Abstract
Sustained hemodynamic stresses, especially sustained high blood flow, result in flow-induced outward vascular remodeling. Mechanisms that link hemodynamic stresses to vascular remodeling are not well understood. Inflammatory cells, known for their release of proteinases, including matrix metalloproteinases (MMPs), are emerging as key mediators for various tissue remodeling. Using a flow-augmented common carotid artery model in rats, we tested whether macrophages play critical roles in adaptive outward vascular remodeling in response to an increase in blood flow. Left common carotid artery ligation caused a sustained increase in blood flow with a gradual increase in luminal diameter in the right common carotid artery. Macrophages infiltrated into the vascular wall that peaked 3 days after flow augmentation. The time course of MMP-9 expression coincided with infiltration of macrophages. Macrophage depletion by liposome-encapsulated dichloromethylene diphosphonate significantly reduced flow-induced outward vascular remodeling, as indicated by the smaller luminal diameter of flow-augmented right common carotid artery in the clodronate-treated group compared with the phosphate-buffered saline-treated group (P < 0.05). These data show critical roles of macrophages in flow-induced outward vascular remodeling. Inflammatory cell infiltration and their subsequent release of cytokines may be key processes for flow-induced outward vascular remodeling.
Keywords: macrophage, vascular remodeling, inflammation, blood flow, matrix metalloproteinase, aneurysm
Introduction
Sustained hemodynamic stresses, especially sustained high blood flow, result in flow-induced outward vascular remodeling. This is an adaptive process of the vascular wall to reduce wall shear stress to physiologic baseline values in response to continuous increase in blood flow (Cox, 1975; Langille et al, 1989; Price and Skalak, 1994; Tronc et al, 2000). This process is characterized by an increase in luminal diameter with relatively small changes in wall thickness. Although flow-induced outward vascular remodeling is a physiologic process to accommodate increased blood flow, the same mechanisms may contribute to pathologic outward vascular remodeling observed in cerebrovascular lesions including intracranial aneurysms and brain arteriovenous malformations (Hashimoto et al, 2001, 2006).
Angiogenic and inflammatory cytokines including matrix metalloproteinases (MMPs) play a critical role in various types of tissue remodeling (Abbruzzese et al, 1998; Tronc et al, 2000). These cytokines are often produced by inflammatory cells such as macrophages. Interestingly, the cerebrovascular lesions that undergo vascular remodeling often show evidence of inflammatory cell infiltration (Chen et al, 2006; Hashimoto et al, 2006).
In this study, we hypothesized that macrophages play critical roles in adaptive outward vascular remodeling in response to an increase in blood flow. To test this hypothesis, we used a model of flow augmentation of common carotid artery (Abbruzzese et al, 1998; Tronc et al, 2000). First, we assessed the infiltration of macrophages into the remodeling right common carotid artery. Second, we assessed the expression of major gelatinases, namely MMP-9 and MMP-2, in the remodeling common carotid artery. Third, we tested whether depletion of macrophages reduces flow-induced outward vascular remodeling.
Materials and methods
Validation of Flow-Induced Outward Vascular Remodeling of Right Common Carotid Artery in Rats
To study flow-induced outward vascular remodeling, we used a flow-augmented common carotid artery model in rats, in which ligation of the left common carotid artery augments the blood flow through the right common carotid artery (De Ciuceis et al, 2005; Schiffers et al, 2000; Tronc et al, 2000). Advantage of this model is that this model does not involve direct surgical manipulation on the blood vessels we are studying, avoiding significant inflammation or surgery-related changes.
To validate this model, we assessed changes in blood flow and luminal diameter of the right common carotid artery after flow augmentation. Adult male Sprague–Dawley rats (300 to 350 g, 10- to 12-week-old) underwent ligation of the left common carotid artery. Blood flow and luminal diameter of the right common carotid artery (flow-augmented side) were measured with a 14MHz ultrasound probe 5mm below the carotid bifurcation. Mean luminal diameter was measured from M-mode image over five cardiac cycles. From the M-mode image, diameter-time-integral (area between anterior and posterior walls) was traced and measured. Mean luminal diameter was calculated by dividing the diameter-time-integral by time over five cardiac cycles. Flow–velocity curve for the right common carotid artery was obtained using the pulse wave Doppler. From the flow–velocity curve, velocity-time-integral was calculated. Blood flow was calculated as velocity-time-integral × 4πr2 × cosθ (r: radius= luminal diameter/2;θ: Doppler beam angle against the blood flow). Luminal diameter and blood flow were measured before surgery (t = 0), immediately after the left common carotid artery ligation, and weekly thereafter until 4 weeks. Shear stress was calculated assuming a constant viscosity and presented as %-baseline. Four animals were studied longitudinally.
