Abstract
Lipid-water interaction plays an important role in the properties of lipid bilayers, cryoprotectants, and membrane-associated peptides and proteins. The temperature at which water bound to lipid bilayers freezes is lower than that of free water. Here, we report a solid-state NMR investigation on the freezing point depression of water in phospholipid bilayers in the presence and absence of cholesterol. Deuterium NMR spectra at different temperatures ranging from -75 to +10°C were obtained from fully 2H2O-hydrated POPC (1-palmitoyl-2-oleoyl-phosphatidylcholine) multilamellar vesicles (MLVs), prepared with and without cholesterol, to determine the freezing temperature of water and the effect of cholesterol on the freezing temperature of water in POPC bilayers. Our 2H NMR experiments reveal the motional behavior of unfrozen water molecules in POPC bilayers even at temperatures significantly below 0°C and show that the presence of cholesterol further lowered the freezing temperature of water in POPC bilayers. These results suggest that in the presence of cholesterol the fluidity and dynamics of lipid bilayers can be retained even at very low temperatures as exist in the liquid crystalline phase of the lipid. Therefore, bilayer samples prepared with a cryoprotectant like cholesterol should enable the performance of multidimensional solid-state NMR experiments to investigate the structure, dynamics, and topology of membrane proteins at a very low temperature with enhanced sample stability and possibly a better sensitivity. Phosphorus-31 NMR data suggest that lipid bilayers can be aligned at low temperatures, while 15N NMR experiments demonstrate that such aligned samples can be used to enhance the signal-to-noise ratio of 15N chemical shift spectra of a 37-residue human antimicrobial peptide, LL-37.
Introduction
Cellular membranes are liquid crystalline lipid bilayers composed of phospholipids, proteins, water, cholesterol, and other components. Water plays an important role in the formation, stability, and function of the bilayer membrane and also in maintaining the liquid crystalline phase of the membrane.1 Many properties of lipid membranes strongly depend on the interaction of lipids with water. Previous studies on model lipid membranes revealed the presence of two different types of water in bilayers: free water and water that is bound to the phospholipid headgroups near the surface of the bilayer.2-4 The number of water molecules bound to phospholipid bilayers depends on the type and the physical phase of lipids. Tightly bound water molecules resist to freeze and maintain significant amount of motional freedom even at subzero temperatures.5-8 An important property of cellular membranes is that they behave as two-dimensional fluids in which molecules can rotate and move in lateral directions. In this process, the fluidity is critical for their function and is determined by the lipid composition and temperature. Therefore, it is important to understand the mobility and freezing temperature of water molecules that are bound to lipids in membranes. Previous studies using thermal analysis and NMR experiments reported that water molecules are mobile even at subzero temperatures under various environments such as water bound to macromolecules, biopolymers, or different compounds present in food.6-10 In this study, we present a solid-state NMR investigation on the mobility of water molecules in lipid bilayers.
Cholesterol, soluble in phospholipid bilayers, plays an important role in controlling the fluidity and stability of biological cellular membranes at different temperatures. Previous studies showed that the addition of cholesterol into phospholipid membranes increased the number of unfreezable water molecules,4 and also lowered and broadened the main phase transition temperature of lipids.11,12 The presence of cholesterol and related changes in the fluidity of a cell membrane are important factors in the function of antimicrobial peptides, toxicity of amyloid peptides like IAPP (islet amyloid polypeptides) and Aβ.13-18 In this study, we have used 2H solid-state NMR techniques to investigate the freezing behavior of water in POPC (1-palmitoyl-2-oleoyl-phosphatidylcholine) bilayers in the presence and absence of cholesterol in order to understand the role of cholesterol in biological membranes. Since the quadrupole coupling is very sensitive to molecular motions, in the present study, 2H NMR experiments are used to study the dynamics of water.19-26 2H quadrupole coupling value of deuterated water is used to study the freezing temperature of pure water, and water present in lipid bilayers with and without cholesterol. POPC bilayers are used as model membranes as this lipid is naturally present in cellular membranes, has a low phase transition temperature, and commonly used in biophysical studies. Our experimental results show that the thermal mobility of water freezes at temperatures much lower than that of the pure water and the presence of cholesterol further lowers the freezing point of water in POPC bilayers.
Materials and methods
Materials
POPC was purchased from Avanti Polar Lipids (Alabaster, AL). Chloroform and methanol were purchased from Aldrich Chemical Company (Milwaukee. WI). D2O was purchased from Cambridge Isotope Lab Inc. (Andover, MA). All chemicals were used without further purification.
Sample preparation
MLVs were prepared by mixing a 5 mg of POPC with 5μl D2O, followed by several freeze in liquid nitrogen and thaw at room temperature cycles. For Cholesterol/ POPC samples, 25% Cholesterol by mole was mixed with POPC and 5 μl D2O was added through several freeze-thaw cycles. The final sample had 42 mole % of water. Sample was stabilized at least an hour before data acquisition at each experimental temperature. Mechanically aligned lipid bilayers were prepared using the naphthalene procedure as described elsewhere.27
Solid-state NMR
All experiments were performed on a Chemagnetics/Varian Infinity 400 MHz solid-state NMR spectrometer operating at resonance frequencies of 400.138, 161.979, 61.424, and 40.55 MHz for 1H, 31P, 2H, and 15N nuclei, respectively. A 5 mm Varian/Chemagnetics double resonance MAS probe was used to acquire the 2H quadrupole coupling NMR spectra of multilamellar vesicles packed in 5 mm glass tube. All experiments on oriented samples were performed with the lipid bilayer normal parallel to the external magnetic field of the spectrometer. The 31P and 15N chemical shift spectra of mechanically aligned samples were obtained using a home-built double resonance probe. Sample temperature was controlled and maintained using a temperature controller unit (Varian/Chemagnetics) with an accuracy of ±0.3°C; some of the low-temperature experiments were also performed in different spectrometers to confirm the accuracy of the measured temperature and spectra. All 2H quadrupole coupling NMR spectra of water in POPC bilayers were acquired using a quadrupole echo pulse sequence (90°-τ-90°- τ-acquire)28 with a 2H 90° pulse length of 2.3 μs. The recycle delay was 1 s and the spectral width was 500 kHz. 31P spectra were obtained using a spin-echo sequence (90°-τ-180°-τ-acq with τ=100 μs), 40 kHz proton-decoupling rf field, 50 kHz spectral width, and a recycle delay of ∼4 seconds. A typical spectrum required the co-addition of 100–1000 transients. The 31P chemical shift spectra are referenced relative to 85% H3PO4 on thin glass plates (0 ppm). 15N chemical shift spectra of a 15N-labeled LL-37 peptide in oriented bilayers were acquired using a ramp cross-polarization sequence29 with a 1H π/2 pulse length of 3.5 μs, 35 kHz cross-polarization RF field strength, and a 1H decoupling of 71 kHz during acquisition. The recycle delay was 3 s and the spectral width was 50 kHz. 15N chemical shift spectra are reference relative to liquid ammonia (at 0 ppm). All spectra were processed using the Spinsight software (Chemagnetics) on a Sun Sparc workstation. The powder spectrum was simulated using a FORTRAN program on a PowerBook G4.
