Abstract
The novel chemopreventive nitric oxide-donating aspirin (NO-ASA) prevents nearly 90% of ductal adenocarcinomas in a animal tumor model. To decipher the mechanism of this effect, we studied in BxPC-3 human pancreatic cancer cells the sequence of signaling events leading from NO-ASA treatment to cell growth inhibition. NO-ASA inhibited the growth of BxPC-3 cells (IC50 = 13 μM), by inhibiting proliferation modestly and inducing apoptosis, necrosis and G1/S cell cycle block. At 15 min of treatment with NO-ASA, the intracellular levels of reactive oxygen species (ROS) began increasing (peak at 8 h, baseline levels by 24 h). ROS activated almost immediately in a time- and concentration-dependent manner the MAPK pathways p38, ERK, and JNK (their activation was abrogated by the antioxidant N-acetylcysteine). MAPK activation induced p21cip-1, which suppressed the levels of cyclin D1 that controls G1/S cell cycle transition. NO-ASA induced COX-2 expression starting 90 min after p21cip-1 was induced. When COX-2 expression was knocked-down using siRNA against cox-2, the expression of p21cip-1 was induced by NO-ASA, regardless of the level of expression of COX-2, suggesting a marginal, if any, role for COX-2 in the growth inhibitory effect of NO-ASA. These findings along with the temporal sequence of individual changes indicate a signaling sequence that involves ROS → MAPKs → p21cip-1 → cyclin D1 → cell death. Our findings establish the critical role of ROS as proximal signaling molecules in the action of anticancer compounds and may be useful in designing mechanism-driven approaches to cancer control.
Keywords: NO-NSAIDs, NO-aspirin, pancreatic cancer, chemoprevention, cell signaling, ROS
INTRODUCTION
Pancreatic cancer represents one of the most lethal human malignancies, with a five year survival of less than 5% [1; 2]. Despite intense efforts in the last twenty years, there persists a paucity of effective agents against pancreatic cancer [3]. We have recently reported that nitric oxide-donating aspirin (NO-ASA) has a remarkable effect on pancreatic carcinogenesis, preventing nearly 90% of ductal adenocarcinomas in a hamster tumor model [4].
NO-ASA (structure in Fig. 1) is a member of the emerging class of NO-NSAIDs that are highly promising agents for the control of various cancers [5]. Their defining pharmacological feature is their ability to release NO. Although their mode of action is not completely understood, redox changes appear to be a significant and early molecular event [6]; it is assumed that ROS activate a signaling cascade that culminates in reduced cell growth [7]. Previous work in colon cancer cells showed that NO-ASA activates by phosphorylation mitogen activated protein kinases (MAPKs), mainly JNK and p38, and that this effect is accompanied by activation of immediate downstream transcription factors (cJun and ATF-2) [8]. Other work on colon cancer cells revealed that NO-ASA inhibits the nitric oxide synthase pathway, Wnt signaling and enhances the expression and catalytic activity of COX-2 [9; 10; 11; 12].
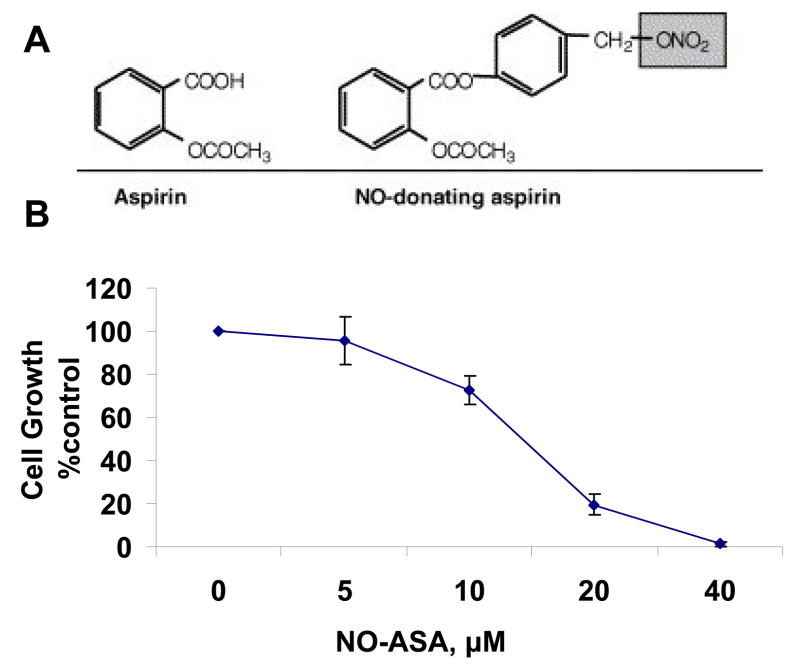
Fig. 1. NO-ASA and its effect on BxPC-3 cell growth.
A: The structural components of NO-ASA: the conventional aspirin moiety is linked by a spacer to the NO-donating group (–ONO2, shaded). B: NO-ASA inhibits the growth of BxPC-3 pancreatic cancer cells, which were treated with various concentrations of NO-ASA for 24 h. Cell growth was assayed using the MTT assay. Data are expressed as percentage of the growth of untreated cells. The results are the average of three independent experiments. Values: mean±SEM. IC50 = 13 μM.
