Abstract
Both serous intraepithelial carcinoma and endometrial glandular dysplasia are associated with uterine serous carcinoma. Recently a candidate serous cancer precursor containing p53 mutations (p53 signature) was described in the fallopian tube. We analyzed normal and neoplastic endometrium for a similar entity. Ten endometrial polyps involved by intraepithelial and/or invasive carcinoma and 137 benign polyps were studied. All were stained for p53 and MIB-1. A subset of p53 signatures and carcinomas were analyzed for γ-H2AX and p53 mutations. p53 signatures were identified in 7 of 10 cases intraepithelial carcinoma and were multicentric in 2. In one case, the signature was in continuity with intraepithelial carcinoma. Six of 137 benign polyps (4%) contained p53 signatures. The MIB-1 fraction in most signatures was less than 5%, and ranged from 50-90% in carcinomas. DNA damage (γ-H2AX) was demonstrated in both p53 signatures and adjacent carcinomas but not in benign polyps. Shared identical p53 mutations were found in paired signatures and carcinomas in two of three cases analyzed, including one case with multiple signatures. In one, a co-existent invasive serous cancer was not found to contain a p53 mutation. In a third, a p53 signature and an invasive cancer harbored two different p53 mutations. This is the first description of p53 signatures adjacent to carcinoma, suggesting a role for this entity in the genesis of serous malignancy. The significance of p53 signatures in benign conditions (polyps) remains to be determined.
Introduction
There are approximately 40,000 new endometrial cancers annually in the United States.1 Although endometrial serous carcinomas comprise a minority of these cancers, they are associated with significant mortality and result in a poor clinical outcome even in patients with early-stage disease.2 3 Until recently, the earliest phase of uterine serous carcinoma was defined as serous intraepithelial carcinoma. However, despite the absence of stromal invasion, serous intraepithelial carcinoma was considered potentially malignant, with the capacity to spread beyond the uterus.4 Recently, atypical glands have been described in the endometrium that are associated with serous intraepithelial carcinoma and contain mutations in the tumor suppressor gene p53.5 6 This entity, termed endometrial glandular dysplasia, has been proposed as a precursor to serous intraepithelial carcinoma. Other mechanisms of serous cancer development, including clonal divergence from an existing endometriod carcinoma have also been described. 7 8
A central molecular event in the pathogenesis of endometrial and pelvic serous carcinomas is a mutation in the tumor suppressor gene p53. Recent studies of pelvic serous cancer have linked a high percentage of early cancers in BRCA+ women and many other ovarian and peritoneal carcinomas to an origin in the distal fallopian tube.9 10 11 12 These early cancers, termed serous tubal intraepithelial carcinomas possess p53 mutations that are identical to co-existing non-contiguous neoplasms, implying that one is a metastasis of the other. Based on these associations, the distal fallopian tube has become a candidate source of many pelvic serous cancers. 13 14
More compelling evidence of the distal fallopian tube as a source of pelvic serous cancer has been the identification of “p53 signatures,” benign appearing mucosa that contains p53 mutations.15 16 17 Moreover, they predominate in the same location as early serous cancers (the fimbria), involve secretory cells, exhibit evidence of DNA damage (γ-H2AX+) and share epidemiologic characteristics with pelvic serous cancer.18 This entity has emerged as a presumptive precursor to many serous cancers.16
Because of the strong association between the p53 signature in the fimbria and STIC, it is conceivable that endometrial serous carcinomas are preceded by a similar series of cellular events. The purpose of this study was to establish a relationship between p53 signatures in the endometrium and the earliest malignant endometrial serous lesion, endometrial intraepithelial carcinoma. We hypothesized that endometrial p53 signatures may represent a potential precursor to some cases of uterine serous carcinoma.
Materials and Methods
Overall study design and rationale
The institutional review board at Brigham and Women’s Hospital approved the study. The goal of the study was to identify the earliest potential precursor of endometrial serous carcinoma and to determine its prevalence in both benign endometrial polyps and in association with serous intraepithelial carcinoma.
Case selection
Two different case groups were obtained from two different sources. The first consisted of 9 uterine specimens (hysterectomies and endometrial biopsies) containing endometrial polyps harboring serous intraepithelial carcinoma, and in some cases, invasive serous carcinoma, that were retrieved by report review of cases at the Department of Surgical Pathology at CellNetix Pathology. An additional hysterectomy specimen harboring only invasive serous carcinoma in a polyp was collected from the Department of Pathology at Brigham and Women’s Hospital. The second case group consisted of consecutively accessioned benign endometrial polyps obtained over a 2-year period in the Department of Pathology at Brigham and Women’s Hospital.
