Abstract
Background
Severe asthma is characterized by persistent airway inflammation and increased formation of reactive oxygen species.
Objectives
Glutathione (GSH) is an important antioxidant in the epithelial lining fluid (ELF). We hypothesized that airway GSH homeostasis was altered in children with severe asthma and was characterized by decreased GSH and increased glutathione disulfide (GSSG) concentrations.
Methods
Bronchoalveolar lavage was obtained from 65 children with severe asthma, including 35 children with baseline airway obstruction evidenced by FEV1 <80%. Control data were obtained from 6 children with psychogenic (habit) cough or vocal cord dysfunction undergoing diagnostic bronchoscopy and 35 healthy adult controls. GSH, GSSG, and other determinants of airway oxidative stress including glutathione S-transferase (GST), glutathione reductase (GR), glutathione peroxidase (GPx), malondialdehyde, 8-isoprostane, and H2O2 were measured in the ELF. The ELF redox potential was calculated from GSH and GSSG by using the Nernst equation. Results: Compared with controls, subjects with severe asthma had lower airway GSH with increased GSSG despite no differences in GST, GR, and GPx activities between groups. This was accompanied by increased malondialdehyde, 8-isoprostane, and H2O2 concentrations in the ELF. GSH oxidation was most apparent in subjects with severe asthma with airway obstruction and was supported by an upward shift in the ELF GSH redox potential.
Conclusion
Children with severe asthma have increased biomarkers of oxidant stress in the ELF that are associated with increased formation of GSSG and a shift in the GSH redox potential toward the more oxidized state.
Keywords: Asthma, children, glutathione, 8-isoprostanes, hydrogen peroxide, malondialdehyde, oxidative stress, redox potential
Severe refractory asthma is a complex disorder characterized by airway hyperresponsiveness, obstructive changes in pulmonary function, and persistent airway inflammation despite high-dose inhaled corticosteroid (ICS) treatment.1–3 Although the inflammatory response is important for the initiation of tissue repair, the exaggerated responses associated with severe asthma result in excessive reactive oxygen species formation and tissue destruction.4 This resulting imbalance between pro-oxidant and antioxidant forces leads to an ongoing cycle of inflammation in the asthmatic airway that ultimately contributes to irreversible airway injury.5
Glutathione (GSH), a tripeptide thiol, is an abundant airway antioxidant6 which reduces organic hydroperoxides and protects the airway from lipid peroxidation.7 In response to hydroperoxides, GSH is released by glutathione S-transferase (GST) and becomes oxidized through a reaction involving glutathione peroxidase (GPx). This process forms glutathione disulfide (GSSG), which can be reduced to GSH by an nicotinamide adenine dinucleotide phosphate (NADPH)–dependent glutathione reductase (GR) reaction (Fig 1). The relationship between GSH and GSSG is a critical regulator of cellular processes and antioxidant defense. With excessive GSSG accumulation, airway GSH homeostasis is altered, resulting in impaired cellular signaling and increased susceptibility to lung injury.8–10
FIG 1.
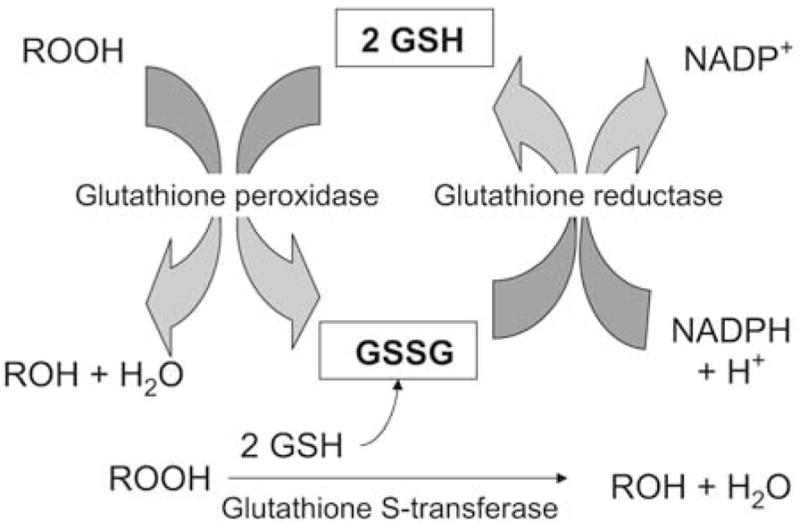
Diagram of airway glutathione homeostasis. ROOH and ROH represent organic hydroperoxides.
Although GSH homeostasis has been previously assessed in the blood,11 sputum,12 and bronchoalveolar lavage (BAL)13 of subjects with mild asthma, no study to date has directly examined airway GSH concentrations in patients with severe asthma. The purpose of this study was to quantify epithelial lining fluid (ELF) GSH homeostasis in children with severe asthma. We hypothesized that children with severe asthma would have increased airway GSSG and greater generalized airway oxidation as measured by increased H2O2, malondialdehyde, and 8-isoprostanes.
METHODS
Sample
A convenience sample of children with severe asthma 5 to 17 years of age attending a difficult asthma clinic at Emory University were recruited for this study. Participants were clinically stable and were under the care of a pediatric pulmonary specialist. Participants underwent flexible bronchoscopy as indicated for persistent asthma symptoms despite appropriate treatment with high-dose inhaled and systemic corticosteroids.14 The subsequent BAL sample was divided between the research and clinical laboratories according to a protocol approved by the local Institutional Review Board. Informed consent was obtained from all caregivers. Children also provided verbal and written assent.
Children with severe asthma met published criteria for persistent asthma15 and had at least a 12% change in FEV1 after short-acting bronchodilator administration. Severe asthma was diagnosed according to criteria developed by the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program2 based on an American Thoracic Society consensus panel report (see this article’s Table E1 in the Online Repository at www.jacionline.org).16 Thresholds for high-dose ICS were adjusted for children and defined as ≥440 μg of fluticasone equivalent per day for children younger than 12 years and ≥880 μg for children 12 to 17 years.15 All children with asthma were treated with a stable dose of ICS or oral corticosteroids for at least 8 weeks before bronchoscopy. Children with immunodeficiency, history of premature birth, or other pulmonary morbidities were excluded. Subjects with asthma were screened for corticosteroid adherence. Known comorbid conditions associated with asthma including sinus infection, sleep disorders, and gastroesophageal reflux were addressed before bronchoscopy.
TABLE E1.