There exists no significant mechanistic work on the effect of NO-ASA on pancreatic cancer. Data from studies in the colon cannot be safely extrapolated to the pancreas, given the significant differences between the two tissues. Thus, in an effort to decipher the mechanism by which NO-ASA prevents pancreatic cancer with such great efficacy, we studied in cultured human pancreatic cancer cells the sequence of signaling events leading from NO-ASA treatment to cell growth inhibition. We focused on ROS and two redox-responsive cascades, MAPKs and COX-2. Studying COX-2 was important because it is considered a key player in pancreatic carcinogenesis [13; 14]. Its significance in pancreatic cancer is based not only on the concept that COX-2 promotes carcinogenesis in general, but also on the evidence that NSAIDs, which inhibit COX, prevent pancreatic cancer [15].
Our data demonstrate that NO-ASA induces a state of oxidative stress, which in turn activates the MAPK cascade, leading to the activation of p21 and inhibition of cell cycle phase transitions. Interestingly, our data show that COX-2 is not required for the growth inhibitory effect of NO-ASA.
MATERIALS AND METHODS
Reagents
NO-ASA [2-(acetyloxy)benzoic acid 4-(nitrooxymethyl)-phenyl ester] was synthesized by us following a standard protocol [16]. 3-(4,5-dimethyl thiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) and N-acetyl-cysteine (NAC) were from Sigma-Aldrich Chemical Co. (Saint Louis, MO).
Cell Culture
BxPC-3 human pancreatic adenocarcinoma cells (American Type Culture Collection, Manassas, VA) were grown in RPMI 1640 medium (with 10% fetal serum, 1000 U/ml penicillin, and 1000 μg/ml streptomycin). Cells were seeded at 5.5×104 cells/cm2 and allowed to attach for 24 h before being treated with NO-ASA. Cell viability was determined using the MTT assay, following the instructions of the manufacturer.
Cell cycle analysis
Following NO-ASA treatment, floating and adherent cells (harvested by trypsinization) were combined, washed with PBS, and fixed in ice-cold 70% ethanol for 30 min. After fixation, cells were washed again with PBS and stained for 30 min at room temperature with the DNA fluorochrome propidium iodide (PI) containing 4 KU/mL RNase type IIA. We measured nuclear PI fluorescence by flow cytometry; in each sample we determined at least 10,000 events. The percentage of cells in G0/G1, S and G2/M was determined from DNA content histograms.
Cell proliferation assay
Cell proliferation was assayed in cells cultured in 96-well plates using 5′-bromo-2′-deoxy-uridine labeling and a commercially available kit (Roche Applied Sciences, Mannheim, Germany) following the instructions of the manufacturer.
Assays for apoptosis and necrosis
Apoptosis and necrosis were assayed in BxPC-3 cells cultured in 96-well plates using the Cell Death Detection ELISAPLUS kit (Roche Applied Sciences, Mannheim, Germany) following the instructions of its manufacturer. The detection of apoptosis was based on a quantitative sandwich-enzyme-immunoassay principle, using mouse monoclonal antibodies directed against DNA and histones, respectively. This allows the specific detection and quantitation of mono- and oligonucleosomes that are released into the cytoplasm of cells that die from apoptosis. Necrosis was assayed using the Cytotoxicity Detection kitPLUS (LDH) (Roche Applied Sciences, Mannheim, Germany), which quantifies of cell death and cell lysis, based on the measurement of lactate dehydrogenase (LDH) activity released from the cytosol of damaged cells into the supernatant.
Determination of reactive oxygen species (ROS)
After NO-ASA treatment, cells were collected by trypsinization, washed with PBS once, resuspended in 10 μM of 5-(and-6)-carboxy-2′,7′-dichlorofluorescine diacetate (DCFDA) (Invitrogen, Carlsbad, CA), incubated at 37 °C for 30 min in the dark. Following this, their fluorescence intensity was determined by flow cytometry (Beckman Coulter Inc., Fullerton, CA). We analyzed a minimum of 10,000 events using the WinMDI software, and expressed the data as fluorescent intensity versus events.
Protein extraction from whole cell lysates
NO-ASA-treated cells were scraped on ice, washed with ice-cold phosphate-buffered saline (PBS) and lysed in lysis buffer (20 mM Hepes, 50 mM NaF, and 1 mM Na3VO4, pH 7.3, with 10% glycerol, 1% Triton X-100, 20 μg/ml leupeptin, 20 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride) and 2.5 mM 4-nitrophenylphosphate). Protein concentration was determined using the Bradford method (Bio-Rad, Hercules, CA).