Immunohistochemistry
Available blocks from all cases of serous intraepithelial and/or invasive serous carcinoma and benign polyps were sectioned and stained for p53 and MIB-1. A positive score for p53 required strong nuclear staining which obscured nuclear detail. In each case, a MIB-1 fraction, or percent of cells with intense nuclear staining, was reported. In all cases of serous intraepithelial carcinoma and in polyps in which discrete segments of p53 positivity were identified in morphologically benign glandular epithelium (p53 signatures), immunostaining for γ-H2AX was performed. γ-H2AX is the phosphorylated form of the core histone H2AX, and is a marker for double-stranded DNA breakage.19 A positive score was based on punctate intranuclear staining.
Antigen retrieval was performed on 5-μm tissue sections in citrate buffer (20 mM, pH 6.0) at 120°C for 30 seconds. Sections were incubated with antibodies directed against p53 (DO-1, Immunotech, Westbrook, ME, USA) at a 1:1200 dilution, MIB-1 (corresponding to Ki-67; M7240, DAKO, Carpintaria, CA, USA) at a 1:200 dilution, or γ-H2AX (JBW301, Upstate Cell Signaling Solution, Charlottesville, VA, USA) at a 1:300 dilution, at room temperature for 40 min as previously described.15 Antigen-antibody complexes were localized using the EnVision system using horseradish peroxidase and 3,3′-diaminobenzidine (DAKO, Carpinteria, CA, USA).
Analysis of p53 mutations
A subset of matched serous intraepithelial carcinomas and/or invasive serous cancers and p53 signatures were analyzed for p53 mutations. Benign-appearing, endometrial glandular epithelium from the same tissue, not exhibiting immunoreactivity for p53, was used as a control. Laser-capture microdissected material was obtained using the PALM microbeam instrument. Genomic DNA was amplified by polymerase chain reaction (PCR) using tailed primers designed to amplify p53 exons 2, 3, 5-9 and 11. Secondary amplification was achieved using primers (T3 and T7) specific to the tail sequence used in the original amplification. Products from both strands were sequenced using T3 and T7 primers. The sequences were analyzed using the Mutation Surveyor program (Soft Genetics, State College, PA). Candidate mutations found were compared to a reference database for cancer-associated p53 mutations (Universal mutation database).15 In order to exclude spurious mutations introduced into somatic DNA as a consequence of formalin-fixation, all p53 mutation-positive exons were re-sequenced from a replicate amplified product. 20
Results
Case characteristics and identification of p53 signatures
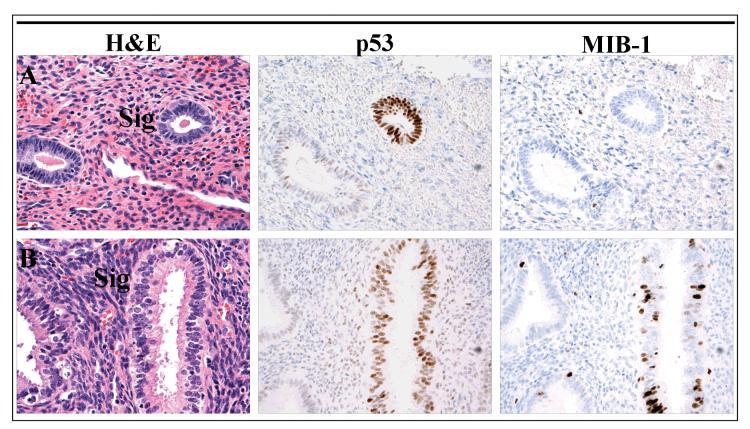
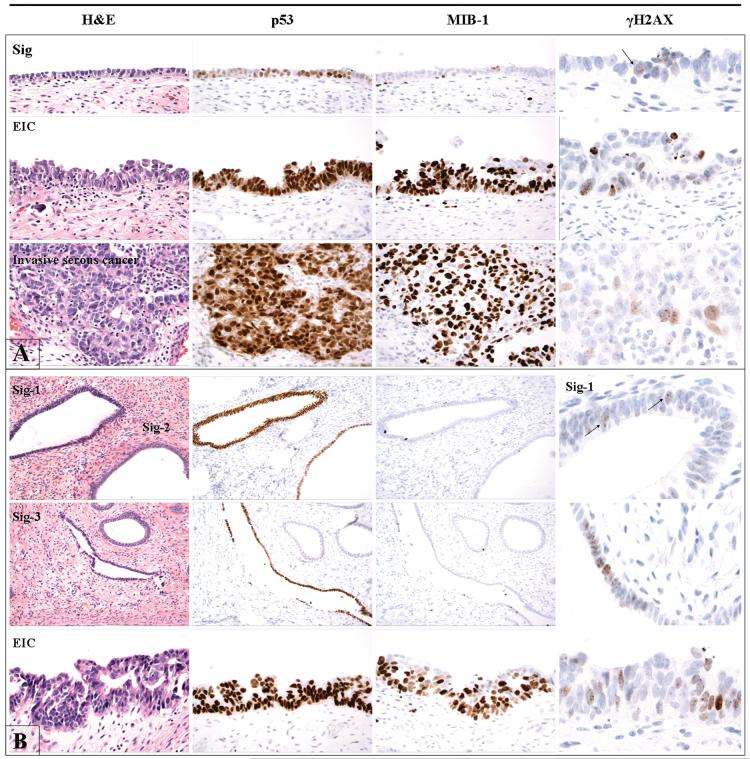
Nine serous intraepithelial carcinomas involving polyps were reviewed, including six which also harbored invasive serous cancer; an additional case which only contained invasive serous cancer in a polyp was also reviewed. p53 signatures were identified in seven of these cases, and were multiple in two (Table 1). Six of 137 benign polyps reviewed were found to harbor p53 signatures. In most of these, the p53 staining was oriented circumferentially in a cross section of a gland (Figure 1, Panels A&B and Figure 2, Panel B). Occasionally, a signature could be seen as a discrete segment of linear p53-staining along the surface of a polyp (Figure 2, Panel A). The MIB-1 fraction in p53 signatures in polyps with and