Abstract
Corticotropin-releasing hormone (CRH; previously known as corticotropin-releasing factor) is the central regulator of the hypothalamic-pituitary-adrenal (HPA) axis, which is the main organizer of the body’s response to stress.1–5 Stress induces the hypothalamic production and release of CRH, which then causes the activation of the CRH receptor (CRHR) type 1 (CRHR-1) in the anterior pituitary to stimulate ACTH release, as well as proopiomelanocortin (POMC) expression and processing. 1,2,6 ACTH stimulates the production and secretion of cortisol (humans) or corticosterone (rodents) by the adrenal cortex. These steroids regulate the body’s response to counteract effects of the stressor and suppress the HPA through the negative feedback mechanism. CRH/POMC expression can also be activated by the cytokines interleukin (IL)-1, IL-6 and tumour necrosis factor (TNF)-α, thus involving the immune system in the central regulation of the HPA axis.7 In addition, CRH together with related urocortin (URC) peptides regulate behavioural, autonomic, endocrine, reproductive, cardiovascular, gastrointestinal and metabolic functions both on the central and on the peripheral levels, and CRH has immunosuppressive effects via the HPA.6,8–12 It is also accepted that peripheral CRH and related peptides have predominantly proinflammatory functions,13,14 and in this way differ from their central immunosuppressive activity.2 However, recent data also suggest that the peripheral CRH may have dual effects: a direct, short-term proinflammatory function and an indirect, remote anti-inflammatory function.15–18
The corticotropin-releasing hormone system in the skin
In this issue of the BJD Ganceviciene et al.,19 report that the complete CRH signalling system is overexpressed in acne-involved skin with a preferential involvement of the sebaceous glands. Furthermore, the authors propose that overactivation of this CRH system can play an important aetiological role in the development of acne vulgaris through stimulation of local inflammatory reactions.
This conclusion is in accordance with the published information that CRH and related URCs are widely produced by human skin in cell type- and anatomical region-specific manners (reviewed).15,20,21 Furthermore, this expression is regulated by environmental stressors that includes ultraviolet radiation and bacterial antigens.15,22,23 The expression is also modified by intrinsic factors such as glucocorticoids or the phase of the hair cycle (reviewed).21
Skin cells of both epidermal and dermal compartments also express the functional CRHRs type 1 and 2 (CRHR-1 and CRHR-2), with CRHR-1 being the predominant (if not the sole) CRHR type detected in human epidermis (keratinocytes and melanocytes).15,24 However, CRHR-2, in addition to CRHR-1, is expressed in dermal cells including hair follicle keratinocytes, melanocytes and follicular papilla fibroblasts, sebaceous and eccrine glands, muscle and dermal blood vessels.15,24–27 Both receptors belong to the group II subfamily of G protein-coupled receptors.6 CRHR-1 binds CHR and URC1 with high affinity but does not bind URC2. CRHR-2 shows preferential activation after binding URC2/3, and also binds CRH although with lower affinity than CRHR-1. The human CRHR1 gene encodes 14 exons and can generate at least seven alternatively spliced isoforms, of which CRHR1α is the most important.6,8,15,28 The CRHR2 gene contains 15 exons and generates several alternatively spliced isoforms with the major forms represented by CRHR2α, β and γ, and with a possibility of generating additional forms because of multiple promoters with intra-intronic location.6,21,29,30 Coupling of different CRHR-1/2 isoforms to different signal transduction systems or their functional assignments represents a major challenge in this field, which, however, could provide mechanistic explanations for organ- and cell type-dependent variability of phenotypic responses to the ligand.6,15,28
Significance of the cutaneous corticotropin- releasing hormone signalling system
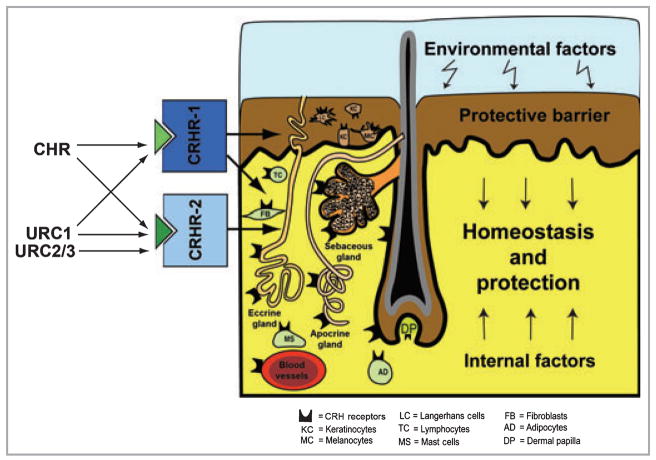
In skin cells, CRH and related peptides exhibit nonendocrine activities regulating cell proliferation, viability, differentiation, secretory and immune activities, thereby defining these peptides as novel important growth factors/pleiotropic cytokines.15,27,31–35 Interestingly, there is a skin compartment- and cell type-dependent variability of phenotypic responses to CRH or URCs.15,24,25,31 These are sometimes opposite for different cell types (e.g. keratinocytes vs. fibroblasts) 31 or for the same cell type but at a different location (e.g. epidermal vs. follicular melanocytes).25,31 These phenotypic effects of CRH and related peptides are secondary to modulation of the intracellular concentrations of cAMP, IP3, Ca2+or NF-κB activity (reviewed).15 We have proposed that the net effect of this diverse CRH/URC-led signalling system(s) is to regulate protective and homeostatic functions of the skin (Fig. 1).