Western blotting
Electrophoresis of protein cell lysates was performed in 10% or 12% SDS-polyacrylamide gel electrophoresis gels as described previously [17]. Proteins were transferred onto nitrocellulose membranes, which were stripped and re-probed as necessary. Antibodies against JNK, p38, ERK1/2, AKT, c-Jun and p21 were from Cell Signaling Technology Inc. (Beverly, MA); those against cyclin D1 and α-tubulin were from Upstate Cell Signaling (Lake Placid, NY); and the antibody against β-actin was from Sigma-Aldrich.
siRNA transfection
COX-2 siRNA was purchased from Dharmacon Inc. (Chicago, IL) and transient transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
RESULTS
NO-ASA inhibits the growth of BxPC-3 pancreatic cancer cells and alters their kinetics
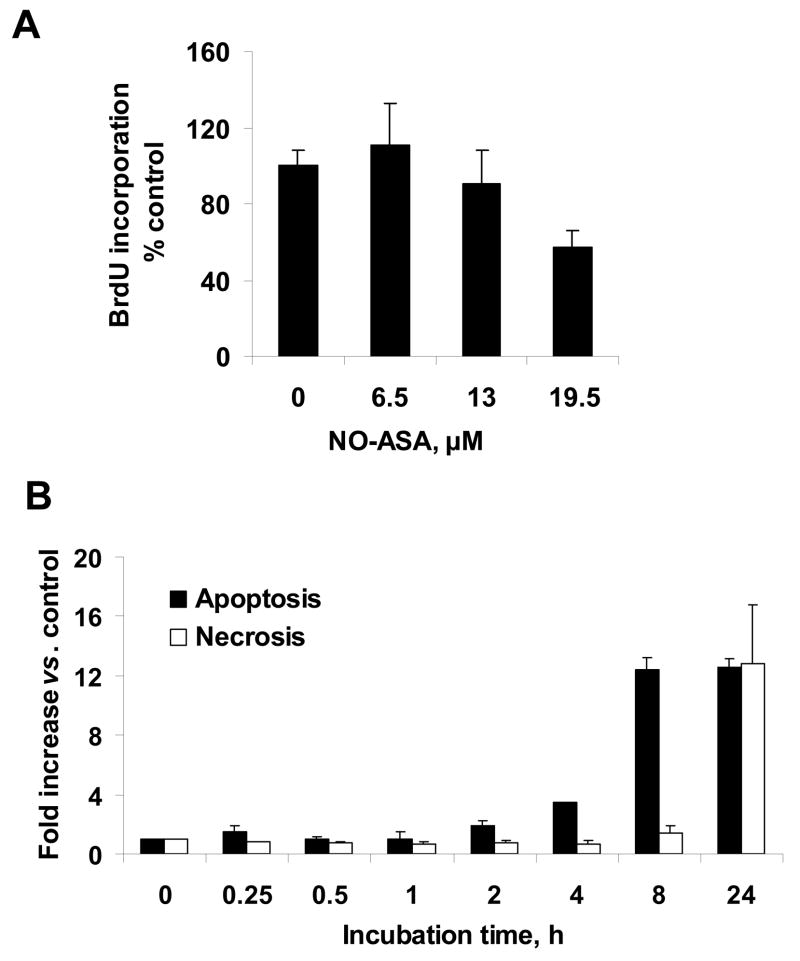
Initially, we evaluated the effect of NO-ASA on the growth of BxPC-3 pancreatic cancer cells and on their cytokinetics. As seen in Fig. 1, the 24 h IC50 of NO-ASA for cell growth inhibition was 13.0 ± 1.0 μM (mean ± S.E.M). Cell proliferation, determined by BrdU incorporation, was marginally, if at all, altered by NO-ASA at or below its IC50 for cell growth, being decreased by 40% at 1.5xIC50. Treatment of BxPC-3 cells with NO-ASA led to both apoptosis and necrosis (Fig. 2). At the latter concentration of NO-ASA, apoptosis started increasing over control (2-fold) after 4 h treatment. The apoptotic rate increased dramatically at 8 h (12-fold over control), reaching a plateau that lasted at least 24 h (the period of observation). In contrast, there was a miniscule increase of necrosis at 8 h, with a 12-fold increase at 24 h. Finally, NO-ASA started blocking the G1/S cell cycle phase transition quite modestly at 4 h and significantly at 8 h (Table 1).
Fig. 2. Effect of NO-ASA on BxPC-3 cytokinetics.
A, BxPC-3 cells were treated with three different concentrations of NO-ASA for 24 h and BrdU incorporation was determined as in Methods. Only the highest NO-ASA concentration produced a statistically significant reduction in BrdU incorporation (P<0.04). B: Apoptosis and necrosis of BxPC-3 cells treated with NO-ASA 19.5 μM was determined as in Methods. Data are expressed as fold change over untreated controls. All values: mean±SEM (n=3).
Table 1.
Cell cycle phase distribution of BxPC-3 cells treated with NO-ASA
| Time (h) | G0/G1 | S Control / NO-ASA treated % total |
G2/M |
|---|---|---|---|
| 0.25 | 50/51 | 29/29 | 20/18 |
| 0.5 | 46/48 | 32/31 | 21/21 |
| 1 | 51/53 | 31/30 | 17/15 |
| 2 | 42/46 | 34/33 | 20/20 |
| 4 | 42/47 | 34/31 | 20/22 |
| 8* | 39/55 | 35/31 | 26/14 |
| 24* | 65/58 | 17/29 | 18/14 |
Representative of two experiments (each point in duplicate), the results of which were within 10%.