National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program criteria for severe asthma in children
|
Major criteria (must have at least 1) |
| Daily high-dose ICS |
| Children <12 y: ≥440 μg fluticasone equivalent/d |
| Children ≥12 y: ≥880 μg fluticasone equivalent/d |
| Daily oral corticosteroid use |
|
Minor criteria (must have at least 2) |
| Treatment with a daily controller medication in addition to ICS |
| Daily short-acting bronchodilator use (at least 5 of 7 days) |
| Airway obstruction with FEV1 <80% predicted at baseline |
| One or more emergency room visits in the previous 12 mo |
| Three or more oral corticosteroid bursts in the previous 12 mo |
| History of worsening symptoms with a reduction in corticosteroid dose |
| History of intubation |
Controls for this study were recruited from 2 populations: (1) children with psychogenic (habit) cough or vocal cord dysfunction, and (2) healthy, nonsmoking adult volunteers. Control children had no family history of asthma, a negative bronchodilator response, no evidence of aeroallergen sensitivity, and normal exhaled nitric oxide (FENO) concentrations. Adults serving as controls were nonsmokers with no known pulmonary disorders and no respiratory symptoms.
Procedures
Spirometry was performed with a portable spirometer (KoKo Legend; Ferraris, Louisville, Colo) according to American Thoracic Society criteria for reproducibility17 and was interpreted according to population reference standards.18 FENO was collected with a reservoir bag at a fixed exhaled flow rate of 0.35 L/s and analyzed offline by chemiluminescence (Sievers NOA 280-I; Ionic Instruments, Boulder, Colo).19 Nicotine exposure was verified in healthy adult controls using a urinary cassette test (Accutest; Jant Pharmacal, Encino, Calif) with a cotinine cutoff of 200 ng/mL. Venipuncture was performed in all participants immediately before bronchoscopy for plasma urea determination.
Bronchoscopy in pediatric participants was performed by pediatric pulmonologists using a laryngeal mask airway or endotracheal tube and inhaled sevoflurane. BAL fluid was collected from the right middle lobe with three 1-mL/kg (50 mL maximum) saline lavages flushed through the suction channel of a flexible bronchoscope (Olympus BF-3C160 [3.7 mm] or BF-P160 [4.9 mm]; Olympus America Inc, Melville, NY). Bronchoscopy was performed in adults by physicians trained in pulmonary and critical care medicine using a flexible bronchoscope (Olympus BF-1T20D, Olympus America) passed transnasally into the right middle lobe. Subjects received intravenous midazolam and fentanyl for the procedure. Three 50-mL saline aliquots were instilled and immediately aspirated. The BAL from all participants was pooled under continuous low pressure suction. In children, the BAL return volume was divided between the research and clinical laboratories. The samples submitted to the clinical laboratories were subjected to standard culture and sensitivity testing, viral respiratory panels, and cytopathological stains for bacteria and fungi.
Bronchoalveolar lavage fluid was centrifuged at 1200 rpm for 7 minutes at 4°C to separate the supernatant and cellular fractions. The supernatant was removed and divided into 250-μL aliquots. To prevent auto-oxidation of the samples during storage, 1 sample aliquot was preserved immediately after collection for GSH and GSSG analysis in a 5% perchloric acid solution containing iodoacetic acid (6.7 μmol/L) and boric acid (0.1 mol/L) with 5 μmol/L γ-glutamyl-glutamate internal standard.20 A second sample aliquot was preserved in 5 μL 2.5 mg/mL butylated hydroxytoluene for 8-isoprostane measurement. Aliquots were stored at −80°C before analysis. The cell pellet was resuspended in 1 mL Dulbecco modified Eagle medium containing 10% FCS. Total cell counts were performed manually with a hemocytometer. Cellular differentials were assessed after Wright staining.
The protein content of the BAL supernatant was assessed using a Coomassie (Bradford) protein assay (Pierce Biotechnology, Rockford, Ill) with a detection limit of 1 μg/mL at an absorbance of 595 nm. Urea nitrogen was measured in plasma and BAL supernatant by using a quantitative colorimetric assay (Pointe Scientific, Canton, Mich) with sensitivity of 0.05 to 150 mg/dL. The dilution of the BAL was calculated from (urea)plasma/(urea)BAL21.
Reduced glutathione and GSSG concentrations were measured in BAL supernatant by reverse-phase high-performance liquid chromatography after derivatization of the samples with dansyl chloride.22 Derivatives were separated on a 10 μm Ultrasil amino-column with detection at 365 nm (Waters Alliance 2690, Waters Corporation, Milford, Mass). Fluorescence detection was recorded by 2 detectors (Waters 474, Waters Corporation, and Gilson 121, Gilson Inc, Middletown, Wis). GSH and GSSG were quantified relative to γ-glutamyl-glutamate by integration.
The redox potential (Eh) of the GSH/GSSG thiol pair in ELF was calculated with the Nernst equation, Eh = Eo + RT/nF ln [disulfide]/([thiol1][thiol2]).23 The Eo is the standard potential for the redox couple, R is the gas constant, T is the absolute temperature, n is 2 for the number of electrons transferred, and F is the Faraday constant. The standard potential Eo for the 2 GSH/GSSG couple was −264 mVat pH =7.4. Adjustment for pH was made by a +5.9 mV change in Eo with every 0.1 decrease in pH.
Enzymatic activities of GST, GR, and GPx were quantified in the BAL supernatant with commercially available assay kits (Cayman Chemical, Ann Arbor, Mich). For GST, samples were concentrated to a molecular weight cutoff of 3000. GST activity was determined after conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) with GSH. GST activity was analyzed over a period of 5 minutes at 340 nm with a CDNB extinction coefficient of 0.0096 μmol/L−1cm−1. GR and GPx samples were concentrated to a 10,000 molecular weight cutoff. GR activity was determined by the rate of NADPH oxidation, and GPx activity was determined through a coupled reaction with GR. GR and GPx activities were analyzed over a period of 5 minutes at 340 nm absorbance with an NADPH extinction coefficient of 0.00622 μmol/L−1cm−1. Each assay had a wavelength accuracy of ±2 nm with repeatability of ±0.2 nm.
Malondialdehyde concentrations were determined in the BAL supernatant with a spectrophotometric assay (Bioxytech MDA-586; Oxis International, Foster City, Calif) at 586 nm absorbance with a detection limit A586 of 0.0088. This assay is based on the reaction of N-methyl-2-phenylindole with malondialdehyde at 25°C. 8-Isoprostane concentrations were obtained by ELISA (Cayman Chemical, Ann Arbor, Mich) with absorbance at 420 nm and detection limit of approximately 2.7 pg/mL. H2O2 concentrations were determined spectrophotometrically from a microplate assay kit (Amplex Red; Molecular Probes, Eugene, Ore) with an excitation of 560 nm, fluorescence emission detection at 590 nm, and sensitivity of 50 nmol/L.