Fig 1.
Corticotropin-releasing hormone (CRH)/urocortin (URC) signalling system regulates protective and homeostatic functions of the skin. CRH receptor (CRHR) type 1 (CRHR-1) is predominantly expressed in the epidermis, while both CRHR-1 and CRHR type 2 (CRHR-2) are expressed in the dermal or adnexal compartments.
Regulation of the cutaneous corticotropin- releasing hormone signalling system
In the context of data presented by Ganceviciene et al.,19 it must be noted that in normal keratinocytes CRH has proinflammatory effects, e.g. CRH activates NF-κB (the master regulator of inflammation), and stimulates expression of intercellular adhesion molecule-1, HLA-DR and cytokine production (TNF-α, IL-1β and IL-6 but not IL-10, which is unaffected).16,34,36,37 Furthermore, lipopolysaccharide (LPS; toll-like receptor-4 agonist) stimulates CRH production, and the LPS-stimulated expression of TNF-α, IL-1β and IL-6 is dependent on the expression of CRHR-1.22 Thus, CRH and its cognate receptor(s) can serve as local amplifiers of inflammatory responses to bacterial antigens and hence be involved in the pathogenesis of acne or other inflammatory dermatoses. Taking into consideration the abundance of data showing proinflammatory actions of CRH in the skin (reviewed),14,15,36 one is facing a dilemma: why can the system designed to protect and stabilize play a destructive role by being involved in the aetiology of inflammatory skin disorders?38
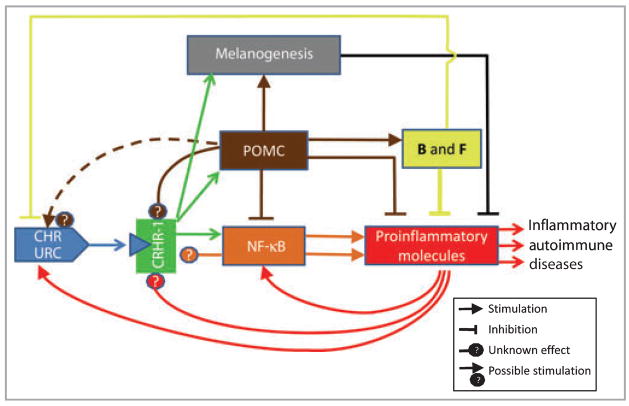
In my opinion, the CRH/URC amplified proinflammatory state is the result of defects in the attenuating homeostatic elements inhibiting either immune activity or CRH/URC signalling or the immune messengers/pathways activated by it (Fig. 2). For example, CRHR-1 activation stimulates cutaneous POMC gene expression and production of potent immunosuppressive ACTH and α-melanocyte-stimulating hormone (α-MSH),39–42 as well as enhancing the production of cortisol (F) and corticosterone (B) that is POMC dependent.32,39,42 This functional algorithm defined in vitro has been confirmed by Ito et al.40 in an ex vivo organ culture system. POMC-derived ACTH and α-MSH (and possibly β-endorphin), as well as corticosteroids, would inhibit directly the proinflammatory chain reaction activated by CRH. The indirect effects would include an immunosuppressive action of melanogenesis intermediates43 induced directly by activation of CRHR-125 or indirectly by activation of melanocortin-1 receptor by ACTH/α-MSH,44 or by action of Th2 cytokines stimulated by α-MSH.45 Thus, in the skin CRH/URC having direct proinflammatory effects can also stimulate indirectly the production of immunosuppressive molecules, which is dependent on the cellular or network context.