Percentages for each cell cycle phase have been adjusted based on the total population of cells in the three phases, excluding sub-diploid cells phases
NO-ASA induces oxidative stress in BxPC-3 cells
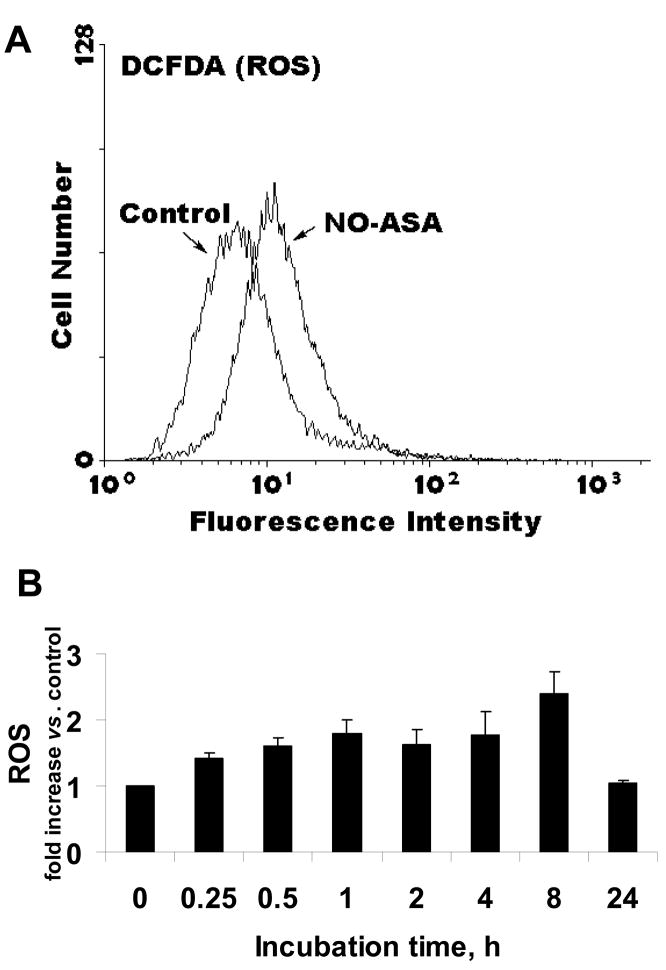
We examined the levels of ROS in response to NO-ASA using the DCFDA probe, a general ROS probe, which reacts with nearly 10 individual ROS (Fig. 3) [18; 19]. The intracellular levels of ROS increased as early as 15 min following exposure to NO-ASA (the earliest time period evaluated), reaching their highest level at 8 h (2.5 fold over untreated control) and returning to baseline levels by 24 h.
Fig. 3. NO-ASA induces ROS production in BxPC-3 cells.
A: Representative flow cytometric histogram of BxPC-3 cells treated with 19.5 μM of NO-ASA for 30 min; ROS levels were measured as in Methods. B: ROS levels at various time points following treatment with NO-ASA 19.5 μM. Values (mean±SEM; n=3) are expressed as fold of untreated controls.
NO-ASA activates MAPKs
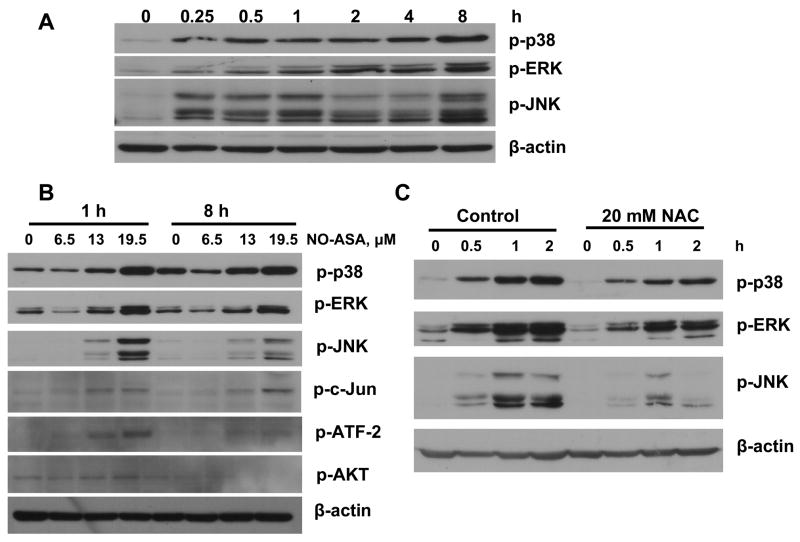
All three MAPK pathways, p38, ERK, and JNK, were activated by NO-ASA. Their activation was dependent on a) the duration of treatment, b) NO-ASA concentration, and c) the redox status of the cell. For all of them, activation began as early as 15 min after treatment of the cells with NO-ASA and continued for the period of observation (8 h). We studied three different concentrations of NO-ASA, corresponding to 0.5x, 1x and 1.5x of its 24 h IC50 for cell growth. As shown in Fig. 4, the concentration dependence of their response is clear. Finally, when we pretreated the cells with NAC, an antioxidant that prevented the generation of ROS (data not shown), the activation of the MAPK pathways was attenuated or even abrogated (in the case of JNK). Consistent with these findings was the observation that c-Jun and ATF-2, the downstream effectors of p38, ERK and JNK, were also activated by NO-ASA, with their response following a similar pattern. Of interest, Akt was marginally, if at all, affected by NO-ASA; this finding was similar to, albeit more pronounced than, what was observed in HT-29 colon cancer cells [8].