Results
2H NMR spectra of pure water
In this study, deuterium NMR spectroscopy is used to determine the mobility of water in lipid bilayers. 2H NMR spectra of deuterated water were collected at several different temperatures to understand the freezing temperature of water and the mobility of water molecules. Under normal experimental conditions, pure liquid water freezes to form ice around 0°C. Due to the fast tumbling motion of water molecules in the liquid state, 2H quadrupole interaction of water is averaged out at temperatures above 0°C and a narrow peak at its isotropic chemical shift frequency is observed (spectra not shown). On the other hand, as the temperature of water is lowered below the freezing temperature, it is expected that water molecules become immobile in the NMR time scale (i.e., the inverse of the quadrupole coupling) and therefore a 2H powder pattern would be observed. While a quadrupole coupling powder pattern from immobile water was observed below 0°C, a narrow line at the isotropic frequency indicating the presence of mobile water molecules was also present in the spectrum (data not shown). The intensity of the narrow peak at the zero-frequency decreased as the temperature lowered further. For example, the spectrum obtained at -15°C in Figure 1A suggests that most water molecules are frozen and resulted in a powder pattern but some water molecules still experienced rapid reorientational motions as evidenced by the presence of an isotropic peak at the center of the spectrum. Representative 2H quadrupole coupling NMR spectra of D2O given in Figure 1 suggest that the isotropic peak disappears at temperatures <-23°C and a typical 2H quadrupole coupling powder (or Pake doublet) spectrum is observed (Figure 1B). The powder pattern line shape was simulated using a Fortran program and the best-fitting spectrum is shown in Figure 1C. The measured magnitudes of the principal components of the 2H quadrupole coupling tensor are 73.0, 90.1, and -163.1 kHz with an asymmetry parameter, η, of 0.1. A typical 2H quadrupole coupling constant for simple crystalline hydrates is known to be about 200 kHz.6 It was previously shown that water molecules in ice reorient with a tetrahedral symmetry.10 The reorientational motion was frozen around 240 K. At the intermediate temperatures (above -15°C), 2H spectra showed the characteristic features of slow molecular reorientation (1/τC<200kHz).
Figure 1.
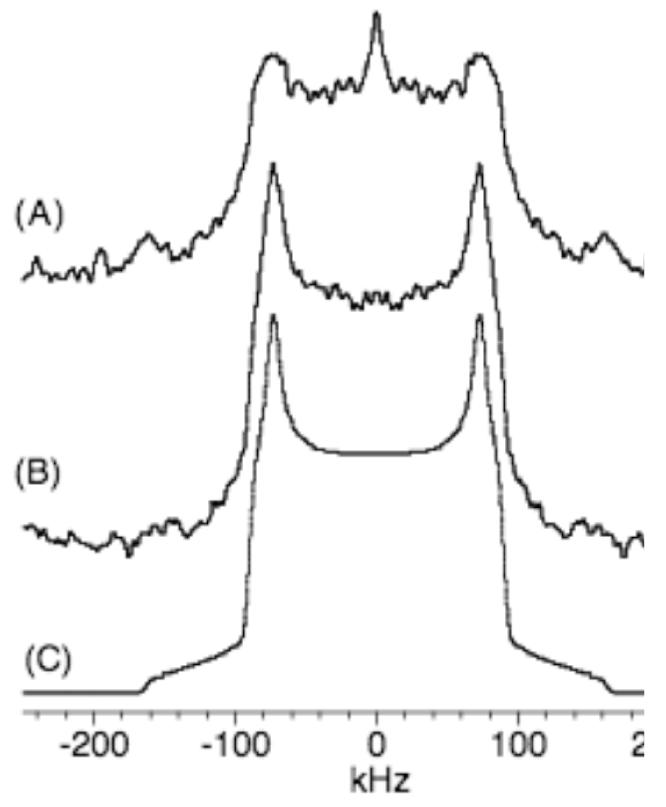
2H quadrupole coupling NMR spectra of deuterated water obtained at -15°C (A), -23°C (B), and by computer simulations (C). The spectrum (C) is the best-fit for the experimental spectrum (B). The magnitudes of the principal components of the quadrupole coupling tensor determined from the best-fit spectrum are 73.0, 90.1 and -163.1 kHz.