Fig 2.
Dynamic feedback interactions between the immune system of the skin and the local corticotropin-releasing hormone (CRH)/urocortin (URC) signalling cascade. POMC, proopiomelanocortin; B, corticosterone; F, cortisol.
The feedback inhibition of CRH production by F or B is well documented in a number of experimental models.46 An opposite effect, the stimulation of CRH/URC, can be exerted by proinflammatory cytokines IL-1, IL-6 and TNF-α,7 or possibly by POMC-derived ACTH, α-MSH or β-endorphin (Fig. 2)15 (e.g. these peptides activate cAMP and Ca signals, which are crucial for CRH/URC production and release).8,12,46–48 The effects of POMC peptides or cytokines on the CRHR activity itself is unknown; however, such a possibility does exist (Fig. 2). For example, factors raising intracellular cAMP levels or activating protein kinase C can regulate alternative splicing of the CRHR-1 with predominant production of the main CRHR1α isoform.28 Furthermore, alternative splicing of CRHR-1 and CRHR-2 can also produce soluble forms that lack transmembrane domains and, therefore, after secretion they can bind and sequester the CRH/URC ligands.15,28 Thus, the cutaneous CRH signalling system represents a dynamic process, being a subject of tight regulation by its downstream regulatory elements (Fig. 2). This regulation is also dependent on the cellular/network context. For example, in keratinocytes CRH is proinflammatory because of poor coupling of the CRHR-1 to cAMP and its dissociation from the POMC activity.15,31 In contrast, in melanocytes it is anti-inflammatory, e.g. CRH inhibits NF-κB via POMC activation,17 increases secretion of immunosuppressive glucocorticoids32 and stimulates melanogenesis, 26 of which intermediates are immunoinhibitory.44 In this model, the deregulation of the downstream immunosuppressive circuitry may have catastrophic consequences, as proinflammatory reactions can self-amplify as proposed in Figure 2. The central role of the CRHR-1 in the above model implies usage of selective receptor antagonists49,50 or alternatively spliced soluble isoforms15 as adjuvants in the therapy of inflammatory skin disorders.
In conclusion, it is proposed that the inefficient local attenuation of the CRH signalling system and/or defective coupling to the downstream immunosuppressive regulatory mechanisms exacerbate or induce proinflammatory responses leading to inflammatory and/or autoimmune disease processes.
Acknowledgments
I thank Drs Edward Wei, Kurt Dohan, Blazej Zbytek and Jackie Granese, and Radomir Slominski for their comments on the manuscript. Writing of this commentary was supported in part by NIH grants AR052190 and AR047079 to A.S.
References
- 1.Vale W, Spiess J, Rivier C, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 3.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 4.Selye H. A syndrome produced by various noxious agents. Nature. 1936;138:32–3. [Google Scholar]
- 5.Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature. 1983;305:325–7. doi: 10.1038/305325a0. [DOI] [PubMed] [Google Scholar]
- 6.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin- releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–86. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 7.Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain Behav Immun. 1995;9:253–75. doi: 10.1006/brbi.1995.1026. [DOI] [PubMed] [Google Scholar]
- 8.Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann NY Acad Sci. 1999;885:312–28. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 9.Reyes TM, Lewis K, Perrin MH, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001;98:2843–8. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis K, Li C, Perrin MH, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA. 2001;98:7570–5. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuperman Y, Chen A. Urocortins: emerging metabolic and energy homeostasis perspectives. Trends Endocrinol Metab. 2008;19:122–9. doi: 10.1016/j.tem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karalis K, Sano H, Redwine J, et al. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254:421–3. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- 14.Theoharides TC, Donelan JM, Papadopoulou N, et al. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–8. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Slominski A, Zbytek B, Zmijewski M, et al. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–48. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone stimulates NF-kappaB in human epidermal keratinocytes. J Endocrinol. 2004;181:R1–7. doi: 10.1677/joe.0.181r001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zbytek B, Pfeffer LM, Slominski AT. CRH inhibits NF-kappaB signaling in human melanocytes. Peptides. 2006;27:3276–83. doi: 10.1016/j.peptides.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski A, Wortsman J, Linton E, et al. The skin as a model for the immunodulatory effects of corticotropin-releasing hormone. In: Schäfer M, Stein C, editors. Mind Over Matter – Regulation of Peripheral Inflammation by the CNS. 1. Basel: Birkhäuser Verlag; 2003. pp. 149–76. [Google Scholar]
- 19.Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345–352. doi: 10.1111/j.1365-2133.2008.08959.x. [DOI] [PubMed] [Google Scholar]