Fig. 4. Effect of NO-ASA on MAPK activation.
BxPC-3 cells were treated with NO-ASA 19.5 μM for various times (A) or with various NO-ASA concentrations for 1 h or 8 h (B) and the expression of the phosphoproteins shown was determined by immunoblotting. C: BxPC-3 cells were treated with 20 mM NAC for 4 h prior to treatment with NO-ASA. Loading control: μ-actin
NO-ASA induces p21 and inhibits cyclin D1
Proliferation, governed at the level of the cell cycle by specific cell cycle regulatory proteins, requires that cyclins activate target cyclin-dependent kinases (CDK). In contrast, the CDK inhibitors p21cip-1 (henceforth denoted p21) limits proliferation by inhibiting cyclin-CDK complexes [20; 21; 22]. A significant downstream effector of MAPKs is p21 [23; 24]. Thus we examined the effect of NO-ASA on p21 levels in these cells.
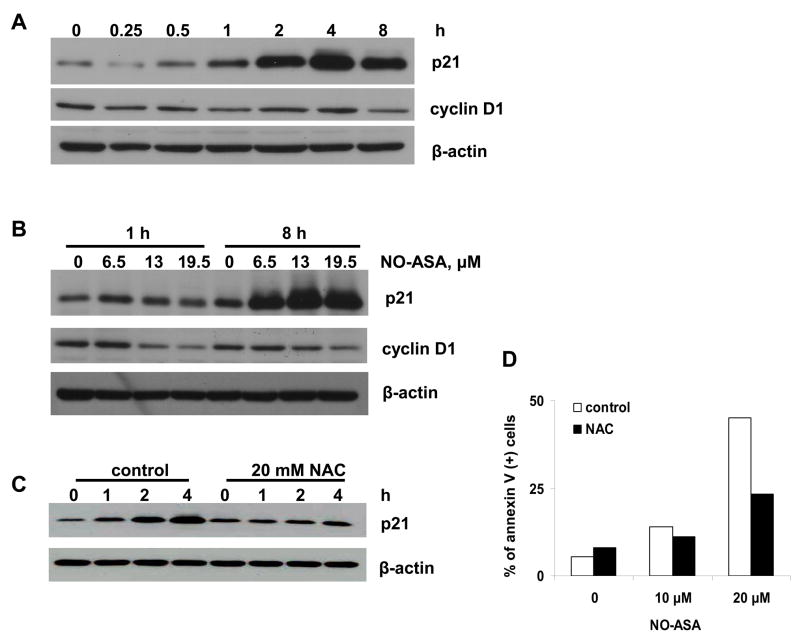
As shown in Fig. 5, NO-ASA induced the expression of p21 in both time- and concentration-dependent fashion. Significant induction of p21 began 2 h post initiation of treatment with NO-ASA, although very modestly increased levels of p21 were noted earlier. NAC, administered as in the previous experiments, prevented the induction of p21. This finding suggests that p21 is either directly responsive to redox changes or affected by factors that are themselves redox sensitive, such as MAPKs.
Fig. 5. Effect of NO-ASA on p21 and cyclin D1 expression.
BxPC-3 cells were treated with NO-ASA 19.5 μM for the indicated periods of time (A) or with three different concentrations of NO-ASA for 1 h or 8 h (B) or were pretreated with 20 mM NAC (C). The expression of proteins was determined by immunoblotting. Loading control: β-actin
Cyclin D1 forms a complex with, and functions as a regulatory subunit of CDK4 or CDK6, whose activity is required for cell cycle G1/S transition [20]. The expression of cyclin D1 is modulated by p21. As shown in Fig. 5, the levels of cyclin D1 were suppressed as those of p21 increased, in agreement with the known regulatory relationship between the two proteins.
It is of interest to note that the antioxidant N-acetyl cysteine (NAC) abrogated not only the induction of signaling molecules, but also cell death (Fig. 5D). This finding makes a strong link between ROS and cell death and supports, however, indirectly, the notion that these signaling pathways may be of some relevance to the induction of cell death.
NO-ASA induces COX-2 expression but COX-2 does not participate in NO-ASA’s growth inhibitory effect
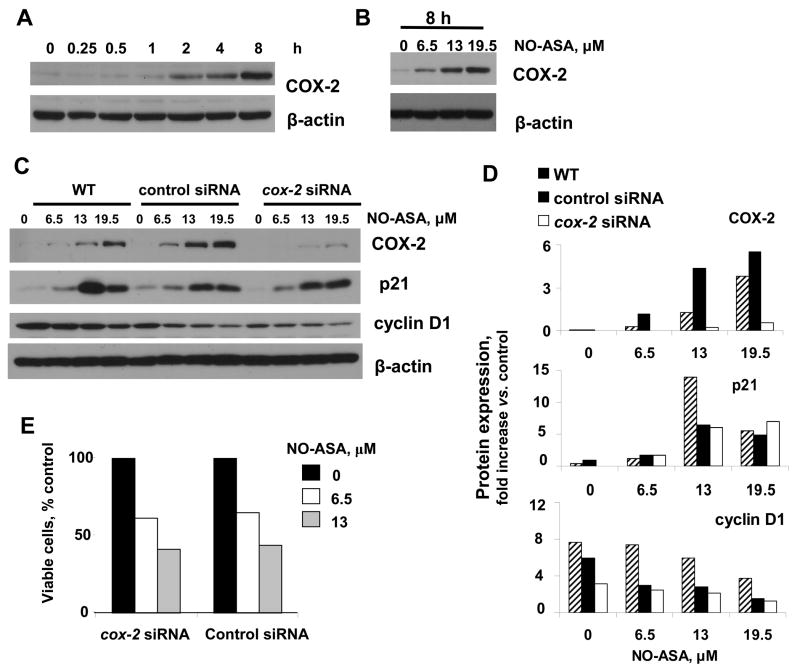
NO-ASA induced COX-2 expression in a concentration- and time-dependent manner (Fig. 6). The induction of COX-2 began at 2 h and increased progressively during the following 6 h. The concentration dependence of this effect was demonstrated at 8 h (NO-ASA concentrations corresponding to its 0.5x, 1x and 1.5x IC50 for growth inhibition). There was no change in COX-2 levels at all three NO-ASA concentrations at 1 h (data not shown).