Statistical analysis
Data were analyzed with SPSS software (Version 15; SPSS Inc, Chicago, Ill). Children with severe asthma were stratified according to the baseline airway obstruction, defined as an FEV1 <80% predicted or an FEV1:forced vital capacity (FVC) <0.80.18 GSH, GSSG, malondialdehyde, 8-isoprostane, and H2O2 concentrations were expressed per milliliter ELF according to the urea dilution21 and were logarithmically transformed before statistical analysis given their nonnormal distribution. GST, GR, and GPx enzymatic activities were also expressed per mL of ELF and logarithmically transformed before analysis. Differences between groups were assessed by ANOVA with Tukey post hoc testing with significance defined as α ≤0.05. Bivariate Pearson correlations were used to examine associations between variables. To test the relationship between GSH oxidation and asthma severity, univariate logistic and linear regression analyses were performed by using the percentage of GSSG as the independent variable and measures of asthma severity, including hospitalization within the previous year, ICS dose, daily prednisone use, FENO, FEV1, and FEV1 bronchodilator reversibility as dependent variables. To evaluate factors that might affect GSH oxidation in the ELF, multivariate backward elimination linear regression was performed by using percent GSSG as the dependent variable and age, sex, ethnicity, ICS dose, prednisone dose, and the percentage of airway eosinophils and neutrophils as predictors. Multicollinearity between predictors was assessed with tolerance statistics. Entry and removal probabilities were set at .05 and .10, respectively.
RESULTS
Seventy-four children with severe asthma, 13 pediatric controls, and 35 healthy adult controls were recruited for this study. After recruitment, 7 of the 13 pediatric controls were diagnosed with BAL colonization (n = 3) or chronic aspiration (n = 4) and were excluded from data analysis. Nine subjects with severe asthma also had BAL colonization with Streptococcus pneumoniae (n = 4), Haemophilus influenzae (n = 2), or Moraxella catarrhalis (n = 3) and were similarly excluded. Features of the excluded children appear in this article’s Tables E2 to E4 in the Online Repository at www.jacionline.org. Although the clinical features of excluded children did not differ from those included in the final analysis, the excluded children did have greater evidence of ELF oxidant stress. Compared with pediatric controls, the children without asthma excluded from data analysis had lower GSH, lower total GSH + GSSG concentrations, a higher percentage of GSSG, and a more oxidized redox potential (Eh) in the ELF (Table E3). Likewise, the subjects with severe asthma excluded from the final analysis had lower GSH, higher GSSG, and a more oxidized redox potential compared with the subjects with severe asthma included in the final data analysis (Table E4).
TABLE E2.
Characteristics of pediatric subjects excluded from data analysis.
| Subject | Asthma status | Sex | Age (y) | ICS | FEV1 (%) | Reason for exclusion |
|---|---|---|---|---|---|---|
| 1 | No asthma | Female | 14 | No | 77 | Chronic aspiration* |
| 2 | No asthma | Male | 9 | Yes | 82 | H influenzae |
| 3 | No asthma | Female | 13 | Yes | 105 | S pneumoniae |
| 4 | No asthma | Female | 15 | Yes | 95 | Chronic aspiration |
| 5 | No asthma | Female | 16 | Yes | 94 | Chronic aspiration |
| 6 | No asthma | Male | 15 | Yes | 78 | Chronic aspiration |
| 7 | No asthma | Female | 14 | Yes | 92 | S pneumoniae |
| 8 | Asthma | Male | 8 | Yes | 104 | H influenzae |
| 9 | Asthma | Male | 9 | Yes | 90 | M catarrhalis |
| 10 | Asthma | Male | 16 | Yes | 106 | S pneumoniae |
| 11 | Asthma | Male | 10 | Yes | 64 | S pneumoniae |
| 12 | Asthma | Male | 19 | Yes | 100 | H influenzae |
| 13 | Asthma | Female | 8 | Yes | 68 | M catarrhalis |
| 14 | Asthma | Female | 7 | Yes | 92 | S pneumoniae |
| 15 | Asthma | Female | 11 | Yes | 82 | S pneumoniae |
| 16 | Asthma | Male | 9 | Yes | 85 | M catarrhalis |
Chronic aspiration was diagnosed according to clinical symptoms and the presence of lipid-laden macrophages and a positive barium swallow or esophageal pH monitoring study.
TABLE E4.
Biomarkers of oxidant stress in children with asthma excluded from data analysis
| Severe asthma AO− included in data analysis (n = 30) | Severe asthma AO+ included in data analysis (n = 25) | Severe asthma excluded from data analysis (n = 9) | |
|---|---|---|---|
| GSH (μmol/L) | 94 ± 107 | 57 ± 95 | 34 ± 51* |
| GSSG (μmol/L) | 39 ± 61 | 72 ± 76 | 16 ± 15† |
| GSH + GSSG (μmol/L) | 134 ± 150 | 129 ± 134 | 50 ± 48 |
| % GSSG | 36 ± 27 | 62 ± 24 | 55 ± 38* |
| Eh GSH:GSSG (mV) | −119 ± 44 | −96 ± 30 | −82 ± 45* |
| H2O2 (μmol/L) | 12 ± 13 | 15 ± 18 | 31 ± 46 |
| MDA (μmol/L) | 38 ± 12 | 51 ± 39 | 95 ± 93 |
| 8-Isoprostanes (μmol/L) | 405 ± 274 | 1073 ± 1929 | 827 ± 1228 |
Data are expressed per mL of epithelial lining fluid (ELF) and represent the mean ± SD. Differences between groups were analyzed with non-parametric Mann-Whitney U statistics. Post-hoc testing was performed on significant variables with ANOVA and Tukey tests.
P < .05 vs severe asthma AO−.
P < .05 vs severe asthma AO+.
TABLE E3.
Biomarkers of oxidant stress in children without asthma excluded from data analysis
| Pediatric controls included in data analysis (n = 6) | Pediatric subjects excluded from data analysis (n = 7) | |
|---|---|---|
| GSH (μmol/L)* | 231 ± 218 | 23 ± 16* |
| GSSG (μmol/L) | 29 ± 30 | 48 ± 47 |
| GSH + GSSG (μmol/L)* | 260 ± 219 | 71 ± 55* |
| % GSSG | 16 ± 14 | 61 ± 23* |
| Eh GSH:GSSG (mV)b | −147 ± 30 | −82 ± 21* |
| H2O2 (μmol/L)* | 6 ± 7 | 11 ± 6* |
| MDA (μmol/L)* | 20 ± 10 | 41 ± 12* |
| 8-Isoprostanes (μmol/L) | 205 ± 100 | 273 ± 17 |
Data are expressed per mL of ELF and represent the means ± SDs. Differences between groups were analyzed with nonparametric Mann-Whitney U statistics.
P < .05 vs pediatric controls included in data analysis.
The resulting pediatric control group contained 6 children. Postbronchoscopy diagnoses in this group included psychogenic (habit) cough (n =4) and vocal cord dysfunction (n =3). None of the children serving as controls were receiving ICS at the time of bronchoscopy. Given the symptomatic nature of these children, 35 healthy adults were also recruited for comparison. Adults in this control group were nonsmokers free of respiratory symptoms and medication use; however, they were significantly older (Table I).