2H NMR spectra of water in POPC bilayers
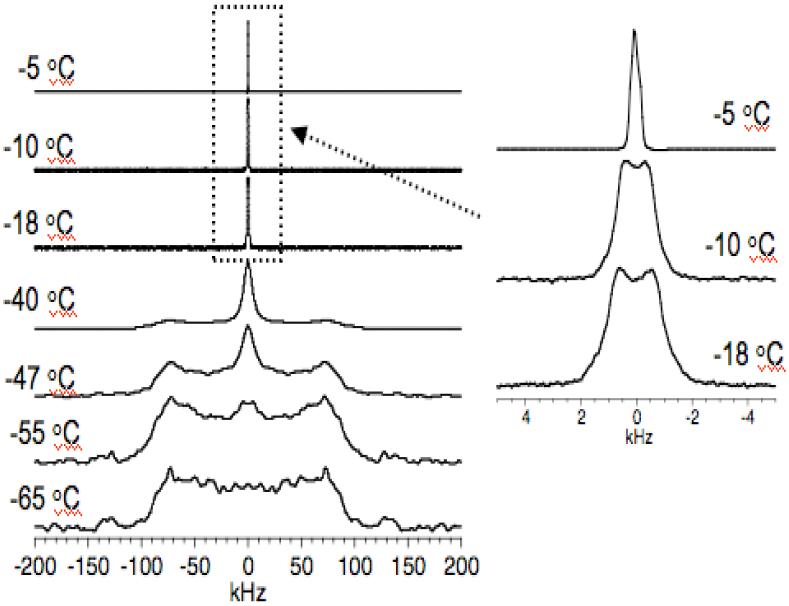
Analysis of 2H NMR spectra of deuterated water molecules in model membranes provides useful information on the motional behavior of water molecules and on the role of water in the stability of lipid bilayer structures. For this purpose, 2H quadrupole coupling spectra of well-hydrated (with D2O) POPC MLVs were obtained at several different temperatures ranging from +25 to -65°C. Representative spectra are given in Figure 2. An isotropic peak was observed for temperatures well above -5°C, indicating that the mobility of water molecules in POPC bilayers averages out the 2H quadrupole interaction. As shown in Figure 2, at -5°C the spectrum shows a combination of an isotropic line at the zero-frequency and a broad line indicating the presence of two types of water molecules. The two different spectral patterns originate from two types of water molecules in the sample: (a) free water molecules resulting in an isotropic peak and (b) water molecules bound to the hydrophilic phospholipid headgroups resulting in a motionally averaged small quadrupole splitting, which is more clear from the line shapes observed at -10°C (Figure 2).
Figure 2.
2H quadrupole coupling NMR spectra of deuterated water in POPC multilamellar vesicles obtained at different temperatures. Typically 250 to 2048 scans were used with a recycle delay of 1s to obtain these spectra.
Previous studies showed similar spectra resulting from the effects of different solutes (DMSO, sorbitol, sucrose, and trehalose) on the hydration of DOPC as a function of temperature.9 During the hydration of lipid molecules, water molecules can form many layers of water molecules near phospholipid headgroups. A previous study showed that only a small fraction of water molecules (<5) per lipid are considerably affected by the interaction with the bilayer surface. For samples with >5 water molecules per lipid, an isotropic peak was observed.30 In this study, a small quadrupole splitting is clearly seen in 2H spectra at temperatures ranging from -10 to -30°C; two representative spectra obtained at temperatures of -10°C and -18°C are shown in Figure 2. The splitting is attributed to the reduction in the mobility of water molecules that are bound to hydrophilic phospholipid headgroups; the rigid quadrupole splitting (∼200 kHz) observed in rigid solid ice is motionally averaged out in hydrated lipid bilayers to a small value, 1.5 kHz.
The peaks in the small doublet broadened as the temperature decreased below -18°C. Similar spectral patterns were reported from dehydrated phospholipid bilayers and studies have reported deuterium spectra of purple membrane associated water as a function of hydration.31 At the same time, the rigid quadrupole coupling pattern also appeared as evidenced by the Pake doublet powder pattern with the maximum intensity at ±80 kHz. Spectrum obtained at -47°C shows both features. A complete freezing of water in POPC vesicles occurs around -65°C (Figure 2). This observation is consistent with the previous DSC (differential scanning calorimetry) data.32 Thus, the temperature to completely freeze the water molecules bound to POPC is lowered by about 40°C compared to that of the pure water. A 2H NMR study that investigated the exchange between deuterium of D2O used to hydrate the sample and hydrogen in phosphatidylcholine headgroups reported a little exchange of deuterons with the hydrogens of the lipid.5 Therefore, this possibility is not considered in the interpretation of 2H spectra in this study.
2H NMR spectra of water in POPC bilayers containing cholesterol
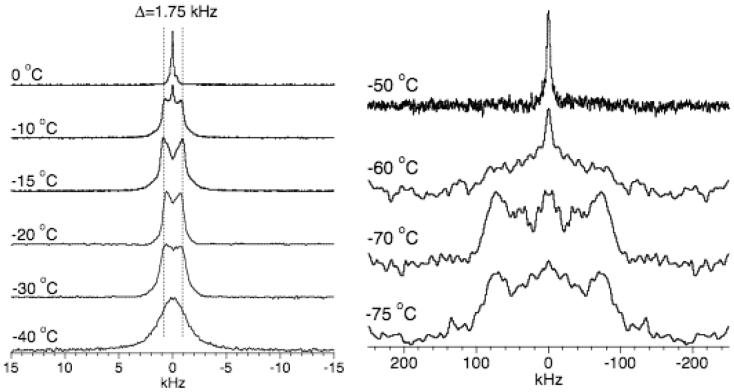
Cholesterol is an important component, naturally occurring in biological cellular membranes. To understand the role of cholesterol on the phase transition temperature of the membrane, in the present study, MLVs composed of 3:1 POPC:Cholesterol were prepared by direct hydration using deuterated water and 2H quadrupole coupling spectra were obtained at several different temperatures. Experimental spectra are given in Figure 3. At 0°C, a single narrow peak dominated the spectrum due to the motional averaging of water molecules, but a small intensity from bound water was also noticeable. The mobility of water molecules is significantly suppressed at -10°C as evidenced by the reduction in the intensity of the isotropic peak and the increase in the intensity of the spectral line from bound water with a splitting of 1.5 kHz as shown in Figure 3. At -15°C, the isotropic peak almost disappeared and the 2H signal broadened out. Figure 3 shows an averaged quadrupole coupling of 1.5 kHz at -20°C, indicating that water at this temperature still experiences a rapid motion that averages out the quadrupole interaction.
Figure 3.