Fig. 6. The role of COX-2 in the effect of NO-ASA on BxPC-3 cells.
BxPC-3 cells were treated with NO-ASA 19.5 μM for the indicated periods of time (A) or with three different concentrations of NO-ASA for 8 h (B) and the expression of COX-2 was determined by immunoblotting. C: BxPC-3 cells were transfected with control siRNA or cox-2 siRNA for 24 h and then treated with three concentrations of NO-ASA for 8 h. Expression of COX-2, p21, and cyclin D1 was determined by immunoblotting. Loading control: β-actin. D: The levels of COX-2, p21, and cyclin D1, determined by densitometry, are presented as fold change over untreated controls (representative of two independent experiments giving similar results).
Since COX-2 overexpression increases the expression of p21 [25], we sought to determined the role, if any, of COX-2 in the regulation of p21. To this end we transfected BxPC-3 cells with siRNA against cox-2. There was no detectable expression of COX-2 even when these cells were treated with NO-ASA up to 19.5 μM. As expected, COX-2 was induced signifcantly over control in these cells when transfected with control siRNA as well as in untransfected cells.
The expression of p21 was, however, induced by NO-ASA, regardless of the level of expression of COX-2, as shown in Fig 6. In all these cases, the expression of cyclin D1 was suppressed by p21, in agreement with current understanding. These data make it clear that the induction of COX-2 by NO-ASA did not affect signaling that controls the G1/S transition that we observed in these cells in response to NO-ASA. Consistent with this conclusion is our finding that the cell growth inhibitory effect of NO-ASA was maintained even when COX-2 expression was knocked down. As shown in Fig. 6E, the number of viable cells in response to two concentrations of NO-ASA was practically the same in cells treated with cox-2 siRNA or scrambled control siRNA.
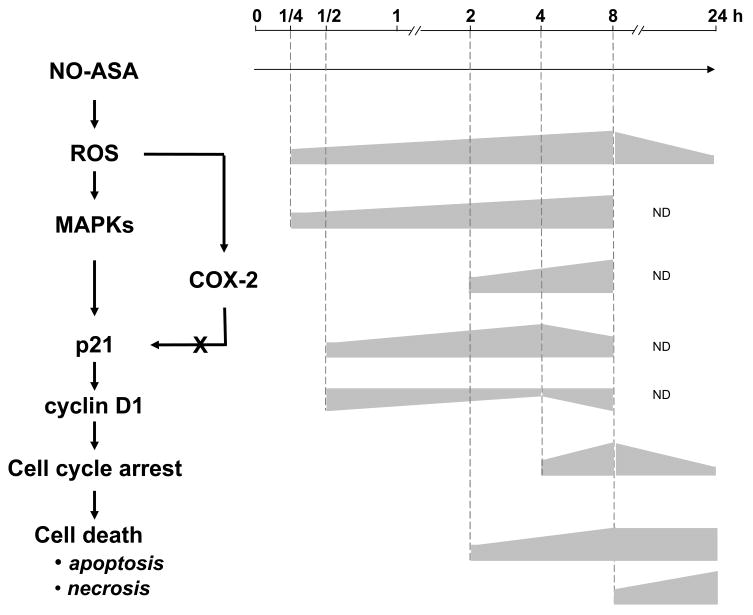
The temporal sequence of signaling changes in response to NO-ASA
Figure 7 depicts a plausible signaling cascade in the effect of NO-ASA on BcPC-3 cells and the time periods during which each change occurred. It stands to reason that changes that precede a given change may be linked etiologically; conversely, a change that follows, for example, the activation of a signaling molecule may not cause it. Applying this line of reasoning it is clear that the sequence of events depicted in this figure is realistic. COX-2 plays no direct role in the changes that we have observed, as its induction occurs after that of p21. This conclusion was supported by the experiments in which its expression was knocked down.
Fig. 7. The signaling cascade initiated by NO-ASA in BxPC-3 cells.
Left: A plausible signaling cascade mediating the effect of NO-ASA on BxPC-3 cells. Right: The time periods during which each change occurred and a rough representation of their relative intensity based on the previously shown data. In this scheme, COX-2 does not modulate the expression of p21.