TABLE I.
Baseline characteristics of controls and subjects with severe asthma with (AO+) and without (AO−) airway obstruction; data represent the means ± SDs or the frequency (%)
| Adult control (n = 35) | Pediatric control (n = 6) | Severe asthma AO− (n = 35) | Severe asthma AO+(n = 30) | |
|---|---|---|---|---|
| Age (y) | 39 ± 11 | 10 ± 6† | 8 ± 5† | 10 ± 5† |
| Male sex | 14 (40%) | 4 (67%) | 21 (60%) | 14 (47%) |
| Ethnicity | ||||
| White | 16 (46%) | 5 (83%) | 26 (74%) | 7 (23%)†‡§ |
| African American | 18 (51%) | 1 (17%) | 9 (26%) | 22 (73%)†‡§ |
| Other | 1 (3%) | 0 | 0 | 1 (3%) |
| Daily ICS dose (fluticasone equivalents) | 0 | 0 | 596 ± 228†‡ | 905 ± 280†‡ |
| Daily asthma medications | ||||
| Budesonide | 0 | 0 | 12 (34%)†‡ | 7 (23%)†‡ |
| Fluticasone | 0 | 0 | 2 (6%) | 2 (7%) |
| Beclomethasone | 0 | 0 | 1 (3%) | 0 |
| Fluticasone/salmeterol | 0 | 0 | 20 (57%)†‡ | 21 (70%)†‡ |
| Montelukast | 0 | 0 | 23 (66%)†‡ | 30 (100%)†‡ |
| Prednisone | 0 | 0 | 0 | 16 (53%)†‡§ |
| Pulmonary function | ||||
| FVC (% predicted) | 96 ± 16 | 92 ± 12 | 100 ± 16 | 83 ± 21†§ |
| FEV1 (% predicted) | 102 ± 16 | 93 ± 9 | 96 ± 17 | 68 ± 20†‡§ |
| FEV1: FVC | 0.87 ± 0.06 | 0.88 ± 0.05 | 0.84 ± 0.11 | 0.72 ± 0.14†‡§ |
| FEV1:FVC (% predicted) | 105 ± 8 | 98 ± 4 | 98 ± 9 | 82 ± 14†‡§ |
| FEF25–75 (% predicted) | 125 ± 33 | 93 ± 11 | 93 ± 28 | 48 ± 26†‡§ |
| FEV1 bronchodilator reversibility (% change)* | 3 ± 6 | 1 ± 2 | 7 ± 4 | 14 ± 9†‡ |
| FENO (offline, ppb) | 6 ± 4 | 6 ± 1 | 14 ± 12†‡ | 14 ± 11†‡ |
| Asthma-related hospitalization (previous year) | 0 | 0 | 8 (23%)†‡ | 30 (100%)†‡§ |
FEF25–75, Forced expiratory flow.
Calculated by [(FEV1 postbronchodilator − FEV1 prebronchodilator)/predicted FEV1]*100.
P < .05 vs adult control.
P < .05 vs pediatric control.
P < .05 vs severe asthma AO−.
Features of the groups appear in Table I. Children with severe asthma were stratified according to the baseline airway obstruction, defined as an FEV1 <80% predicted or an FEV1 to FVC ratio <0.80.18 Subjects with severe asthma with airway obstruction (AO+) had increased bronchodilator reversibility with albuterol despite higher ICS doses and a greater prevalence of hospitalization within the previous year (Table I).
Flexible bronchoscopy with BAL was well tolerated in all participants. Bronchospasm greater than 15 seconds was observed in 3 children with severe asthma and responded immediately to bronchodilators and positive airway pressure. No subject required overnight hospitalization or prolonged observation.
The composition of the BAL is presented in Table II. BAL samples from adult controls were characterized by larger return volumes and higher cell counts. Adult and pediatric controls also had significantly less protein and fewer neutrophils and eosinophils than subjects with severe asthma with (AO+) and without (AO−) airway obstruction (P <.05; Table II).
TABLE II.
Composition of the BAL in controls and subjects with severe asthma with (AO+) and without (AO−) airway obstruction; data represent the means ± SDs
| Adult control (n = 35) | Pediatric control (n = 6) | Severe asthma AO− (n = 35) | Severe asthma AO+ (n = 30) | |
|---|---|---|---|---|
| BAL recovery (% of volume instilled) | 44 ± 13 | 38 ± 16 | 30 ± 15‡ | 31 ± 19‡ |
| Total leukocyte cell count (×106) | 9.30 ± 5.52 | 4.25 ± 3.55‡ | 3.26 ± 2.75‡ | 3.38 ± 3.06‡ |
| Cellular differential (%) | ||||
| Macrophages/monocytes | 90.6 ± 3.6 | 91.8 ± 5.5 | 89.6 ± 6.6 | 86.9 ± 7.0 |
| Neutrophils | 2.4 ± 1.5 | 2.4 ± 2.1 | 5.0 ± 4.9‡§ | 4.9 ± 4.3‡§ |
| Eosinophils | 0.6 ± 0.6 | 0.2 ± 0.5 | 1.2 ± 1.6‡§ | 1.9 ± 3.7‡§ |
| Lymphocytes | 5.6 ± 2.8 | 5.4 ± 6.3 | 5.9 ± 4.6 | 6.4 ± 6.0 |
| Protein (μg/dL) | 120.8 ± 70.3 | 173.1 ± 97.9 | 229.3 ± 85.6‡§ | 224.3 ± 175.7‡§ |
| Urea (mg/dL) | 0.41 ± 0.50 | 0.35 ± 0.48 | 0.49 ± 0.41 | 0.37 ± 0.39 |
| Plasma urea (mg/dL) | 10.5 ± 3.7 | 11.7 ± 3.7 | 13.6 ± 3.2 | 14.2 ± 2.7 |
| Urea dilution factor* | 74 ± 47 | 75 ± 57 | 72 ± 94 | 73 ± 40 |
| ELF volume recovered† (mL) | 0.64 ± 0.31 | 0.20 ± 0.08 | 0.31 ± 0.40 | 0.41 ± 0.71 |
| pH (log [H+]) | 6.82 ± 0.39 | 6.90 ± 0.27 | 7.01 ± 0.44 | 6.92 ± 0.60 |
Calculated from (ureaplasma)/(ureaBAL).
Calculated from mL of BAL return/urea dilution factor.
P < .05 vs adult control.
P < .05 vs pediatric control.