Detection of Macrophages and Gelatinase Activity
Presence of macrophages in the right common carotid arteries was assessed by immunohistochemistry using ED1 antibody (Serotec). To localize gelatinase activity including MMP activity in the remodeling right common carotid artery tissues, we performed in situ zymography using gelatin as a substrate. Wound tissue was used as the positive control. Treatment of the tissue sections with MMP inhibitor, 1,10-phenanthroline monohydrate (1 mmol/L, 1 h), was used to assess contribution of MMP activity to the gelatinase activity. Gelatin zymogram gels were used to measure gelatinase levels, mainly MMP-9 and MMP-2. In some samples, ethylenediamine tetraacetic acid (nonspecific MMP inhibitor, 20 mmol/L), phenylmethylsulfonyl fluoride (serine protease inhibitor, 1 mmol/L), or pepstatin A (aspartic protease inhibitor, 1 µmol/L) was added to the homogenized samples before loading them to zymogram gels to confirm the identity of proteolytic bands. To verify pro and active forms of MMPs, some samples were pretreated with 4-aminophenyl mercuric acetate (pro-MMP activator, 0.1 mmol/L) for 1 h at 37°C.
Macrophage Depletion
Macrophage depletion was achieved by series of liposome-encapsulated dichloromethylene diphosphonate (clodronate liposome) injections as previously described (Van Rooijen and Sanders, 1994). Clodronate was a gift from Roche Diagnostics GmbH (Mannheim, Germany). Animals received clodronate liposomes (0.7 mg/kg) intravenously 2 days before right common carotid artery flow-augmentation by left common carotid artery ligation or sham surgery, then every week until the end of experiment as previously described (Danenberg et al, 2002; van Rooijen et al, 1997). This regimen was reported to cause a reduction of macrophages to less than 10% of baseline (Danenberg et al, 2002; van Rooijen et al, 1997). Animals in the control group received the same volume of phosphate-buffered saline-containing liposome (PBS liposome).
To verify the efficacy and specificity of the macrophage depletion treatment, we assessed the efficacy of the macrophage depletion by examining macrophages in the spleen and liver using immunohistochemistry. Examination of macrophages in the spleen and liver has been used to assess the degree of systemic depletion of a macrophage/monocyte population (Danenberg et al, 2002; van Rooijen et al, 1997). In addition, complete blood counts with differential leukocytes were performed using HEMAVET 950 (Drew Scientific, Dallas, TX, USA). Blood samples were collected 1 day before the initial injection, 1 day after the ligation, and then between each injection. Automated counts were verified by manual counts in selected samples.
Four groups were studied: Group 1, intact macrophage + sham surgery; Group 2, macrophage depletion+ sham surgery; Group 3, intact macrophage+ flow augmentation; Group 4, macrophage depletion+ flow augmentation (n = 6 for each group). Luminal diameter of the right common carotid artery was measured at 4 weeks after flow augmentation or sham surgery, a time point at which flow-induced outward vascular remodeling reached plateau in control rats of our preliminary experiments. Weight, right common carotid artery blood flow, and systolic blood pressure were measured at the baseline, 2, and 4 weeks after surgery. Systolic blood pressure was measured using non-invasive tail cuff system (Kent Scientific, Torrington, CT, USA) under the awake condition, after 3 days of training.
Statistical Analysis
To compare the luminal diameter, blood flow, and blood pressure among the groups at each time point, we used analysis of variance followed by Bonferroni–Dunn post hoc test. To compare the luminal diameter and blood flow longitudinally, we used analysis of variance with a repeated measure design. All the data were presented as mean±s.d. Statistical significance was taken at P < 0.05.
Results
Validation of Flow-Augmented Right Common Carotid Artery Model
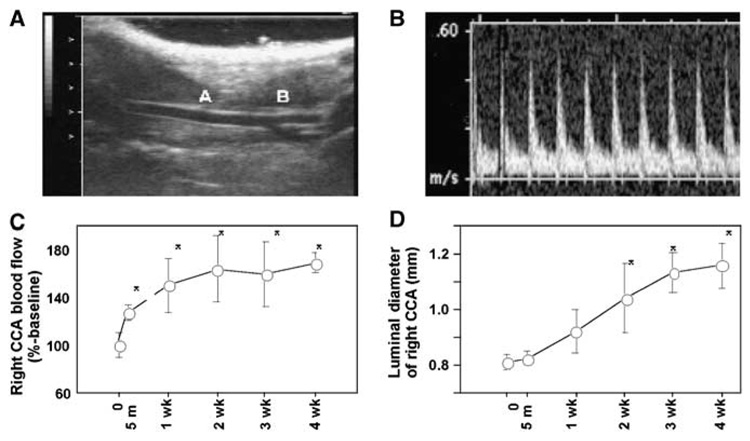
Figure 1A shows a representative view of the right common carotid artery and its bifurcation. Blood velocity was measured 5mm below the carotid bifurcation using the pulse wave Doppler (Figure 1B). As shown in Figure 1C, there was an immediate increase in blood flow in the right common carotid artery 5 mins after left common carotid artery ligation. Blood flow through the right common carotid artery further increased to 150% of baseline at 1 week, and the increase in flow was sustained for at least up to 4 weeks. Although the right common carotid artery blood flow levels at 1, 2, 3, and 4 weeks were higher than at baseline (P < 0.05), there were no differences in the right common carotid blood flow levels among 1, 2, 3, and 4 weeks.