Another conclusion from these findings is that in this cell line the initial (and fairly rapid) detectable effect is the induction of ROS by NO-ASA, which is followed quickly by the activation of MAPKs. Within 1 h the signal is propagated to p21 and the cell cycle machinery, although it requires an additional 3 h before a cell cycle block (G1/S) is detectable. Apoptosis is apparent at 2 h and reaches its plateau at 8 h, while necrosis is clearly a late event.
DISCUSSION
Our data demonstrate a sequence of signaling events in a pancreatic cancer cell line treated with NO-ASA that culminates in cell death by apoptosis and necrosis. The highlights of this effect are a) the rapid onset of signaling changes, b) the proximity and key role of ROS induction, c) the time required for the initial signal to translate in the desired cytokinetic outcome (cell death; it begins at 4h), and d) the absence of any vital role of COX-2.
The mechanism of action of NO-NSAIDs, of which NO-ASA is the best-studied member, remains incompletely understood [5]. As our study confirms, in agreement with previous data from colon cancer cell lines [6], the induction of ROS is a pivotal event in their action. In fact, the notion has been advanced recently that the induction of ROS is a mechanistic property of many anticancer agents used in both prevention and treatment of cancer [7]. This is not surprising as it is being increasingly appreciated that ROS are important signaling molecules, which normally regulate multiple cell functions. The damaging effects of ROS, some associated with carcinogenesis, appear at high concentrations, when they generate the irreversible stage of oxidative stress [26].
It is clear form our findings that the induction of ROS by NO-ASA is quickly followed by the activation of the MAPK signaling cascade, which is well recognized as redox-responsive [23; 24]. Key signaling downstream molecules that were tested (p21, cyclin D1) were activated in response to MAPK activation, ultimately leading to cell cycle deceleration and the activation of cell death. This appears to be a rather slow effect. The slowest response that we observed was the induction of cell necrosis, which was barely detectable 4 h after the onset of apoptosis. This lag likely reflects the time needed to develop secondary necrosis (an “end-stage” of apoptosis) or the need for high ROS concentrations (they peaked at 8 h) before the mechanism of cell necrosis was activated. Of note, cell death was the predominant cytokinetic effect of NO-ASA; both cell proliferation and the cell cycle block were quantitatively weak effects.
A discordant finding was the non-participation of COX-2 in pancreatic cancer cell death induced by NO-ASA. This finding has been reported previously by us and others in cell lines of different tissue origin [10] and adds to the accumulating data that COX-2 plays a rather subtle, if not limited, role in carcinogenesis [27]. In fact, COX-2 may be one of many players in cancer and possibly not the dominant player whose inactivation would control cancer. Thus, NSAIDs may exert their chemopreventive effect against pancreatic cancer by non-COX mechanisms.
Fig. 7 outlines a plausible signaling cascade mediating the effect of NO-ASA on BxPC-3 cells and represents schematically the time periods during which each change occurred along with a rough representation of their relative intensity. However, activation by NO-ASA of this linearly direct signaling sequence may not be the sole mechanism by which NO-ASA exerts its anticancer effect [5]. For example, ROS could activate additional signaling pathways such as NF-κB, a known redox sensitive transcriptional factor; oxidation of a cysteinyl residue of its p50 subunit inhibits its DNA binding [28]. In fact, NO-ASA inhibits NF-κB activation in pancreatic cancer cells [9], but whether such additional effects branch off the pathway of Fig. 7 or are independent events remains to be determined.
In conclusion, our work presented here documents a signaling sequence by which NO-ASA can induce the death of the pancreatic cancer cell. Obtaining detailed knowledge of the mechanism by which a pharmacological agent such as NO-ASA kills the pancreatic cancer cell is critical to the rational design of therapeutic strategies for this lethal cancer. Additional work will be required to incorporate such information into a comprehensive assessment of cell death so that they may ultimately be translated into therapeutic applications.