GSH, GSSG, and GSH/GSSG redox potential in ELF
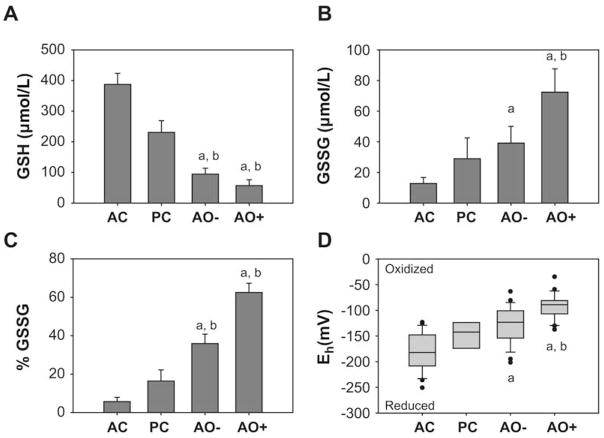
Compared with adult and pediatric control subjects, children with severe asthma had significantly lower total GSH + GSSG concentrations in the ELF (adult control: 436 ± 249 μmol/L; pediatric control: 260 ± 230 μmol/L; severe asthma [AO−]: 134 ±150 μmol/L; severe asthma [AO+]: 129 ± 134 μmol/L; P < .001 for severe asthma AO± vs adult controls, P=.07 for severe asthma AO± vs pediatric controls). In subjects with severe asthma with airway obstruction, the majority (~60%) of the total pool was in the oxidized (GSSG) form (Fig 2). Using the Nernst equation, the oxidative redox potential (Eh) for the GSH/GSSG pair was significantly more reduced in the adult controls compared with the other groups, with the greatest oxidation apparent in subjects with severe asthma with airway obstruction (Fig 2).
FIG 2.
Airway glutathione characteristics. A, GSH. B, GSSG. C, % GSSG. D, The redox potential (Eh) of the GSH/GSSG pair in the ELF. Data represent the means ± SEMs per mL of ELF with AC = adult control (n = 31), PC = pediatric control (n = 6), AO− = severe asthma without airway obstruction (n = 31), and AO+ = severe asthma with airway obstruction (n = 25). *P < .01 vs adult controls; †P < .05 vs pediatric controls.
Airway GST, GR, and GPx activities
To determine whether altered GSH-dependent enzymatic activities might account for increased GSH oxidation in severe asthma, GST, GR, and GPx were quantified in the ELF. GST activities (expressed per milliliter ELF) were similar between groups (adult control: 1.25 ± 0.94; pediatric control: 1.00 ± 0.99; severe asthma [AO−]: 1.02 ± 1.14; severe asthma [AO+]: 1.19 ± 1.65 nmol/min/mL; P=.305). GR activities (adult control: 0.87 ± 1.00; pediatric control: 0.83 ± 1.18; severe asthma [AO−]: 0.99 ± 0.68; severe asthma [AO+]: 0.89 ± 0.82 nmol/min/mL; P = .406) and GPx activities (adult control: 1.40 ± 1.25; pediatric control: 2.04 ± 1.33; severe asthma (AO−): 1.83 ± 1.10; severe asthma (AO+): 2.06 ± 1.23 nmol/min/mL; P=.719) were also not different between groups.
Associations between GSH and other oxidative biomarkers
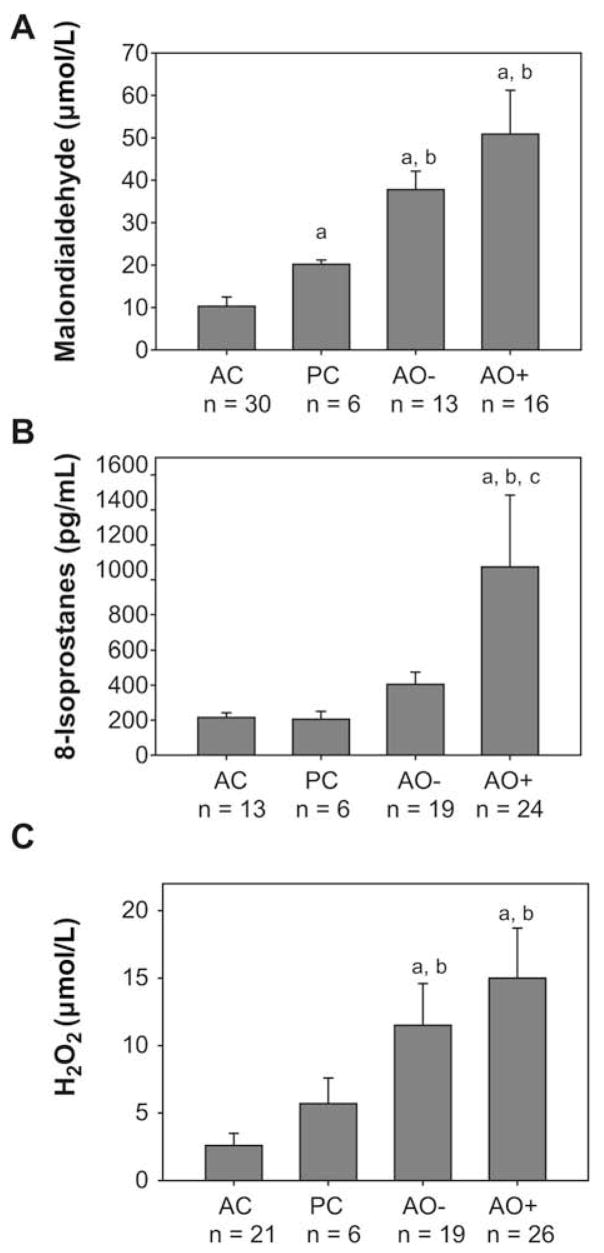
Malondialdehyde, 8-isoprostanes, and H2O2 were significantly elevated in the ELF of subjects with severe asthma compared with adult and pediatric controls (Fig 3). In subjects with severe asthma with and without airway obstruction, malondialdehyde, 8-isoprostanes, and H2O2 were positively correlated with the percentage of airway GSSG and the oxidative redox potential (Eh) of the GSH:GSSG couple (P ≤.05 for each association; see this article’s Table E5 in the Online Repository at www.jacionline.org).
FIG 3.
Airway biomarkers of oxidant stress. A, Malondialdehyde. B, 8-isoprostane. C, H2O2 concentrations in the ELF. Data represent the means ± SEMs per mL of ELF with AC = adult controls, PC = pediatric controls, AO− = severe asthma without airway obstruction, and AO+ = severe asthma with airway obstruction. *P < .01 vs adult controls; †P < .05 vs pediatric controls; ‡P < .05 vs subjects with severe asthma without airway obstruction.
TABLE E5.
| GSH:GSSG Eh | % GSSG | H2O2 | 8-Isoprostanes | Malondialdehyde | |
|---|---|---|---|---|---|
| GSH:GSSG Eh | 1 | 0.854 | 0.299 | 0.315 | 0.509 |
| P < .001 | P = .049 | P = .027 | P = .031 | ||
| % GSSG | 1 | 0.351 | 0.485 | 0.343 | |
| P < .020 | P = .005 | P = .024 | |||
| H2O2 | 1 | 0.338 | 0.343 | ||
| P = .050 | P = .049 | ||||
| 8-Isoprostanes | 1 | 0.698 | |||
| P < .001 | |||||
| MDA | 1 |
Data are from the combined sample of subjects with severe asthma with and without airway obstruction. Adult and pediatric controls were excluded from this analysis.