Figure 1.
(A) Representative view of the right common carotid artery. Right side is the cephalic side (head), and the left side is the caudal side (tail). A: Right common carotid artery. B: Carotid bifurcation. (B) Representative view of the pulse wave Doppler tracing of the right common carotid artery. (C) Blood flow of the right common carotid artery after flow augmentation. There was an immediate increase in blood flow in the right common carotid artery 5 minutes after the left common carotid artery ligation. Blood flow in the right common carotid artery further increased at 1 week, and the increase was sustained for at least up to 4 weeks. (D) Luminal diameter of right common carotid artery after left common carotid artery ligation. Before surgery to 3 weeks, there was a gradual increase in luminal diameter, which thereafter appeared to plateau. Luminal diameter at 1, 2, 3, and 4 weeks was greater than at baseline (mean±s.d). *P<0.05 compared with baseline (t=0). CCA: common carotid artery.
Figure 1D shows a time course of the mean luminal diameter of the right common carotid artery after flow augmentation by left common carotid artery ligation. From baseline to 3 weeks, there was a gradual increase in mean luminal diameter, which thereafter appeared to plateau. Luminal diameter at 2, 3, and 4 weeks was greater than baseline (P < 0.05).
In this flow-augmented carotid remodeling model, there was a sustained increase in blood flow with a gradual increase in luminal diameter, consistent with previous findings by others (De Ciuceis et al, 2005; Schiffers et al, 2000; Tronc et al, 2000). Animals that received sham surgery did not show any significant change in blood flow or luminal diameter of right common carotid artery (data not shown).
Presence of Macrophages in Remodeling Common Carotid Artery
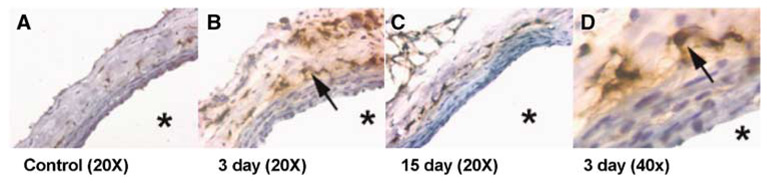
In the right common carotid artery without flow augmentation (control: sham surgery), macrophages were undetectable (Figure 2A). However, in right common carotid arteries 3 days after flow augmentation, numerous macrophages could be seen in the adventitia (Figures 2B and 2D). This pattern of preferential distribution of macrophages in the adventitia in the carotid artery was previously described in different types of vascular remodeling (see discussion; Barker et al, 1994; Capers et al, 1997; Gavrila et al, 2005; Ivan et al, 2002; Weiss and Taylor, 2008; Zhang et al, 2008). At day 15, most of the macrophages in the adventitia had disappeared (Figure 2C).
Figure 2.
Macrophage infiltration into the flow-augmented right common carotid artery. (A) In the control common carotid artery, macrophages were undetectable. (B) In the flow-augmented right common carotid arteries, numerous macrophages could be seen in the adventitia at day 3. (C) At day 15, most of the macrophages in the adventitia had disappeared. (D) Macrophages in the adventitia at day 3 with higher magnification. Macrophages were detected using ED1 antibody (Serotec). *: lumen.
Colocalization of Macrophages with Gelatinase Activity
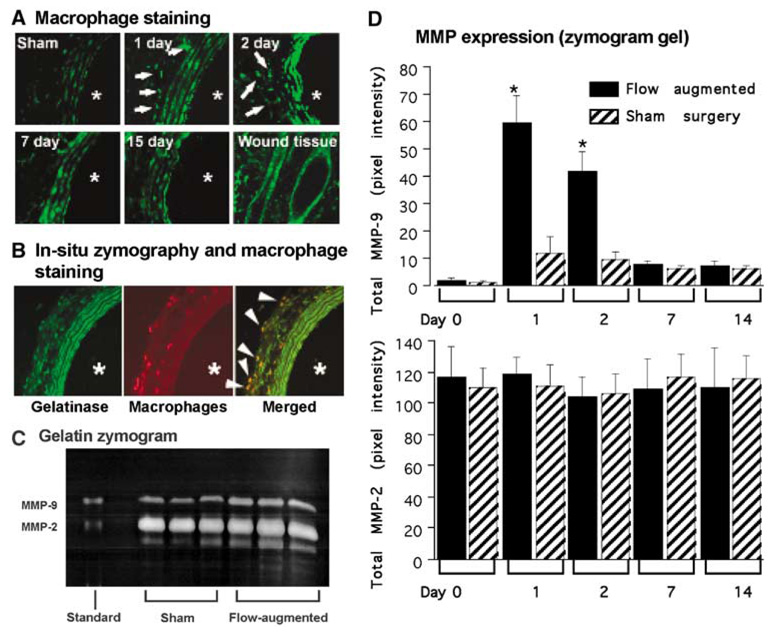
As macrophages can secrete various proteinases including matrix metalloproteinases, we performed in situ zymography using gelatin as a substrate on the remodeling right common carotid artery at 1, 2, 3, 7, and 15 days after flow augmentation or sham surgery (Figure 3A). Wound tissue was used as positive control. The remodeling carotid artery showed gelatinase activity between elastic laminae and in the adventitia. Among the time points we studied, gelatinase activity was most prominent at days 2 and 3. Sham surgery group showed low levels of gelatinase activity only between elastic laminae, probably reflecting constitutive expression of MMP-2 by smooth muscle cells as previously described (Southgate et al, 1999; Zaltsman and Newby, 1997). Treatment of the tissue sections with MMP inhibitor, 1,10-phenanthroline monohydrate had inhibited gelatinase activity (data not shown).