Acknowledgments
NIH R01 CA101011902
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Samra JS, Gananadha S, Hugh TJ. Surgical management of carcinoma of the head of pancreas: extended lymphadenectomy or modified en bloc resection? ANZ J Surg. 2008;78:228–36. doi: 10.1111/j.1445-2197.2008.04426.x. [DOI] [PubMed] [Google Scholar]
- Hart AR, Kennedy H, Harvey I. Pancreatic cancer: a review of the evidence on causation. Clin Gastroenterol Hepatol. 2008;6:275–82. doi: 10.1016/j.cgh.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Boeck S, Heinemann V. The role of second-line chemotherapy after gemcitabine failure in patients with advanced pancreatic cancer. Future Oncol. 2008;4:41–50. doi: 10.2217/14796694.4.1.41. [DOI] [PubMed] [Google Scholar]
- Ouyang N, Williams JL, Tsioulias GJ, Gao J, Iatropoulos MJ, Kopelovich L, Kashfi K, Rigas B. Nitric oxide-donating aspirin prevents pancreatic cancer in a hamster tumor model. Cancer Res. 2006;66:4503–11. doi: 10.1158/0008-5472.CAN-05-3118. [DOI] [PubMed] [Google Scholar]
- Rigas B. The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr Opin Gastroenterol. 2007;23:55–9. doi: 10.1097/MOG.0b013e32801145b0. [DOI] [PubMed] [Google Scholar]
- Gao J, Liu X, Rigas B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc Natl Acad Sci U S A. 2005;102:17207–12. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas B, Sun Y. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br J Cancer. 2008;98:1157–60. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley TR, Rigas B. Nitric oxide-donating aspirin inhibits colon cancer cell growth via mitogen-activated protein kinase activation. J Pharmacol Exp Ther. 2006;316:25–34. doi: 10.1124/jpet.105.091363. [DOI] [PubMed] [Google Scholar]
- Williams JL, Ji P, Ouyang N, Liu X, Rigas B. NO-donating aspirin inhibits the activation of NF-{kappa}B in human cancer cell lines and Min mice. Carcinogenesis. 2008 doi: 10.1093/carcin/bgm275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JL, Nath N, Chen J, Hundley TR, Gao J, Kopelovich L, Kashfi K, Rigas B. Growth inhibition of human colon cancer cells by nitric oxide (NO)-donating aspirin is associated with cyclooxygenase-2 induction and beta-catenin/T-cell factor signaling, nuclear factor-kappaB, and NO synthase 2 inhibition: implications for chemoprevention. Cancer Res. 2003;63:7613–8. [PubMed] [Google Scholar]
- Spiegel A, Hundley TR, Chen J, Gao J, Ouyang N, Liu X, Go MF, Tsioulias GJ, Kashfi K, Rigas B. NO-donating aspirin inhibits both the expression and catalytic activity of inducible nitric oxide synthase in HT-29 human colon cancer cells. Biochem Pharmacol. 2005;70:993–1000. doi: 10.1016/j.bcp.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Nath N, Kashfi K, Chen J, Rigas B. Nitric oxide-donating aspirin inhibits beta-catenin/T cell factor (TCF) signaling in SW480 colon cancer cells by disrupting the nuclear beta-catenin-TCF association. Proc Natl Acad Sci U S A. 2003;100:12584–9. doi: 10.1073/pnas.2134840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XZ, Tong WG, Adrian TE. Cyclooxygenases and lipoxygenases as potential targets for treatment of pancreatic cancer. Pancreatology. 2001;1:291–9. doi: 10.1159/000055827. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Adsule S, Li Y, Padhye S. Back to the future: COX-2 inhibitors for chemoprevention and cancer therapy. Mini Rev Med Chem. 2007;7:599–608. doi: 10.2174/138955707780859431. [DOI] [PubMed] [Google Scholar]
- Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res. 2003;37:1–24. doi: 10.1159/000071364. [DOI] [PubMed] [Google Scholar]
- Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson Gd, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J Med Chem. 1997;40:1347–65. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- Yeh RK, Chen J, Williams JL, Baluch M, Hundley TR, Rosenbaum RE, Kalala S, Traganos F, Benardini F, del Soldato P, Kashfi K, Rigas B. NO-donating nonsteroidal antiinflammatory drugs (NSAIDs) inhibit colon cancer cell growth more potently than traditional NSAIDs: a general pharmacological property? Biochem Pharmacol. 2004;67:2197–205. doi: 10.1016/j.bcp.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130:1910–7. [PubMed] [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–31. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Santamaria D, Ortega S. Cyclins and CDKS in development and cancer: lessons from genetically modified mice. Front Biosci. 2006;11:1164–88. doi: 10.2741/1871. [DOI] [PubMed] [Google Scholar]
- Pei XH, Xiong Y. Biochemical and cellular mechanisms of mammalian CDK inhibitors: a few unresolved issues. Oncogene. 2005;24:2787–95. doi: 10.1038/sj.onc.1208611. [DOI] [PubMed] [Google Scholar]
- Weiss RH. p21Waf1/Cip1 as a therapeutic target in breast and other cancers. Cancer Cell. 2003;4:425–9. doi: 10.1016/s1535-6108(03)00308-8. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. Faseb J. 2008;22:954–65. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Pimienta G, Pascual J. Canonical and alternative MAPK signaling. Cell Cycle. 2007;6:2628–32. doi: 10.4161/cc.6.21.4930. [DOI] [PubMed] [Google Scholar]
- Zahner G, Wolf G, Ayoub M, Reinking R, Panzer U, Shankland SJ, Stahl RA. Cyclooxygenase-2 overexpression inhibits platelet-derived growth factor-induced mesangial cell proliferation through induction of the tumor suppressor gene p53 and the cyclin-dependent kinase inhibitors p21waf-1/cip-1 and p27kip-1. J Biol Chem. 2002;277:9763–71. doi: 10.1074/jbc.M106307200. [DOI] [PubMed] [Google Scholar]
- Frein D, Schildknecht S, Bachschmid M, Ullrich V. Redox regulation: a new challenge for pharmacology. Biochem Pharmacol. 2005;70:811–23. doi: 10.1016/j.bcp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Rigas B, Kashfi K. Cancer prevention: a new era beyond cyclooxygenase-2. J Pharmacol Exp Ther. 2005;314:1–8. doi: 10.1124/jpet.104.080564. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ueno Y, Okamoto T. Oxidoreductive regulation of nuclear factor kappa B. Involvement of a cellular reducing catalyst thioredoxin. J Biol Chem. 1993;268:11380–8. [PubMed] [Google Scholar]