Coefficients for H2O2, 8-isoprostanes, and malondialdehyde were obtained after logarithmic transformation.
Clinical predictors of GSH oxidation in subjects with severe asthma
Within the group of subjects with severe asthma, total GSH + GSSG concentrations were not associated with baseline FEV1 or the presence of airway eosinophils or neutrophils. Total GSH + GSSG concentrations, the percentage of GSSG, and the redox potential (Eh) of the GSH:GSSG couple were significantly associated with baseline FENO (GSH + GSSG: r = −0.60, P=.003; % GSSG: r = 0.480, P=.032; Eh GSH:GSSG: r = 0.43, P=.047). To test the association between altered GSH homeostasis and clinical markers of asthma severity, univariate logistic and linear regression analyses were performed by using the percentage of GSSG as the predictor and hospitalization within the previous year, prednisone use, ICS dose, FENO, baseline FEV1, and FEV1 bronchodilator reversibility as dependent variables. Using this approach, hospitalization within the previous year (−2 log L = 61.49; P = .001) and baseline FENO (R2 = 0.230; P = .032) were significantly predicted by the percentage of GSSG (see this article’s Tables E6 and E7 in the Online Repository at www.jacionline.org).
TABLE E6.
Results of univariate logistic linear regression of the percentage of GSSG in the ELF on clinical features of severe asthma*
| Dependent variable modeled | Regression coefficient (β) | SE | e^β | P value | 95% CI |
|---|---|---|---|---|---|
| Hospitalization within the previous year† | |||||
| % GSSG | 0.043 | 0.012 | 1.044 | .001 | (1.019–1.070) |
| Constant | −2.267 | 0.706 | 0.104 | .001 | |
| Daily prednisone use† | |||||
| % GSSG | 0.007 | 0.015 | 1.007 | .615 | (0.979–1.037) |
| Constant | −1.215 | 0.935 | 0.297 | .194 | |
Data are from the combined sample of subjects with severe asthma with and without airway obstruction, excluding adult and pediatric controls.
Modeled as yes = 1, no = 0.
TABLE E7.
Results of univariate linear regression of the percentage of GSSG in the ELF on clinical features of severe asthma*
| Dependent variable modeled | Regression coefficient (β) | SE | t | P value | 95% CI |
|---|---|---|---|---|---|
| FENO† | |||||
| % GSSG | 0.012 | 0.005 | 2.320 | .032 | (0.001, 0.022) |
| Constant | 1.810 | 0.286 | 6.323 | <.001 | |
| ICS dose (μg/d) | |||||
| % GSSG | 1.795 | 2.484 | 0.723 | .477 | (−3.332, 6,922) |
| Constant | 671.034 | 151.425 | 4.431 | <.001 | |
| Baseline FEV1 | |||||
| % GSSG | −0.148 | 0.092 | −1.596 | .116 | (−0.333, 0.038) |
| Constant | 91.548 | 5.147 | 17.787 | <.001 | |
| FEV1 reversibility after bronchodilator (%) | |||||
| % GSSG | 0.010 | 0.096 | 0.106 | .917 | (−0.194, 0.214) |
| Constant | 11.000 | 5.686 | 1.934 | .072 | |
Data are from the combined sample of subjects with severe asthma with and without airway obstruction, excluding adult and pediatric controls.
Data were logarithmically transformed for analysis.
To evaluate factors that might affect airway GSSG formation, stepwise linear regression analysis was performed on children with severe asthma with the percentage of GSSG as the dependent variable and age, sex, ethnicity, ICS dose, prednisone dose, and the percentage of airway eosinophils and neutrophils as predictors. With the multivariate model, only sex (P=.038) and airway eosinophils (P=.031) were predictive of airway GSH oxidation (final model R2 = 0.322, P=.045; see this article’s Table E8 in the Online Repository at www.jacionline.org).
TABLE E8.
Results of backward linear regression of selected clinical features on the percentage of glutathione disulfide (GSSG) in the ELF*
| Model | Regression coefficient | SE | t | P value | 95% CI |
|---|---|---|---|---|---|
| Included variables† | |||||
| Constant | 76.604 | 10.850 | 7.060 | <.001 | (53.603, 99.604) |
| Sex | −27.726 | 12.254 | −2.263 | .038 | (−53.703, −1.749) |
| Airway eosinophils (%)3 | −16.469 | 6.957 | −2.367 | .031 | (−31.218, −1.720) |
| Excluded variables | |||||
| Ethnicity | 0.114 | 0.491 | .631 | ||
| Prednisone dose (mg) | 0.064 | 0.234 | .818 | ||
| Age (y) | −0.017 | −0.061 | .952 | ||
| Airway neutrophils (%)‡ | −0.169 | −0.717 | .484 | ||
| ICS dose | 0.355 | 1.515 | .151 | ||
Data are from the combined sample of subjects with severe asthma with and without airway obstruction, excluding adult and pediatric controls.
Sum of squares (model/total) =4170.2/12,966.1; R2 = 0.322; P = .045.
Data were logarithmically transformed for analysis.
DISCUSSION
This is the first study to provide a detailed assessment of airway GSH homeostasis in children with severe asthma. Total ELF GSH + GSSG concentrations were significantly lower in symptomatic children than in adult controls and were accompanied by greater GSH oxidation as measured by GSSG. GSSG was increased nearly 2-fold in subjects with severe asthma with airway obstruction and was further associated with increased malondialdehyde, 8-isoprostane, and H2O2 concentrations. Enzymatic activities of GST, GR, and GPx were similar between groups and were not associated with ELF GSH levels. These results suggest that GSH homeostasis is altered in children with severe asthma as a function of increased ELF oxidation, which favors the conversion of GSH to GSSG and signals compromised antioxidant function.
GSH was first characterized in the ELF of healthy, nonsmoking adults nearly 20 years ago. In the classic study by Cantin et al,6 total GSH + GSSG concentrations in the alveolar ELF of healthy adults were approximately 430 μmol/L, with less than 5% of the total pool in the oxidized (GSSG) form. Although smoking initially increases GSH concentrations,6 long-term smoking has been associated with increased GSSG formation.24 Similar increases in GSSG with resulting declines in GSH have been noted in adults with chronic alcoholism,25,26 human immunodeficiency virus,27 pulmonary fibrosis,28,29 and acute respiratory distress syndrome.30,31 These studies suggest that ELF GSH homeostasis is disturbed in a variety of pulmonary disorders and may account for some of the respiratory morbidity associated with pulmonary disease.