2H quadrupole coupling NMR spectra of deuterated water in 3:1 POPC:cholesterol multilamellar vesicles obtained at different temperatures. 256 to 2048 scans were used with a recycle delay of 1s.
Around -40°C, the splitting is merged to a single broad line at the center of the spectrum with a full-width at a half-maximum of 4 kHz (Figure 3). However, water molecules are not frozen and their motion is still fast to average the rigid quadrupole splitting of 200 kHz to 4 kHz. The increase in the observed 2H quadrupole splitting from 1.5 kHz at -30°C to 4 kHz at -40°C could be due to the change in the lipid phase from Lα to gel phase.25 For temperatures below -40°C and above -50°C, bound water molecular motion is frozen in the NMR time scale to result in a rigid 2H quadrupole coupling powder pattern and the signal intensity increases as the temperature decreases to -75°C (Figure 3). Therefore, our results infer that the presence of 33 mole % cholesterol decreases the temperature at which the mobility of water molecules in POPC bilayers freezes by about 15°C.
31P Chemical shift spectra of mechanically aligned bilayers at low temperature
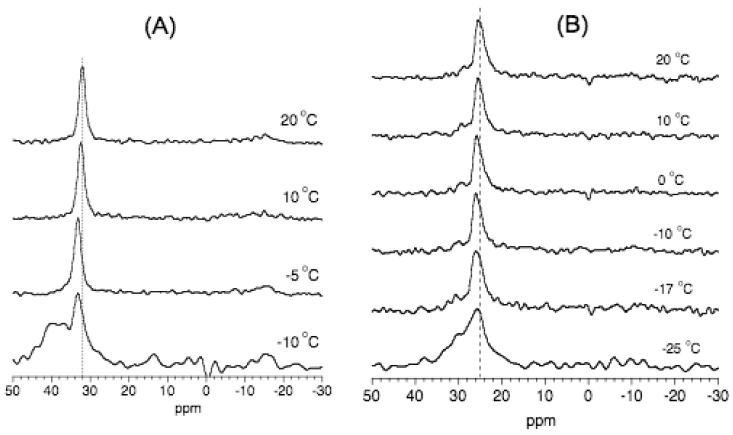
As shown in Figure 4, for temperatures down to -30°C, bound water molecules undergo motion that results in an averaged quadrupole splitting of 1.5 kHz. Therefore, lipid molecules should be in a fluid phase even at this low temperature, which can be determined from 31P NMR. Since mechanically aligned lipid bilayers are commonly used in NMR studies of model membranes,33-37 we explored the possibility of aligning lipids at a low temperature and the possibility of using cholesterol to align lipid samples below the main phase transition temperature of the lipid. 31P chemical shift spectra of uniaxially aligned POPC bilayers without (Figure 5A) and with cholesterol (Figure 5B) obtained at different temperatures are given in Figure 5. The appearance of a single peak ∼32 ppm in Figure 5A suggests that the pure POPC sample is well aligned for temperatures as low as the phase transition temperature (∼-5°C) of the lipid. As the temperature of the sample decreases the frequency of the observed peak increases: for example, 32 ppm at 20°C to 34 ppm at -5°C. This is because a decrease in the sample temperature reduces the lipid head group motion and therefore decreases the span of 31P chemical shift; the low frequency edge of the broad low intensity signal at the noise level arising from unaligned lipids can be seen to change from ∼ -14 ppm at 20°C to ∼ -16 ppm at -5°C. Therefore, the observed peak from aligned lipids in the sample is always at the parallel (or low-field) edge of the 31P powder pattern suggesting that the lipid orientation did not change even at -5°C. However, at -10°C, that is below the main phase transition temperature of the lipid, a broad signal ∼40 ppm indicating the contribution from lipids in gel phase is observed (Figure 5A). On the other hand, interestingly, alignment of lipid molecules are retained for temperatures as low as -17°C for POPC bilayers containing cholesterol as indicated by the appearance of a narrow peak ∼25 ppm (Figure 5B). The difference in the frequency of peaks observed in the pure (at ∼32 ppm) and cholesterol-containing (∼25 ppm) POPC bilayers could be attributed to the additional motional averaging experienced by the lipid head group in the presence of cholesterol. Since cholesterol increases the fluidity of lipid bilayers, more motional averaging is expected for water bound to lipids as observed by the relatively less 2H quadrupole splitting in the presence of cholesterol (Figures 2-4) as well as by the reduced 31P chemical shift anisotropy span of the lipid head group.
Figure 4.
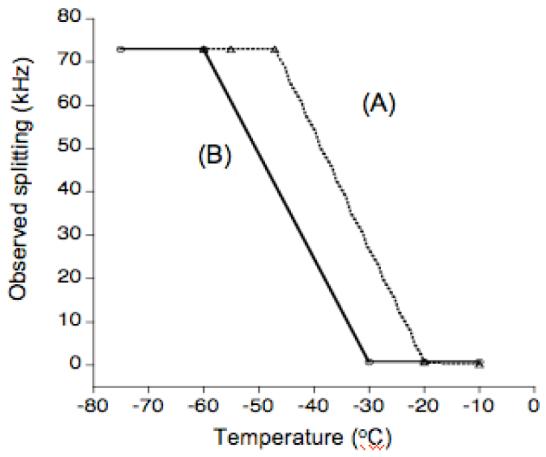
A comparison of experimentally measured frequencies of the 2H quadrupole splitting at the maximum intensity (A) D2O in POPC MLVs and (B) D2O in 3:1 POPC:cholesterol MLVs.
Figure 5.
31P chemical shift spectra of the uniaxially aligned POPC bilayers at different temperatures without (A) and with (B) cholesterol. Each spectrum was the resultant of 128 scans with a recycle delay of 3s.