- 20.Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 21.Slominski A, Wortsman J, Pisarchik A, et al. Cutaneous expression of corticotropin releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–93. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 22.Zbytek B, Slominski AT. CRH mediates inflammation induced by lipopolysaccharide in human adult epidermal keratinocytes. J Invest Dermatol. 2007;127:730–2. doi: 10.1038/sj.jid.5700607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone–proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20:2539–47. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski A, Pisarchik A, Tobin DJ, et al. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–50. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kauser S, Slominski A, Wei ET, et al. Modulation of the human hair follicle pigmentary unit by corticotropin-releasing hormone and urocortin peptides. FASEB J. 2006;20:882–95. doi: 10.1096/fj.05-5257com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause K, Schnitger A, Fimmel S, et al. Corticotropin-releasing hormone skin signaling is receptor-mediated and is predominant in the sebaceous glands. Horm Metab Res. 2007;39:166–70. doi: 10.1055/s-2007-961811. [DOI] [PubMed] [Google Scholar]
- 27.Zouboulis CC, Seltmann H, Hiroi N, et al. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci USA. 2002;99:7148–53. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–6. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 29.Catalano RD, Kyriakou T, Chen J, et al. Regulation of corticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: identification of multiple splice variants. Mol Endocrinol. 2003;17:395–410. doi: 10.1210/me.2002-0302. [DOI] [PubMed] [Google Scholar]
- 30.Lovenberg TW, Liaw CW, Grigoriadis DE, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–40. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slominski A, Zbytek B, Pisarchik A, et al. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206:780–91. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slominski A, Wortsman J, Tuckey RC, et al. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;266:143–9. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donelan J, Boucher W, Papadopoulou N, et al. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA. 2006;103:7759–64. doi: 10.1073/pnas.0602210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quevedo ME, Slominski A, Pinto W, et al. Pleiotropic effects of corticotropin releasing hormone on normal human skin keratinocytes. In Vitro Cell Dev Biol Anim. 2001;37:50–4. doi: 10.1290/1071-2690(2001)037<0050:peocrh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Slominski AT, Roloff B, Zbytek B, et al. Corticotropin releasing hormone and related peptides can act as bioregulatory factors in human keratinocytes. In Vitro Cell Dev Biol Anim. 2000;36:211–16. doi: 10.1290/1071-2690(2000)036<0211:CRHARP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.O’Kane M, Murphy EP, Kirby B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp Dermatol. 2006;15:143–53. doi: 10.1111/j.1600-0625.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 37.Zbytek B, Mysliwski A, Slominski A, et al. Corticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytes. Life Sci. 2002;70:1013–21. doi: 10.1016/s0024-3205(01)01476-x. [DOI] [PubMed] [Google Scholar]
- 38.Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest. 2007;117:3166–9. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slominski A, Zbytek B, Szczesniewski A, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–6. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 40.Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–4. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 41.Rousseau K, Kauser S, Pritchard LE, et al. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007;21:1844–56. doi: 10.1096/fj.06-7398com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slominski A, Zbytek B, Semak I, et al. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Slominski A, Paus R, Mihm MC. Inhibition of melanogenesis as an adjuvant strategy in the treatment of melanotic melanomas: selective review and hypothesis. Anticancer Res. 1998;18:3709–15. [PubMed] [Google Scholar]
- 44.Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 45.Böhm M, Luger TA, Tobin DJ, et al. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 2006;126:1966–75. doi: 10.1038/sj.jid.5700421. [DOI] [PubMed] [Google Scholar]
- 46.Orth DN. Corticotropin-releasing hormone in humans. Endocr Rev. 1992;13:164–91. doi: 10.1210/edrv-13-2-164. [DOI] [PubMed] [Google Scholar]
- 47.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 48.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin- releasing factor. Pharmacol Rev. 1991;43:425–73. [PubMed] [Google Scholar]
- 49.Gulyas J, Rivier C, Perrin M, et al. Potent, structurally constrained agonists and competitive antagonists of corticotropin-releasing factor. Proc Natl Acad Sci USA. 1995;92:10575–9. doi: 10.1073/pnas.92.23.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koerber SC, Gulyas J, Lahrichi SL, et al. Constrained corticotropin-releasing factor (CRF) agonists and antagonists with i-(i + 3) GluXaa-DXbb-Lys bridges. J Med Chem. 1998;41:5002–11. doi: 10.1021/jm980350k. [DOI] [PubMed] [Google Scholar]