Few studies have examined GSH homeostasis in asthma, and fewer still have measured GSH in the ELF. In an earlier study, Smith et al32 noted that total GSH + GSSG concentrations in the ELF were higher in adults with mild asthma than controls. However, GSH concentrations were inversely correlated with bronchial hyperresponsiveness, suggesting a relationship between antioxidant defense and pulmonary function.32 Although similar studies of steroid-naive subjects with mild asthma have shown no differences in baseline GSH concentrations as compared with controls,13,33 baseline GSSG is elevated13 and increases further with segmental antigen challenge.33 These data suggest that airway oxidant stress is a defining feature of asthma that may be present in spite of normal clinical features.
The redox potential is a measure of the capacity to reduce reactive oxygen species where the more negative the number, the greater the reduction potential. We observed redox potentials in symptomatic children that were significantly lower than those of healthy adults. Children with severe asthma had ELF redox potentials that, on average, were approximately 75 mV more oxidized than adult controls. Other studies of plasma have suggested that the GSH redox potential increases in an age-dependent manner by 0.7 mV per year.34,35 If a similar trend is present in ELF, then the oxidant stress observed in our sample of children with severe asthma is more similar to that of an older adult. Furthermore, a 75 mV shift in the GSH redox potential is more than sufficient to cause a 17-fold change in the ratios of reduced to oxidized forms of proteins such as thioredoxins.23
This study has a number of limitations. Because bronchoscopy cannot be performed on asymptomatic children solely for research purposes, our pediatric control group was limited to symptomatic children with psychogenic cough or vocal cord dysfunction undergoing bronchoscopy for diagnostic purposes. It is therefore possible that the GSH concentrations we observed are not representative of those from healthy asymptomatic children. Similarly, because we were limited to a convenience sample of children with severe asthma, it is unclear whether ELF GSH concentrations differ in subjects with severe asthma who are well controlled on high doses of ICS and do not undergo bronchoscopy. The fact that our adult control group was significantly older also raises the question whether age is a determinant of antioxidant defense across the lifespan. Additional studies are needed before these questions can be adequately addressed.
It is also possible the differences in GSH homeostasis that we observed could be attributed to the confounding effects of asthma treatment or other clinical features. Although ICS dose was highly associated with GSH and oxidative biomarkers, ICS dose failed to predict GSH oxidation in multivariate analysis. The fact that ICS dose is a surrogate of severe asthma and the major criterion for diagnosis16 likely accounts for this relationship. Alternatively, these data may indicate that corticosteroids alone are insufficient to counteract airway oxidant stress in this population. The elevated malondialdehyde, 8-isoprostane, and H2O2 concentrations observed in subjects with severe asthma with airway obstruction also question the steroid sensitivity of this population. Additional studies are needed to examine thoroughly the relationship between GSH homeostasis, corticosteroid treatment, and clinical indicators of severe asthma in children.
For this study, we asked pediatric participants and their caregivers to list all current medications. We then questioned children and their parents about the specific dosages and frequency of administration. Although this method of medication assessment likely yielded accurate information regarding current asthma treatment, it may also have underestimated the use of over-the-counter medications such as acetaminophen. In a recent report from the International Study of Asthma and Allergies in Childhood, acetaminophen (paracetamol) use was associated with a dose-dependent increased risk of asthma symptoms in children 6 to 7 years of age.36 This finding warrants further study. Acetaminophen is metabolized by cytochrome P450 to form the toxic species N-acetyl-p-benzoquinone imine, which is detoxified by GSH. With repeated acetaminophen use or excessive dosages, liver GSH stores are depleted, resulting in liver damage.37 Future studies should consider the effects of acetaminophen use on altered airway GSH homeostasis, particularly in patients with severe asthma.
In summary, we have demonstrated significant alterations of GSH homeostasis in children with severe refractory asthma characterized by decreased GSH, increased GSSG, and greater oxidation as measured by the GSH redox potential, malondialdehyde, 8-isoprostane, and H2O2 concentrations in the ELF. Given the antioxidant properties of GSH, further studies are needed to understand the impact of altered GSH homeostasis on asthma severity. Although previous studies have provided some support for the role of dietary antioxidants in allergic airway disease, the results of antioxidant supplementation studies have been largely disappointing.38 However, these previous studies focused on clinical outcomes such as pulmonary function and did not take into account the effects of antioxidant therapy on extracellular antioxidant balance or intracellular signaling.38 Given our findings of altered GSH homeostasis in severe asthma, additional studies are warranted to understand better the effects of airway GSH on respiratory morbidity. Ultimately, these data argue for interventions to increase ELF GSH concentrations in this population of children who are otherwise very difficult to treat.
Acknowledgments
We acknowledge Brian Fitzpatrick for his assistance with database formation and data analysis, Leandrea Burwell for her assistance with glutathione measurement, and Eric Hunter for his assistance with subject characterization.
Supported by the National Institutes of Health (NIH)/National Institute for Nursing Research (NINR) KO1 NR010584, NIH/National Center for Research Resources (NCRR) K12 RR017643, NIH/National Heart, Lung, and Blood Institute (NHLBI) Severe Asthma Research Program RO1 HL69170, and the American Nurses Foundation.
Abbreviations used
- AO
Airway obstruction
- BAL
Bronchoalveolar lavage
- CDNB
1-Chloro-2,4-dinitrobenzene
- ELF
Epithelial lining fluid
- FENO
Fraction of exhaled nitric oxide
- FVC
Forced vital capacity
- GPx
Glutathione peroxidase
- GR
Glutathione reductase
- GSH
Glutathione (reduced form)
- GSSG
Oxidized glutathione
- GST
Glutathione S-transferase
- ICS
Inhaled corticosteroid
Footnotes
*The Severe Asthma Research Program is a multicenter asthma research group funded by the National Heart, Lung, and Blood Institute and consisting of the following contributors (Steering Committee members†): Brigham & Women’s Hospital—Elliot Israel, † Bruce D. Levy, Gautham Marigowda; Cleveland Clinic Foundation—Serpil C. Erzurum, † Raed A. Dweik, Suzy A. A. Comhair, Marcelle Baaklini, Daniel Laskowski, Jacqueline Sharp; Emory University—W. Gerald Teague, † Anne M. Fitzpatrick, Eric Hunter; Imperial College School of Medicine—Kian Fan Chung, † Mark Hew, Alfonso Torrego, Sally Meah, Mun Lim; National Jewish Medical and Research Center—Sally E. Wenzel, † Diane Rhodes; University of Pittsburgh—William J. Calhoun, † Renee Folger, Bill T. Ameredes, Melissa P. Clark, Rebecca Z. Wade; University of Virginia—Benjamin Gaston, † Peter Urban; University of Wisconsin—William W. Busse, † Nizar Jarjour, Erin Billmeyer, Cheri Swenson, Gina Crisafi; Wake Forest University—Eugene R. Bleecker, † Deborah Meyers, Wendy Moore, Stephen Peters, Annette Hastie, Gregory Hawkins, Jeffrey Krings, Regina Smith; Washington University in St Louis—Mario Castro, † Leonard Bacharier, Iftikhar Hussain, Jaime Tarsi; Data Coordinating Center—James R. Murphy, † Douglas Curran-Everett; National Heart, Lung, and Blood Institute—Patricia Noel. †
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
Clinical implications: Children with severe asthma have significant airway oxidant stress despite treatment with inhaled and oral corticosteroids. Additional therapies to decrease oxidant stress may be warranted in children with severe asthma.