15N Chemical shift spectra of a peptide embedded in mechanically aligned bilayers at low temperature
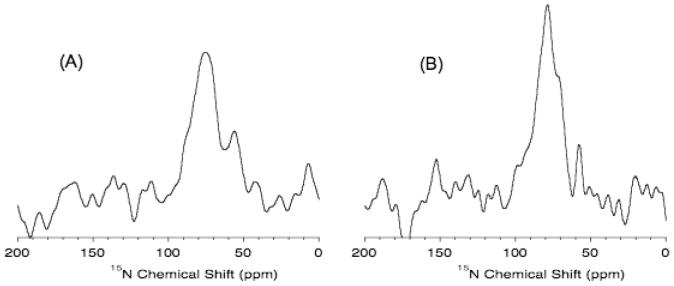
Since lipid bilayers can be aligned at low temperatures as demonstrated by 31P experiments (Figure 5), it should be possible to align peptides and proteins at very low temperatures and therefore it could be possible to increase the sensitivity of solid-state NMR experiments.38 To demonstrate this point, experiments were performed on mechanically aligned POPC bilayers containing a human antimicrobial peptide, LL-3739,40 at different temperatures. Sample 15N chemical shift spectra given in Figure 6 suggest that it is possible to align samples at low temperatures and the observed signal-to-noise ratio is ∼1.3 times better than that observed at a higher temperature. Lowering the sample temperature suppresses molecular motions, which average dipolar couplings that are needed to transfer proton magnetization to nitrogen in a cross-polarization experiment, and therefore enhances the signal-to-noise ratio of the observed 15N spectrum of the peptide embedded in lipid bilayers. Previous NMR studies have reported the high-resolution structure,41 topology,36,37 and mechanism of membrane disruption of LL-37.42 Previous solid-state NMR studies have shown that this peptide is oriented near the surface of lipid bilayers and functions via carpet/toroidal-pore mechanism to disrupt bacterial membranes.
Figure 6.
15N chemical shift spectra of 2 mole % 15N-Phe-6-LL-37 in POPC bilayers. A 6.6 mg of LL-37 was used in the sample. 27,000 scans with a 3 s delay and a ramp-cross-polarization contact time of 250 μs were used to obtain the spectra at (A) 37°C and (B) 2°C.
Discussion
There is considerable current interest in understanding the role of water-lipid interactions in the structure, folding, and function of a variety of molecules embedded in the cell membrane. Particularly, the structural and dynamical differences between the free water molecules and lipid-bound water molecules are interesting. In this study, we have investigated the dynamics of water in pure water and when it is bound to lipids using 2H NMR spectroscopy.
Freezing point of water in phospholipid vesicles
Previous studies have shown that deuterated water and regular water have the same swelling behavior of POPC.5,43 Thus, the results obtained from lipid samples hydrated by deuterated water are comparable with those obtained from the lipid samples hydrated by H2O. In the present study, 2H solid-state NMR spectra of water in phospholipid bilayers were obtained at several different temperatures in order to see their motional properties and phase behaviors. Pure water undergoes a phase transition from liquid to solid around 0°C. However, as suggested by 2H NMR results (Figure 1), the motional properties of water molecules still exist even at temperatures below the normal freezing point of water. The temperature at which water molecular motions freeze depends on its environment, such as membranes, macromolecules, and other ultrastructural elements in cells, tissues, or food products.2 Table 1 summarizes the approximate freezing temperatures of water in several different systems. As seen from Table 1, the freezing temperature of water in biological environments is less than that of the pure water.
Table 1.
Temperatures at which water molecular motions freeze in different environments.
Molecular origin of the isotropic 2H NMR signal of deuterated water bound to lipid membranes below subzero temperature is not clear. However, it has been explained in a few different ways. In the case of pure water, one study proposed that naturally occurring small defect in ice crystalline could cause free rotation of water molecules,11 which results in the observed isotropic peak in the 2H quadrupole coupling spectrum. Another study of ice using 2H NMR showed that water molecules in normal hexagonal ice experiences tetrahedral jumps even at subzero temperatures.10 The latter results were used to explain the conductivity and polarizability of ice.10 These experimental results supported Pauling’s assumption that ice can exist in a large number of configurations in ice, each corresponding to certain orientations of water molecules. These results also confirmed Pauling’s calculation of residual entropy of ice, which depends on the motional contribution and contributions from the geometrical disorder in hydrogen bondings of molecules in lattice. Tetrahedral reorientational motion has been used to explain the behavior of water molecules in pure crystalline ice.10 Wolfe and collaborators analyzed unfreezable water in terms of quantitative measurements of the interaction among membranes or macromolecules at the freezing temperature.2 Three effects were used to explain the observed freezing point depression:2 (i) due to the presence of small solutes, (ii) due to the presence of macromolecules, membranes and other hydrophilic ultrastructure, (iii) due to the effect of viscosity. Wu and coworkers studied freezing and dynamics of non-freezable water in fully-hydrated sphingomyelin bilayers at subzero temperatures using 13C, 2H, and 31P NMR techniques and DSC experiments.44 They observed that the bound water molecular motion in phospholipid bilayers freezes at -70°C, which is in good agreement with the results presented in this study. However, 2H quadruple coupling line shape was not the same as observed for the hexagonal phase in normal ice. In addition, they proposed that freezing of hydrated lipid bilayers composed of lipid and water occurred through several steps. During the phase transition of a lipid from liquid crystalline to gel phase, the phase of the hydrocarbon chains of lipid molecules changes first and followed by the bulk water molecules present between lipid bilayers. As the temperature of the sample continuously decreased, phospholipid headgroups freeze and finally water molecules bound to the headgroup freeze. The temperature of each event in the freezing process depends on the types of lipids in the sample. As shown in Figure 2, our results are in excellent agreement with this explanation. Bulk water molecules moving freely between lipid bilayers freeze first as noted by the disappearance of the isotropic peak in the 2H NMR spectrum, and followed by the freezing of water molecules weakly bound to lipids as evidenced by the small quadrupole splitting. DSC and 2H NMR study on water in waxy corn starch showed that a large fraction of water was as mobile as the molecules in the liquid water even at a temperature around -32°C.8 Hansen and coworkers7 studied hydrated soybean protein and showed that water bound to soy protein and carbohydrates freeze below -70°C. Frozen water in this system also did not adopt ice structure due to the interaction with these macromolecules. The present study of water in POPC and POPC/Cholesterol showed the typical 2H quadrupole coupling powder pattern spectra as those observed from ice. This suggests that the bound water in POPC bilayers exists in the ice structure. Studies have reported the different states of water in polymers.48 A deuterium NMR study of water organization in cellulose reported the presence of water molecules that undergo fast (107 s-1) 180° flips around the bisector axis.47