Figure 3.
MMP expression in the remodeling common carotid artery. (A) In situ zymography (gelatin) in flow-augmented right common carotid artery (× 20). The remodeling carotid artery showed abundant gelatinase activity between elastic laminae and scattered gelatinase activity in the adventitia (white arrows). Among the time points we studied, gelatinase activity was most prominent at day 2. Sham surgery group showed gelatinase activity only between elastic laminae, probably reflecting constitutive expression of MMP-2 by smooth muscle cells as previously described. (B) In situ zymography and immunofluorescence staining for macrophages in flow-augmented right common carotid artery at day 3 (× 20). Wound tissue was used as a positive control. *Indicates the lumen. Gelatinase activity was between elastic laminae and in the adventitia (left panel, green). Macrophages were seen mostly in the adventitia (middle panel, red). Macrophages were colocalized with gelatinase activity in the adventitia (right panel, yellow). White arrows indicate colocalization of gelatinase activity with macrophages. (C) Gelatin zymogram gel using flow-augmented right common carotid artery at day 2. Flow-augmented right common carotid arteries had higher levels of MMP-2 and MMP-9 at day 2 than those from sham surgery. Although pro-MMP-9, pro-MMP-2, and active MMP-2 bands were prominent, active MMP-9 bands were very faint. (D) Time course of MMP-9 and MMP-2 expression in flow-augmented right common carotid artery. In the flow-augmented right common carotid artery, upregulation of total MMP-9 was detected as early as day 1, after which MMP-9 levels gradually returned to baseline. In sham surgery group, there was a slight increase in MMP-9 at day 1, probably reflecting systemic inflammation caused by the sham surgery. The time course of MMP-9 expression coincided with infiltration of macrophages. MMP-2 was expressed in both sham and flow-augmented right common carotid artery at all time points that we studied. There was no difference in MMP-2 levels between sham and flow-augmented right common carotid arteries at any time point. Moreover, there was no significant temporal change of MMP-2 levels in flow-augmented common carotid artery. (Mean±s.d.; *P<0.05, compared with the baseline.)
To examine colocalization of gelatinase activity with macrophages, we performed immunofluorescence staining for macrophages combined with in situ zymography using gelatin as a substrate (Figure 3B). Again, the remodeling carotid artery 3 days after flow augmentation showed gelatinase activity between elastic laminae and in the adventitia (Figure 3B, left panel). Macrophages were mostly seen in the adventitia (Figure 3B, middle panel). Macrophages were colocalized with the gelatinase activity in the adventitia (yellow color in the right panel of Figure 3B), indicating that macrophages, which were present in the remodeling common carotid arteries, were expressing gelatinases including MMPs.
Expression of Gelatinases in Flow-Augmented Common Carotid Artery
Gelatin zymograms using homogenized samples from flow-augmented common carotid artery at day 2 showed higher levels of pro-MMP-9, pro-MMP-2, and active MMP-2, compared with those from the sham surgery group (Figure 3C). Active MMP-9 bands were present in flow-augmented common carotid artery samples, but very faint.
Figure 3D shows a temporal change in total MMP-9 and total MMP-2 levels. In the flow-augmented right common carotid artery, upregulation of total MMP-9 was detected as early as day 1, after which MMP-9 levels gradually returned to baseline. In sham surgery group, there was a slight increase in MMP-9 at day 1, probably reflecting systemic inflammation caused by the sham surgery. The time course of MMP-9 expression coincided with infiltration of macrophages.
MMP-2 was expressed in both sham and flow-augmented right common carotid artery at all time points that we studied. There was no difference in MMP-2 levels between sham and flow-augmented right common carotid arteries at any time point. Moreover, there was no significant temporal change of MMP-2 levels in flow-augmented common carotid artery. These findings indicate that MMP-2 expression observed in sham and flow-augmented common carotid arteries reflect constitutive expression of MMP-2 as previously described (Southgate et al, 1999; Zaltsman and Newby, 1997).