References
- 1.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school-age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–25. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164:1376–81. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 4.Comhair SAA, Ricci KS, Arroliga M, Lara AR, Dweik RA, Song W, et al. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am J Respir Crit Care Med. 2005;172:306–13. doi: 10.1164/rccm.200502-180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comhair SAA, Xu W, Arroliga M, Khatri SB, Thunnissen FBJM, Almasan A, et al. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am J Pathol. 2005;166:663–74. doi: 10.1016/S0002-9440(10)62288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63:152–7. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 7.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16:534–54. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- 8.Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1 alpha and NF-kappa B redox sensitivity: evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem. 2000;275:21130–9. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- 9.Rahman I, Mulier B, Gilmour PS, Watchorn T, Donaldson K, Jeffery PK, MacNee W. Oxidant-mediated lung epithelial cell tolerance: the role of intracellular glutathione and nuclear factor-kappaB. Biochem Pharmacol. 2001;62:787–94. doi: 10.1016/s0006-2952(01)00702-x. [DOI] [PubMed] [Google Scholar]
- 10.Koike Y, Hisada T, Utsugi M, Ishizuka T, Shimizu Y, Ono A, et al. Glutathione redox regulates airway hyperresponsiveness and airway inflammation in mice. Am J Respir Cell Mol Biol. 2007;37:322–9. doi: 10.1165/rcmb.2006-0423OC. [DOI] [PubMed] [Google Scholar]
- 11.Mak JCW, Leung HCM, Ho SP, Law BKW, Lam WK, Tsang KWT, et al. Systemic oxidative and antioxidative status in Chinese patients with asthma. J Allergy Clin Immunol. 2004;114:260–4. doi: 10.1016/j.jaci.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Dauletbaev N, Rickmann J, Viel K, Buhl R, Wagner T-O-F, Bargon J. Glutathione in induced sputum of healthy individuals and patients with asthma. Thorax. 2001;56:13–8. doi: 10.1136/thorax.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–3. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 14.Payne D, McKenzie SA, Stacey S, Misra D, Haxby E, Bush A. Safetyand ethics of bronchoscopy and endobronchial biopsy in difficult asthma. Arch Dis Child. 2001;84:423–6. doi: 10.1136/adc.84.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Heart, Lung and Blood Institute, National Asthma Education and Prevention Program. The NAEPP Expert Panel Report: guidelines for the diagnosis and management of asthma—update on selected topics 2002. Pub no. 02-5075. Bethesda (MD): National Institutes of Health; 2002. [Google Scholar]
- 16.American Thoracic Society. Proceedings of the American Thoracic Society Workshop on Refractory Asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–51. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 18.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society and the European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 20.Jones DP, Carlson JL, Samiec PS, Sternberg P, Mody VC, Reed RL, et al. Glutathione measurement in human plasma: evaluation of sample collection, storage, and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;27:175–84. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 21.Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston O, Martin PG, et al. Estimation of volume of epithelial lining fluid recovered by lavage using urea as a marker of dilution. J Appl Physiol. 1986;60:532–8. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 22.Reed DJ, Babson JR, Beatty PW, Brodie EA, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Ann Biochem. 1980;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 23.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 24.Nagai K, Betsuyaku T, Kondo T, Nashuhara Y, Nishimura M. Long term smoking with age builds up excessive oxidative stress in bronchoalveolar lavage fluid. Thorax. 2006;61:496–502. doi: 10.1136/thx.2005.049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LAS. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161:414–9. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 26.Yeh MY, Burnham EL, Moss M, Brown LAS. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med. 2007;176:1–7. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacht ER, Diaz P, Clanton T, Hart J, Gadek JE. Alveolar fluid glutathione decreases in asymptomatic HIV-seropositive subjects over time. Chest. 1997;112:785–8. doi: 10.1378/chest.112.3.785. [DOI] [PubMed] [Google Scholar]
- 28.Behr J, Degenkolb B, Maier K, Braun B, Beinert T, Krombach F, et al. Increased oxidation of extracellular glutathione by bronchoalveolar inflammatory cells in diffuse fibrosing alveolitis. Eur Respir J. 1995;8:1286–92. doi: 10.1183/09031936.95.08081286. [DOI] [PubMed] [Google Scholar]
- 29.Beeh KM, Beier J, Haas IC, Kornmann O, Micke P, Buhl R. Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:1119–23. doi: 10.1183/09031936.02.00262402. [DOI] [PubMed] [Google Scholar]
- 30.Bunnell E, Pacht ER. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;148:1174–8. doi: 10.1164/ajrccm/148.5.1174. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt R, Luboeinski T, Markart P, Ruppert C, Daum Grimminger F, et al. Alveolar antioxidant status in patients with acute respiratory distress syndrome. Eur Respir J. 2004;24:994–9. doi: 10.1183/09031936.04.00120703. [DOI] [PubMed] [Google Scholar]
- 32.Smith LJ, Houston M, Anderson J. Increased levels of glutathione in bronchoalveolar lavage fluid from patients with asthma. Am Rev Respir Dis. 1993;147:1461–4. doi: 10.1164/ajrccm/147.6_Pt_1.1461. [DOI] [PubMed] [Google Scholar]
- 33.Comhair SAA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet. 2000;355:624. doi: 10.1016/S0140-6736(99)04736-4. [DOI] [PubMed] [Google Scholar]
- 34.Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Reed RL, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 35.Jones DP, Mody VC, Carlson JL, Lynn MJ, Sternberg P. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 36.Beasley R, Clayton T, Crane J, von Mutius Lai CKW, Montefort S, et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from phase three of the ISAAC programme. Lancet. 2008;372:1039–48. doi: 10.1016/S0140-6736(08)61445-2. [DOI] [PubMed] [Google Scholar]
- 37.Potter WZ, Thorgeirsson SS, Jollow DJ, Mitchell JR. Acetaminophen-induced hepatic necrosis, V: correlation of hepatic necrosis, covalent binding and glutathione depletion in hamsters. Pharmacology. 1974;12:129–43. doi: 10.1159/000136531. [DOI] [PubMed] [Google Scholar]
- 38.Devereux G. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115:1109–17. doi: 10.1016/j.jaci.2004.12.1139. [DOI] [PubMed] [Google Scholar]