Effect of cholesterol on the physical property of lipid bilayers
Cholesterol molecules insert into the hydrophobic acyl chain region of cellular membranes, and play a very important role in controlling the fluidity of the cell membrane.49 At lower temperatures, they act as an efficient cryoprotectant, which protects the lipid bilayer structure from damaging under severe conditions. This study was aimed at addressing the role of cholesterol on the mobility of water molecules bound to lipid bilayers. Our experimental results show that cholesterol affects the fluidity of POPC bilayers in such a way that the bound water freezes at a significantly lower temperature than the pure water (Figures 1-4). The results are in excellent agreement with previous studies.26,32 This depression of the freezing point of water could be explained by the structural basis of cholesterol molecules in lipid bilayers. Cholesterol is naturally found in both leaflets of biological cellular membranes. The OH group of cholesterol is known to form a strong hydrogen bond with the carbonyl group of a phospholipid. In addition, it may also form a hydrogen bond with water generating more space between lipid molecules.49 As shown in a previous study,11 cholesterol increases the fluidity of lipid membranes and therefore water in cholesterol-containing POPC bilayers can be prevented from freezing at low temperatures. A study on the water penetration depth in bilayer membranes and the role of cholesterol in modulating this penetration depth showed that cholesterol might decrease the depth of water penetration by about 2.5 angstroms.50 Another study51 on the effect of cholesterol in the lipid bilayer and water proton magnetization transfer showed that the pseudo-first-order magnetization exchange rate was increased as cholesterol concentration increased. It was suggested that the cholesterol-induced 1H magnetization exchange may be related either to longer correlation times of the lipid or to an increase in the number of water molecules associated with bulk water.51 As shown in the present study, water in cholesterol-containing POPC bilayers freezes at a much lower temperature than the pure water and the water in POPC bilayers. This observation provides an explanation to the role of cholesterol as a cryoprotectant. The molecular behavior of water at reduced temperatures may further be compared with the dehydration effect of lipids. As the temperature decreases, free mobile water molecules located between lipid bilayers form ice and the number of water molecules bound to the lipid headgroups decreases. Cholesterol could support the bilayer membrane to hold more hydration layers near lipid headgroups and change the conformation of the lipid headgroup, and thus protect the lipid membranes.
Low-temperature solid-state NMR experiments on lipid bilayers
Although solid-state NMR has been shown as an useful tool in structural and dynamical studies of biomolecules embedded in lipid bilayers, there are still intrinsic difficulties such as low sensitivity and stability of mechanically aligned lipid bilayers need to be overcome. Aligned model membranes are commonly used to determine the orientation or topology and the structure of membrane proteins using time-consuming multidimensional solid-state nuclear magnetic resonance experiments.34,38,52,53 Due to the long experimental time of multidimensional experiments, the phospholipid bilayers can be damaged by dehydration, which can further cause misalignments of the aligned lipid bilayers. It was demonstrated in our previous study that some of the difficulties in solid-state NMR experiments could be overcome by conducting experiments on the mechanically aligned lipid bilayers at reduced temperatures.38 The results obtained from the present study further prove that incorporation of cholesterol into POPC bilayers can stabilize lipid bilayers in fluid state at much reduced temperatures by decreasing the freezing point of water. Thus, as demonstrated in this study, cholesterol or other cryoprotectants could be added to phospholipid bilayers in the form of mechanically aligned lipid samples for solid-state NMR experiments to increase the sensitivity of solid-state NMR experiments and also to enhance the stability of model membranes.
Conclusion
Here, we have investigated the mobility of water and freezing temperature of fully 2H2O hydrated MLVs of POPC in the presence and absence of cholesterol using solid-state 2H NMR techniques. A series of 2H quadrupole coupling NMR spectra were obtained from lipid bilayers at several different temperatures to understand the freezing behavior of water molecules in POPC lipid bilayers and the effect of cholesterol in the freezing temperature of water bound to lipids in bilayers. Our experimental results show that water molecules in POPC bilayers exhibited motional behavior even at temperatures as low as -20°C and freeze at a much lower temperature than the pure water. With the introduction of cholesterol into POPC bilayers, the temperature at which the dynamical motion of water freezes is further reduced. The degree of freezing point depression was dependent on the concentration of cholesterol in the sample. These observations provide insights into the cryoprotectant role of cholesterol in the cell membrane. In addition, our results suggest that cholesterol, as a cryoprotectant, can be used to prepare model membranes for structural studies using solid-state NMR spectroscopy to enhance the sensitivity of the experiment and stability of model membranes.54-61 A temperature-dependent 2H spin-lattice relaxation time study, as reported for amorphous ices, could provide information on dynamical differences between different states of water in lipid bilayers.62
Acknowledgement
This work was supported by Korea Research Foundation Grant funded by Korea Government (MOEHRD, Basic Research Promotion Funds, KRF-2005-003-C00090 and KRF-2006-331-C00153), and by research funds from the National Institutes of Health (AI054515 to A.R.). We thank Dr. Katherine Henzler-Wildman for the synthesis and purification of LL-37 peptide used in this study and Kevin Hallock for fruitful discussions.