Effects of Macrophage Depletion on Flow-Induced Outward Vascular Remodeling of Right Common Carotid Artery
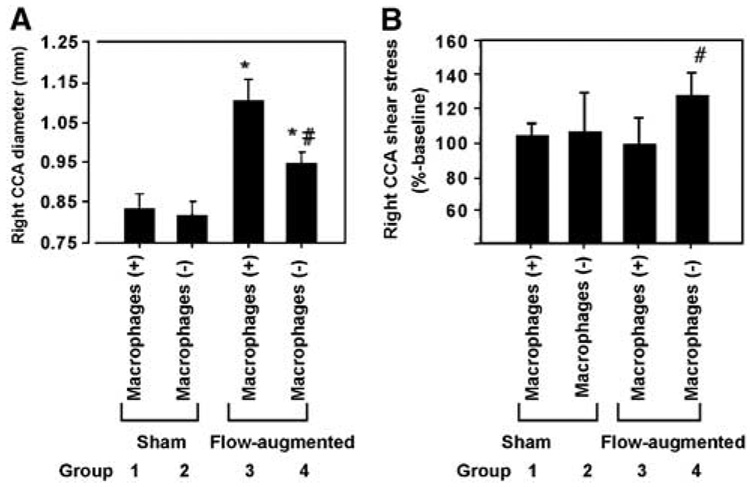
Effects of macrophage depletion on flow-induced outward vascular remodeling were assessed by measuring the mean luminal diameter of right common carotid artery at 4 weeks after flow-augmentation or sham surgery in animals that received the macrophage depletion treatment by clodronate liposome or PBS liposome (Figure 4A).
Figure 4.
(A) Right common carotid artery diameter at 4 weeks after flow augmentation or sham surgery. Macrophage depletion reduced flow-induced outward vascular remodeling. Between the macrophage intact groups, flow augmentation by left common carotid artery ligation increased the luminal diameter of the right common carotid artery (Group 1 versus Group 3). In the macrophage-depleted group, less outward vascular remodeling occurred in response to flow augmentation. (Group 3 versus Group 4: 1.10±0.06 versus 0.95±0.03 mm, P<0.05). (B) Shear stress levels common carotid artery diameter at 4 weeks after flow augmentation or sham surgery. Shear stress levels were presented as % control using the baseline values as control. Shear stress levels in flow-augmented common carotid artery in macrophage-depleted mice were significantly higher than other three groups of animals, reflecting that reduced outward vascular remodeling in macrophage-depleted mice could not normalize shear stress levels that were increased by flow augmentation. (Mean±s.d.; *P<0.05, compared with the sham groups; #P±0.05, compared with Group 3; n=6 in each group.)
Flow augmentation by left common carotid artery ligation increased the luminal diameter of the right common carotid artery in macrophage intact groups (Group 1 versus Group 3, P < 0.05). Macrophage depletion did not have any significant effect on the luminal diameter of the right common carotid artery without flow augmentation (sham surgery, Group 1 versus Group 2). Macrophage depletion significantly reduced flow-induced outward vascular remodeling (Group 3 versus Group 4: 1.10±0.06 versus 0.95±0.03 mm, P < 0.05).
Figure 4B shows right common carotid artery shear stress levels at 4 weeks after flow augmentation presented as %-baseline. Shear stress levels in flow-augmented common carotid artery in macrophage-depleted mice were significantly higher than other three groups of animals, reflecting that reduced outward vascular remodeling in macrophage-depleted mice could not normalize shear stress levels that were increased by flow augmentation.
Table 1 shows weight, right common carotid artery blood flow, and blood pressure at the baseline, 2, and 4 weeks after surgery. There were no differences in body weight between four groups at any time points. Consistent with our validation data, left common carotid artery ligation significantly increased the right common carotid artery blood flow at 2 and 4 weeks. There were no effects of macrophage depletion on the right common carotid artery blood flow. In addition, there were no differences in systolic blood pressure among four groups at any time points. Neither left common carotid ligation nor macrophage depletion had any effects on systolic blood pressure.
Table 1.
Body weight, right common carotid artery blood flow, and systolic blood pressure
| Body weight (g) |
Right CCA blood flow (% baseline) |
Systolic blood pressure (mmHg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Macrophage | Surgery | Baseline | 2 weeks | 4 weeks | Baseline | 2 weeks | 4 weeks | Baseline | 2 weeks | 4 weeks |
| 1 Intact | Sham | 311±13 | 352±10 | 369±10 | 100±0 | 103±11 | 109±7 | 136±14 | 136±18 | 142±11 |
| 2 Depleted | Sham | 307±9 | 353±8 | 375±6 | 100±0 | 108±17 | 110±10 | 147±6 | 135±16 | 137±11 |
| 3 Intact | Flow augmentation | 307±10 | 351±14 | 375±11 | 100±0 | 159±14*# | 160±13*# | 139±10 | 141±15 | 145±14 |
| 4 Depleted | Flow augmentation | 311±14 | 355±8 | 372±7 | 100±0 | 165±11*# | 166±10*# | 146±9 | 135±14 | 133±16 |
Data are presented as mean±s.d.
P<0.05 compared with Group 1.
P<0.05 compared with Group 2.
