the HMG box domain of LEF-1 and forms a co-immunoprecipitation complex. β-Catenin has also been shown to co-immunoprecipitate with Sox-9, leading to inhibition of signaling, similar to our findings with TGF-β [Akiyama et al., 2004].
Unraveling the manner in which chondrocytes integrate multiple complex signaling pathways in the local environment and determine cell fate is critical for an understanding of endochondral ossification. Among the most challenging features of endochondral ossification is identification of factors that prime chondrocytes for progression through maturation in the absence of inhibitory signals. β-Catenin partially impairs TGF-β signaling, as measured by activation of the p3TP-Luc promoter, but TGF-β has dominant effects as a regulator of maturation. Thus, although β-Catenin is a critical regulator of chondrocyte maturation effects are completely dependent on the presence or absence of TGF-β signaling. Our study defines β-Catenin as one of the elusive intracellular signals priming chondrocytes for progression through maturation in the absence of inhibitory signals.
Acknowledgments
The authors express their appreciation to Dr. Leboy, Dr. Massague, Dr. Westendorf, Dr. Tabin, and Dr. Lassar for providing essential reagents for this study.
Grant sponsor: National Health Service Award (to RJO); Grant number: AR38945.
References
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18(9):1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballock R, Mita BC, Zhou X, Chen DH, Mink LM. Expression of thyroid hormone receptor isoforms in rat growth plate cartilage in vivo. J Bone Miner Res. 1999;14(9):1550–1556. doi: 10.1359/jbmr.1999.14.9.1550. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Oulad-Abdelghani M, Ward SJ, Bronner S, Chambon P, Dolle P. A new mouse member of the Wnt gene family, mWnt-8, is expressed during early embryogenesis and is ectopically induced by retinoic acid. Mech Dev. 1996;58(1–2):141–152. doi: 10.1016/s0925-4773(96)00569-2. [DOI] [PubMed] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress wingless signalling activity. Nature. 1998;395(6702):604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- Church V, Nohno T, Linker C, Marcelle C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115(Pt 24):4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Iwamoto M, Mukudai Y, Kawakami Y, Nohno T, Higuchi Y, Takemoto S, Ohuchi H, Noji S, Kurisu K. Bone morphogenetic signalling is required for maintenence of differentiated phenotype, control of proliferation, and hypertrophy in chondrocytes. J Cell Biol. 1998;140:409–418. doi: 10.1083/jcb.140.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, Koike T, Okada H, Komori T, Yoneda T, Church V, Francis-West PH, Kurisu K, Nohno T, Pacifici M, Iwamoto M. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002;251(1):142–156. doi: 10.1006/dbio.2002.0802. [DOI] [PubMed] [Google Scholar]
- Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O’Keefe RJ. Smad 2 and 3 mediate TGF-β1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;141:4728–4735. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- Ferguson CM, Schwarz EM, Puzas JE, Zuscik MJ, Drissi H, O’Keefe RJ. Transforming growth factor-beta1 induced alteration of skeletal morphogenesis in vivo. J Orthop Res. 2004;22(4):687–696. doi: 10.1016/j.orthres.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Fischer L, Boland G, Tuan RS. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002;277(34):30870–30878. doi: 10.1074/jbc.M109330200. [DOI] [PubMed] [Google Scholar]
- Grimsrud CD, Romano PR, D’Souza M, Puzas JE, Reynolds PR, Rosier RN, O’Keefe RJ. BMP-6 is an autocrine stimulator of chondrocyte differentiation. J Bone Miner Res. 1999;14(4):475–482. doi: 10.1359/jbmr.1999.14.4.475. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127(14):3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104(3):341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Moon RT. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech Dev. 1998;71(1–2):119–129. doi: 10.1016/s0925-4773(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech. 1994;28(6):505–519. doi: 10.1002/jemt.1070280606. [DOI] [PubMed] [Google Scholar]
- Ionescu AM, Schwarz EM, Zuscik MJ, Drissi H, Puzas JE, Rosier RN, O’Keefe RJ. ATF-2 cooperates with Smad3 to mediate TGF-beta effects on chondrocyte maturation. Exp Cell Res. 2003;288(1):198–207. doi: 10.1016/s0014-4827(03)00181-2. [DOI] [PubMed] [Google Scholar]
- Johnson R, Tabin C. Molecular models of vertebrate limb development. Cell. 1997;90:979–990. doi: 10.1016/s0092-8674(00)80364-5. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Im DS, Kim SH, Ryu JH, Hwang SG, Seong JK, Chun CH, Chun JS. Beta-catenin regulates expression of cyclooxygenase-2 in articular chondrocytes. Biochem Biophys Res Commun. 2002;296(1):221–226. doi: 10.1016/s0006-291x(02)00824-0. [DOI] [PubMed] [Google Scholar]
- Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci USA. 2000;97(15):8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher RK, Church VL, Allen S, Robson L, Abdelfattah A, Brown NA, Hattersley G, Rosen V, Luyten FP, Dale L, Francis-West PH. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. Dev Biol. 2000;218(2):183–198. doi: 10.1006/dbio.1999.9586. [DOI] [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Lutz A, Vortkamp A, Pirro A, Karperien M, Defize LHK, Ho C, Mulligan R, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM. PTH/PTHrp receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest. 1999;104(4):399–407. doi: 10.1172/JCI6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JL, Bonner J, Modrell M, Ragland JW, Moon RT, Dorsky RI, Raible DW. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131(6):1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- Li X, Schwarz EM, Zuscik MJ, Rosier RN, Ionescu AM, Puzas JE, Drissi H, Sheu TJ, O’Keefe RJ. Retinoic acid stimulates chondrocyte differentiation and enhances bone morphogenetic protein effects through induction of smad1 and smad5. Endocrinology. 2003;144(6):2514–2523. doi: 10.1210/en.2002-220969. [DOI] [PubMed] [Google Scholar]
- Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4(5):349–360. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Matsunaga S, Yamamoto T, Fukumura K. Temporal and spatial expressions of transforming growth factor-betas and their receptors in epiphyseal growth plate. Int J Oncol. 1999;14(6):1063–1067. doi: 10.3892/ijo.14.6.1063. [DOI] [PubMed] [Google Scholar]
- Miller JR. The Wnts. Genome Biol. 2002;3(1):ReviewS3001. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18(55):7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13(4):157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW. Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann’s organizer. Nature. 2000;403(6771):781–785. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- Nusse R. WNT targets. Repression and activation. Trends Genet. 1999;15(1):1–3. doi: 10.1016/s0168-9525(98)01634-5. [DOI] [PubMed] [Google Scholar]
- Pelton R, Dickinson M, Moses H, Hogan B. In situ hybridization analysis of TGF-beta 3 RNA expression during mouse development: Comparative studies with TGF-beta 1 and TGF-beta 2. Development. 1990;110:609–620. doi: 10.1242/dev.110.2.609. [DOI] [PubMed] [Google Scholar]
- Ramel MC, Lekven AC. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development. 2004;131(16):3991–4000. doi: 10.1242/dev.01277. [DOI] [PubMed] [Google Scholar]
- Rudnicki JA, Brown AM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol. 1997;185(1):104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- Sakou T, Onishi T, Yamamoto T, Nagamine T, Sampath T, Ten Dijke P. Localization of Smads, the TGF-beta family intracellular signaling components during endochondral ossification. J Bone Miner Res. 1999;14(7):1145–1152. doi: 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal muscle promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139(2):541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Karaplis A, Sohn P. Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor beta (TGF-beta) on endochondral bone formation. J Cell Biol. 1999;145(4):783–794. doi: 10.1083/jcb.145.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+signaling by Wnt-5A. Dev Biol. 1997;182(1):114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162(5):899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufan AC, Daumer KM, DeLise AM, Tuan RS. AP-1 transcription factor complex is a target of signals from both WnT-7a and N-cadherin-dependent cell-cell adhesion complex during the regulation of limb mesenchymal chondrogenesis. Exp Cell Res. 2002;273(2):197–203. doi: 10.1006/excr.2001.5448. [DOI] [PubMed] [Google Scholar]
- Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278(42):41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- Volk SW, Lu Valle P, Leask T, Leboy PS. A BMP responsive transcriptional region in the chicken type X collagen gene. J Bone Min Res. 1998;13(10):1521–1529. doi: 10.1359/jbmr.1998.13.10.1521. [DOI] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J Biol Chem. 1996;271(8):4468–4476. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Moon RT. When Wnts antagonize Wnts. J Cell Biol. 2003;162(5):753–755. doi: 10.1083/jcb.200307181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153(1):35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130(5):1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- Zhou S, Eid K, Glowacki J. Cooperation between TFG-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19(3):463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]