References
- 1.Isaraelachvili J, Wennerstrom H. Nature. 1996;379:219–225. doi: 10.1038/379219a0. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe J, Bryant G, Koster K. CryoLetters. 2002;23:157–166. [PubMed] [Google Scholar]
- 3.Ueda I, Tseng HS, Kaminoh Y, Ma SM, Kamaya H, Lin SH. Molecular Pharmacology. 1986;29:582–588. [PubMed] [Google Scholar]
- 4.Miller IR, Bach D, Wachtel EJ, Eisenstein M. Bioelectrochemistry. 2002;58:193–196. doi: 10.1016/s1567-5394(02)00035-x. [DOI] [PubMed] [Google Scholar]
- 5.Yan Z, Pope J, Wolfe J. J. Chem. Soc. Faraday Trans. 1993;15:2583–2588. [Google Scholar]
- 6.Salsbury NJ, Drake A, Chapman D. Chem. Phys. Lipids. 1972;8:142–151. doi: 10.1016/0009-3084(72)90026-6. [DOI] [PubMed] [Google Scholar]
- 7.Hansen JR. J. Agric. Food Chem. 1976;24(6) [Google Scholar]
- 8.Li S, Dickinson LC, Chinachoti P. J. Agric. Food Chem. 1998;46:62–71. doi: 10.1021/jf9609441. [DOI] [PubMed] [Google Scholar]
- 9.Yoon YH, Pope JM, Wolfe J. Biophy. J. 1998;74:1949–1965. doi: 10.1016/S0006-3495(98)77903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittebort RJ, Usha MG, Ruben DJ, Wemmer DE, Pines A. J. Am. Chem. Soc. 1988;110:5668–5671. [Google Scholar]
- 11.Gally HU, Seelig A, Seelig J. Hoppe-Seylers Zeischrift fur Physiologische Chemie. 1976;357:1447–1450. [PubMed] [Google Scholar]
- 12.Tran R, Ho S, Dea P. Biophy. Chem. 2004;110:39–47. doi: 10.1016/j.bpc.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Ramamoorthy A, Thennarasu S, Tan A, Lee DK, Clayberger C, Krensky AM. Biochim. Biophys. Acta. 2006;1758:154–163. doi: 10.1016/j.bbamem.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Epand RF, Ramamoorthy A, Epand RM. Prot. Pept. Lett. 2006;13:1–5. [PubMed] [Google Scholar]
- 15.Kakio A, Nishimoto SI, Yanagisawa K, Kozutsumi Y, Matsuzaki K. J. Biol. Chem. 2001;276:24985–24990. doi: 10.1074/jbc.M100252200. [DOI] [PubMed] [Google Scholar]
- 16.Jayasinghe SA, Langen R. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2007;1768:2002–2006. doi: 10.1016/j.bbamem.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood AI, Pan J, Mills TT, Nagle JF, Epand RM, Tristram-Nagle S. Biochim Biophys Acta. 2008;1778:1120–1130. doi: 10.1016/j.bbamem.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Epand RM. Biochim Biophys Acta. 2008;1778:1576–1582. doi: 10.1016/j.bbamem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Santos JS, Lee DK, Hallock KJ, Ramamoorthy A. Recent Research Developments in Physical Chemistry. Transworld Research Network. 2002;6:179–211. [Google Scholar]
- 19.Kurze V, Steinbauer B, Huber T, Beyer K. Biophs. J. 2000;78:2442–2451. doi: 10.1016/S0006-3495(00)76788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenk MR, Alk T, Seelig A, Seelig J. Biophs. J. 1997;72:1719–1731. doi: 10.1016/S0006-3495(97)78818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bechinger B, Seelig J. Biochemistry. 1991;30:3923–3929. doi: 10.1021/bi00230a017. [DOI] [PubMed] [Google Scholar]
- 22.Bechinger B, Seelig J. Chemistry & Physics of lipids. 1991;58:1–5. doi: 10.1016/0009-3084(91)90105-k. [DOI] [PubMed] [Google Scholar]
- 23.Brown MF, Seelig J. Nature. 1977;269:721–723. [Google Scholar]
- 24.Brown MF, Seelig J. Biochemistry. 1978;17:381–384. doi: 10.1021/bi00595a029. [DOI] [PubMed] [Google Scholar]
- 25.Marassi FM, Macdonald PM. Biochemistry. 1992;31:10031–10036. doi: 10.1021/bi00156a024. [DOI] [PubMed] [Google Scholar]
- 26.Chochina SV, Avdulov NA, Igbavboa U, Cleary JP, O’hare EO, Wood WG. J. Lipid Res. 2001;42:1292–1297. [PubMed] [Google Scholar]
- 27.Hallock KJ, Henzler KA, Lee DK, Ramamoorthy A. Biophys. J. 2002;82:2499–2503. doi: 10.1016/S0006-3495(02)75592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis JH, Jeffrey KR, Bloom M, Valic MI, Higgs TP. Chem. Phys. Lett. 2006;42:390–394. [Google Scholar]; Schmidt-Rohr K, Spiess HW. Multidimensional Solid-state NMR and Polymers. Academics Press; 1994. [Google Scholar]
- 29.Metz G, Wu X, Smith SO. J. Magn. Reson. 1994;A110:219–223. [Google Scholar]
- 30.Volke F, Eisenblatter S, Galle J, Klose G. Chem. and Phys. Lipids. 1994;70:121–131. doi: 10.1016/0009-3084(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 31.Moraes CM, Bechinger B. Mag. Resosn. Chem. 2004;42:155–161. doi: 10.1002/mrc.1321. [DOI] [PubMed] [Google Scholar]; Bechinger B, Weik M. Biophys. J. 2003;85:361–369. doi: 10.1016/S0006-3495(03)74480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bach D, Miller IR. BBA. 1998;1368:216–224. doi: 10.1016/s0005-2736(97)00179-x. [DOI] [PubMed] [Google Scholar]; Bach D, Miller IR. Chem. Phys Lipids. 2005;136:67–72. doi: 10.1016/j.chemphyslip.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Moll FD, Cross TA. Biophys. J. 1990;57:351–361. doi: 10.1016/S0006-3495(90)82536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramamoorthy A, Marassi FM, Zasloff M, Opella SJ. J. Biomol. NMR. 1995;6:329–334. doi: 10.1007/BF00197814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marassi FM, Ramamoorthy A, Opella SJ. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8551–8556. doi: 10.1073/pnas.94.16.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Denny JK, Tian C, Kim S, Mo Y, Kovacs F, Song Z, Nishimura K, Gan Z, Fu R, Quine JR, Cross TA. J. Magn. Reson. 2000;144:162–167. doi: 10.1006/jmre.2000.2037. [DOI] [PubMed] [Google Scholar]