Complete blood counts showed that monocyte population in the treatment group were under the detection level 2 days after the treatment. Animals that received PBS liposome (control) had normal monocyte counts (0.5±0.2K per microliter). There were no significant differences in platelets (862±21 versus 833±62 count per microliter), total white blood cells (15.0±4.3 versus 13.0±4.0K per microliter), neutrophils (5.7±1.7 versus 4.8±1.3K per microliter), lymphocytes (8.5±2.9 versus 7.5±3.1K per microliter), eosinophils (0.1±0.1 versus 0.1±0.1K per microliter), and red blood cells counts (7.5±0.3 versus 7.3±0.2 count per microliter) between the baseline and at day 2. In all samples, basophils were below the detectable levels.
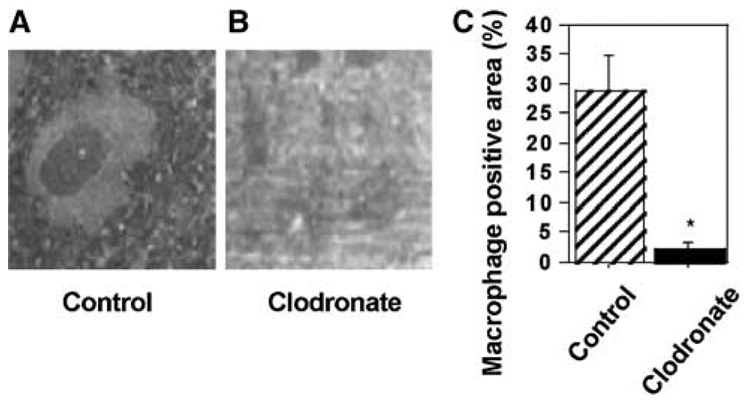
Immunohistochemistry was used to visualize macrophages in the spleen. At 2 weeks into the clodronate liposome treatment, PBS-treated animals (control) had abundant macrophages in the spleen (Figure 5A), whereas clodronate-treated animals only had few macrophages (Figure 5B). Macrophage-positive area (brown-colored ED1-positive area) in the spleen from clodronate-treated animals was significantly lower than in PBS-treated animals, represented by an approximate 92% reduction in the macrophage-positive area in the spleen (Figure 5C).
Figure 5.
Depletion of macrophages by a clodronate liposome treatment. (A, B) PBS-treated animals (A) had abundant macrophages in the spleen whereas clodronate-treated animals (B) only had few macrophages (brown colored) present. (C) Macrophage-positive area. Macrophage-positive area (brown-colored ED1-positive area) in the spleen from clodronate-treated animals was significantly lower than PBS-treated animals, representing an approximate 92% reduction. Macrophages were detected using ED1 antibody (Serotec).
Discussion
In this study, we have shown critical roles of macrophages in flow-induced outward vascular remodeling of common carotid artery in rats. First, we have shown that macrophages have infiltrated into the flow-augmented common carotid artery (Figure 2). Second, the time course of macrophage infiltration coincided with increased gelatinase activity (Figure 3). Third, pharmacological depletion of macrophages reduced flow-induced outward vascular remodeling (Figure 4). These findings strongly indicate that macrophages play critical roles in flow-induced outward vascular remodeling.
Consistent with our findings, Tronc et al (2000) showed increased levels of MMPs in the rabbit common carotid arteries that underwent flow-induced outward vascular remodeling using a surgically created arteriovenous fistula model. They also showed that MMP inhibition by doxycycline and BB-94 reduced outward vascular remodeling, possibly by preventing fragmentation of elastic laminae. Combined with our results, cytokine-producing-inflammatory cells, especially macrophages, seem to be critical for flow-induced outward vascular remodeling.
An intriguing finding of this study was an emergence of macrophages in the adventitia after flow augmentation (Figure 2 and Figure 3). We were not able to detect macrophages in endothelial or smooth muscle cell layers at any time point. Adventitial macrophages have been observed in other types of vascular remodeling including models of hypertrophic vascular remodeling in response to systemic hypertension (Capers et al, 1997; Weiss and Taylor, 2008), atherosclerotic remodeling (Barker et al, 1994; Zhang et al, 2008), and abdominal aortic aneurysm formation (Gavrila et al, 2005). Interestingly, in these cases, macrophage infiltration into the adventitia often occurs before significant numbers of macrophages appear in the intima or media (Gossl et al, 2007; Maiellaro and Taylor, 2007; Moulton et al, 2003; Pagano and Gutterman, 2007). Inflammation in these vascular lesions appears to progress bidirectionally from both luminal and adventitial sides (Maiellaro and Taylor, 2007; Pagano and Gutterman, 2007). Inflammatory cell infiltration in the adventitia is considered to be a key element of inward progression of inflammation in these lesions (Langheinrich et al, 2007; Maiellaro and Taylor, 2007; Pagano and Gutterman, 2007). Our model of vascular remodeling in response to increased flow augmentation may represent the transient, inward progression of inflammation that quickly returns to a quiescent and stabilized state. Adventitial macrophages may have a unique role in controlling inward vascular inflammation that couples with vascular remodeling.
Our data from the macrophage depletion study clearly showed the critical role of macrophages in flow-induced outward vascular remodeling in this model. However, our in situ zymography indicated that the contribution of macrophages to the overall MMP activity was rather small. In addition to gelatinase activity in the adventitia, we detected significant gelatinase activity in the media, especially between elastic laminas (Figure 3). The gelatinase activity in the media may be from the constitutive expression of MMP-2 by smooth muscle cells as previously described (Southgate et al, 1999; Zaltsman and Newby, 1997). This is consistent with our zymogram gel data that showed constitutive expression of MMP-2. It may not be the total MMP activity, but a coordinated temporal–spatial distribution of MMPs that determines flow-induced outward vascular remodeling in our model. Macrophages can modulate extracellular matrix and other cell behaviors through non-MMP-dependent mechanisms. Further studies will be needed to establish a causal relationship between macrophage-derived MMPs and outward vascular remodeling.
In this study, macrophage depletion treatment was unable to completely suppress remodeling. This may be because of the incomplete depletion of macrophages. Alternatively, these data may suggest that there are other cell types or other mechanisms independent of macrophages that compensate for the relative lack of macrophages. Other inflammatory cells such as neutrophils can secrete MMPs and may play a critical role in outward vascular remodeling. Both smooth muscle cells and endothelial cells can be alternative sources for MMPs. Orchestrated effects of multiple cell types and multiple systems may be controlling outward vascular remodeling.
Although macrophage depletion suppressed outward vascular remodeling in response to flow augmentation, there was no significant difference in the bulk blood flow rates between macrophage-depleted mice and mice with intact macrophages. A lack of effects of the smaller diameter of flow-augmented common carotid artery on the bulk blood flow rate in the macrophage-depleted mice is probably reflecting that the bulk blood flow rate in the common carotid artery is a function of systemic blood pressure and the downstream resistance that is primarily determined by the downstream resistance blood vessels.
There are several limitations to our study. In our study, clodronate liposome treatment did not have effects on other leukocyte subpopulation, platelets, or red blood cell, and the animals that received clodronate liposome did not show any apparent signs of adverse effects. However, it is still possible that the clodronate liposome treatment may have unknown side effects that might confound our results. Clodronate liposome itself is innocuous unless clodronate is released inside the cell membrane after phagocytosis. Free clodronate that is released upon cell death may affect other nontarget cells.
Leukocyte or MMP accumulation in the vascular wall may be partly affected by surgical procedure. To discriminate from the confounding effects of surgery, we used proper control groups for every time point. Although the right common carotid arteries from sham surgery animals did show a small increase in MMP-9 levels 1 day after surgery—probably as part of a systemic inflammation—the increase in the sham surgery group was far smaller than that of the flow-augmented group.
An alternative approach for testing roles of macrophages in flow-induced outward vascular remodeling is to use genetically engineered mice that have reduced number or function of macrophages. For example, mice lacking macrophage chemoattractant protein-1 are unable to activate monocytes and macrophages, and monocyte recruitment is impaired (Lu et al, 1998). However, experiments using these knockout mice may be confounded by known and unknown developmental abnormalities caused by genetic manipulation and potential long-term compensation by other cell types or systems.
In summary, our data showed critical roles of macrophages in flow-induced outward vascular remodeling. Inflammatory cell infiltration into the flow-augmented blood vessels and their subsequent release of cytokines such as MMPs may be key processes for flow-induced outward vascular remodeling.
Acknowledgements
The authors would like to thank Erik Tsou, Ryo Ota and Yasuhisa Kanematsu for assistance in preparation of the paper.
This study was funded by NIH R01NS055876 (TH), American Heart Association Grant-in-Aid 0755102Y (TH), and NIH P01NS044155 (WLY, TH).
Footnotes
Disclosure
The authors state no conflict of interest.