- 37.Ramamoorthy A, Kandasamy SK, Lee DK, Kidambi S, Larson RG. Biochemistry. 2007;46:965–975. doi: 10.1021/bi061895g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee DK, Henzler K, Ramamoorthy A. J. Am. Chem. Soc. 2004;126:2318–2319. doi: 10.1021/ja039077o. [DOI] [PubMed] [Google Scholar]
- 39.Katherine A, Wildman K. A. Henzler, Lee DK, Ramamoorthy A. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 40.Dürr UHN, Sudheendra US, Ramamoorthy A. BBA-Bimembranes. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 41.Porcelli F, Verardi R, Shi L, Henzler-Wildman KA, Ramamoorthy A, Veglia G. Biochemistry. 2008;47:5565–5572. doi: 10.1021/bi702036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henzler-Wildman KA, Martinez GV, Brown MF, Ramamoorthy A. Biochemistry. 2004;43:8459–8469. doi: 10.1021/bi036284s. [DOI] [PubMed] [Google Scholar]; Ramamoorthy A, Lee D.k., Santos JS, Henzler-Wildman KA. J. Am. Chem. Soc. 2008;130:11023–11029. doi: 10.1021/ja802210u. [DOI] [PubMed] [Google Scholar]
- 43.Klose G, Konig B, Paltauf F. Chem. Phys. Lipids. 1992;61:265. [Google Scholar]
- 44.Wu WG, Chi LM, Yang TS, Fang SY. J. Biol. Chem. 1991;266:13602–13606. [PubMed] [Google Scholar]
- 45.Ladbrooke BD, Chapman D. Chem. Phys. Lipids. 1969;3:304–367. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh CH, Wu WG. Biopys. J. 1995;69:4–12. doi: 10.1016/S0006-3495(95)79885-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radloff D, Boeffel C, Spiess HW. Macromolecules. 1996;29:1528–1534. [Google Scholar]
- 48.Smyth G, Quinn FX, McBrierty VJ. Macromolecules. 1988;21:3198. [Google Scholar]; Hofmann K, Hatakayama H. Polymer. 1994;35:2749. [Google Scholar]
- 49.Kurze V, Steinbauer B, Huber T, Beyer K. Biophys. J. 2000;78:2441–2451. doi: 10.1016/S0006-3495(00)76788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon SA, McIntosh TJ, Latorre R. Science. 1982;216:65–67. doi: 10.1126/science.7063872. [DOI] [PubMed] [Google Scholar]
- 51.Fralix TA, Ceckler TL, Wolff SD, Simon SA, Balaban RS. Magn. Reson. Med. 2005;18:214–223. doi: 10.1002/mrm.1910180122. [DOI] [PubMed] [Google Scholar]
- 52.Wu CH, Ramamoorthy A, Opella SJ. J. Magn. Res. 1994;A109:270–272. [Google Scholar]; Ramamoorthy A, Wei Y, Lee DK. Ann. Rep. NMR Spectrosc. 2004;52:1–52. [Google Scholar]
- 53.Ramamoorthy A, Wu CH, Opella SJ. J. Magn. Res., B. 1995;107:88–90. doi: 10.1006/jmrb.1995.1063. [DOI] [PubMed] [Google Scholar]; Ramamoorthy A, Gierasch LM, Opella SJ. J. Magn. Res., B. 1995;109:112. doi: 10.1006/jmrb.1995.1157. [DOI] [PubMed] [Google Scholar]; Ramamoorthy A, Gierasch LM, Opella SJ. J. Magn. Res., B. 1996;110:102. doi: 10.1006/jmrb.1996.0016. [DOI] [PubMed] [Google Scholar]
- 54.Kawamura I, Ohmine M, Tanabe J, Tuzi S, Saito H, Naito A. BBA Biomembranes. 2007;1768:3090–3097. doi: 10.1016/j.bbamem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Toke O, Cegelski L, Schaefer J. BBA Biomembranes. 2006;1758:1314–1329. doi: 10.1016/j.bbamem.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 56.Lange C, Muller SD, Walther TH, Burck J, Ulrich AS. BBA Biomembranes. 2007;1768:2627–2634. doi: 10.1016/j.bbamem.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 57.Varga K, Tian L, McDermott AE. Biochim. Biophys. Acta. 2007;1774:1604–1613. doi: 10.1016/j.bbapap.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 58.Baldus M. J. Biomol. NMR. 2007;39:73–86. doi: 10.1007/s10858-007-9177-3. [DOI] [PubMed] [Google Scholar]; Ramamoorthy A, Wei YF, Lee DK. Ann. Rep. NMR Spectrosc. 2004;52:1–52. [Google Scholar]
- 59.Varga K, Aslimovska L, Watts A. J. Biomol. NMR. 2008;41:1–4. doi: 10.1007/s10858-008-9235-5. [DOI] [PubMed] [Google Scholar]; Traaseth NJ, Buffy JJ, Zamoon J, Veglia G. Biochemistry. 2006;45:13827–13834. doi: 10.1021/bi0607610. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Berthold DA, Gennis RB, Rienstra CM. Prot. Sci. 2008;17:199–204. doi: 10.1110/ps.073225008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dürr UHN, Waskell L, Ramamoorthy A. BBA Biomembranes. 2007;1768:3235–3259. doi: 10.1016/j.bbamem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Aisenbrey C, Bechinger B. J. Am. Chem. Soc. 2004;126:16676–16683. doi: 10.1021/ja0468675. [DOI] [PubMed] [Google Scholar]; Aisenbrey C, Cusan M, Larnbotte S, Jasperse P, Georgescu J, Harzer U, Bechinger B. Chem.Bio.Chem. 2008;9:944–51. doi: 10.1002/cbic.200700507. [DOI] [PubMed] [Google Scholar]
- 62.Scheuermann M, Geil B, Winkel K, Fujara F. J. Chem. Phys. 2006;124:224503. doi: 10.1063/1.2204911. [DOI] [PubMed] [Google Scholar]