References
- Abbruzzese TA, Guzman RJ, Martin RL, Yee C, Zarins CK, Dalman RL. Matrix metalloproteinase inhibition limits arterial enlargements in a rodent arterio-venous fistula model. Surgery. 1998;124:328–334. discussion 334–335. [PubMed] [Google Scholar]
- Barker SG, Tilling LC, Miller GC, Beesley JE, Fleetwood G, Stavri GT, Baskerville PA, Martin JF. The adventitia and atherogenesis: removal initiates intimal proliferation in the rabbit which regresses on generation of a ‘neoadventitia’. Atherosclerosis. 1994;105:131–144. doi: 10.1016/0021-9150(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Capers Q, Alexander RW, Lou P, De Leon H, Wilcox JN, Ishizaka N, Howard AB, Taylor WR. Monocyte chemoattractant protein-1 expression in aortic tissues of hypertensive rats. Hypertension. 1997;30:1397–1402. doi: 10.1161/01.hyp.30.6.1397. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fan Y, Poon KYT, Achrol AS, Lawton MT, Zhu Y, McCulloch CE, Hashimoto T, Lee C, Barbaro NM, Bollen AW, Yang G-Y, Young WL. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci. 2006;11:3121–3128. doi: 10.2741/2037. [DOI] [PubMed] [Google Scholar]
- Cox RH. Arterial wall mechanics and composition and the effects of smooth muscle activation. Am J Physiol. 1975;229:807–812. doi: 10.1152/ajplegacy.1975.229.3.807. [DOI] [PubMed] [Google Scholar]
- Danenberg HD, Fishbein I, Gao J, Monkkonen J, Reich R, Gati I, Moerman E, Golomb G. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106:599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice. Evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25:2106–2113. doi: 10.1161/01.ATV.0000181743.28028.57. [DOI] [PubMed] [Google Scholar]
- Gavrila D, Li WG, McCormick ML, Thomas M, Daugherty A, Cassis LA, Miller FJ, Jr, Oberley LW, Dellsperger KC, Weintraub NL. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1671–1677. doi: 10.1161/01.ATV.0000172631.50972.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossl M, Versari D, Mannheim D, Ritman EL, Lerman LO, Lerman A. Increased spatial vasa vasorum density in the proximal LAD in hypercholesterolemia—implications for vulnerable plaque-development. Atherosclerosis. 2007;192:246–252. doi: 10.1016/j.atherosclerosis.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Meng H, Young WL. Intracranial aneurysms: links between inflammation, hemodynamics and vascular remodeling. Neurol Res. 2006;28:372–380. doi: 10.1179/016164106X14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Mesa-Tejada R, Quick CM, Bollen AW, Joshi S, Pile-Spellman J, Lawton MT, Young WL. Evidence of increased endothelial cell turnover in brain arteriovenous malformations. Neurosurgery. 2001;49:124–131. doi: 10.1097/00006123-200107000-00019. discussion 131–132. [DOI] [PubMed] [Google Scholar]
- Ivan E, Khatri JJ, Johnson C, Magid R, Godin D, Nandi S, Lessner S, Galis ZS. Expansive arterial remodeling is associated with increased neointimal macrophage foam cell content: the murine model of macrophage-rich carotid artery lesions. Circulation. 2002;105:2686–2691. doi: 10.1161/01.cir.0000016825.17448.11. [DOI] [PubMed] [Google Scholar]
- Langheinrich AC, Michniewicz A, Bohle RM, Ritman EL. Vasa vasorum neovascularization and lesion distribution among different vascular beds in ApoE−/−/LDL−/− double knockout mice. Atherosclerosis. 2007;191:73–81. doi: 10.1016/j.atherosclerosis.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Langille BL, Bendeck MP, Keeley FW. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol. 1989;256(4 Part 2):H931–H939. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano PJ, Gutterman DD. The adventitia: the outs and ins of vascular disease. Cardiovasc Res. 2007;75:636–639. doi: 10.1016/j.cardiores.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RJ, Skalak TC. Circumferential wall stress as a mechanism for arteriolar rarefaction and proliferation in a network model. Microvasc Res. 1994;47:188–202. doi: 10.1006/mvre.1994.1015. [DOI] [PubMed] [Google Scholar]
- Schiffers PM, Henrion D, Boulanger CM, Colucci-Guyon E, Langa-Vuves F, van Essen H, Fazzi GE, Levy BI, De Mey JG. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:611–616. doi: 10.1161/01.atv.20.3.611. [DOI] [PubMed] [Google Scholar]
- Southgate KM, Mehta D, Izzat MB, Newby AC, Angelini GD. Increased secretion of basement membrane-degrading metalloproteinases in pig saphenous vein into carotid artery interposition grafts. Arterioscler Thromb Vasc Biol. 1999;19:1640–1649. doi: 10.1161/01.atv.19.7.1640. [DOI] [PubMed] [Google Scholar]
- Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol. 2000;20:E120–E126. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, Bakker J, Sanders A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends Biotechnol. 1997;15:178–185. doi: 10.1016/s0167-7799(97)01019-6. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Weiss D, Taylor WR. Deoxycorticosterone acetate salt hypertension in apolipoprotein E−/− mice results in accelerated atherosclerosis: the role of angiotensin II. Hypertension. 2008;51:218–224. doi: 10.1161/HYPERTENSIONAHA.107.095885. [DOI] [PubMed] [Google Scholar]
- Zaltsman AB, Newby AC. Increased secretion of gelatinases A and B from the aortas of cholesterol fed rabbits: relationship to lesion severity. Atherosclerosis. 1997;130:61–70. doi: 10.1016/s0021-9150(96)06046-7. [DOI] [PubMed] [Google Scholar]
- Zhang LN, Velichko S, Vincelette J, Fitch RM, Vergona R, Sullivan ME, Croze E, Wang YX. Interferon-beta attenuates angiotensin II-accelerated atherosclerosis and vascular remodeling in apolipoprotein E deficient mice. Atherosclerosis. 2008;197:204–211. doi: 10.1016/j.atherosclerosis.2007.03.019. [DOI] [PubMed] [Google Scholar]