without serous cancer ranged from 0% to 20%, but was less than 5% in most cases. In contrast, in serous intraepithelial carcinomas, the MIB-1 fraction ranged from 50-90%. Nuclear γ-H2AX dot-like immunostaining, indicative of DNA damage, was seen at least focally in all p53 signatures in cases with concurrent serous endometrial intraepithelial carcinoma. Serous intraepithelial and invasive carcinomas exhibited more intense γ-H2AX staining (Figure2). Of the six p53 signatures found in benign polyps, four showed no γ-H2AX staining, and two showed non-specific staining that was indistinct from the surrounding p53-negative glands.
Table 1. Characteristics of cases with serous cancer in an endometrial polyp.
| Case | Age | EIC present | Invasive cancer present | p53 signature | Extrauterine tumor |
|---|---|---|---|---|---|
| 1 | 63 | yes | yes | no | cervix |
| 2 | 74 | yes | yes | 1 signature | omentum |
| 3 | 77 | yes | yes | 3 signatures | |
| 4 | 64 | yes | no | no | |
| 5 | 67 | yes | yes | 1 signature | |
| 6 | 82 | yes | no | 2 signatures | omentum |
| 7 | 78 | yes | yes | no | |
| 8 | 57 | yes | yes | 1 signature | ovary, peritoneum |
| 9 | 65 | yes | no | no | |
| 10 | 82 | no | yes | 1 signature | ovary, bowel serosa |
EIC = endometrial intraepithelial carcinoma
Figure 1.
p53 signatures in benign polyps. Immunostaining for p53 highlights discrete glands which are morphologically indiscernible from the surrounding glands. The proliferative fraction of the p53 signature in Panel A is 0%, similar to an adjacent p53-negative gland, while the signature in Panel B exhibits a higher proliferative index.
Figure 2.
Immunoprofile of matched endometrial p53 signatures and serous cancers in two cases (Panel A and Panel B). In both cases, the p53 signatures share intense nuclear p53 staining with the serous cancers. The proliferative indices of the p53 signatures (evidenced by MIB-1 staining) are consistently lower, however, than either serous endometrial intraepithelial carcinoma or the invasive cancer. Evidence of DNA damage (punctate nuclear γ-H2AX staining) is seen in the p53 signature as well as in the serous cancers.
p53 mutation analysis
Four cases with serous intraepithelial carcinoma and p53 signatures were analyzed for p53 mutations by the methods described above. Genomic DNA amplification was successful in three cases. The results are summarized in Table 2. In two cases, shared mutations were identified between p53 signatures and concurrent serous intraepithelial carcinomas, including one case with 3 signatures. In one of these cases, an invasive serous cancer was present in which no p53 mutation was found. The morphologic features and immunoprofiles of these two cases are illustrated in Figure 2. In a third case, a p53 signature was found to harbor a different mutation from the concurrent invasive serous cancer. There were no mutations found in the normal (control) epithelium in any of the three cases.
Table 2. p53 mutations in endometrial p53 signatures and serous cancers.
| Case | Epithelium | p53 mutation | Mutation effect |
|---|---|---|---|
| 3 | Normal | None | |
| EIC | 818G>A | Arg to His | |
| p53 signature 1 | 818G>A | Arg to His | |
| p53 signature 2 | 818G>A | Arg to His | |
| p53 signature 3 | 818G>A | Arg to His | |
| 8 | Normal | None | |
| Invasive serous cancer | None | ||
| EIC | 961delA | frameshift | |
| p53 signature | 961delA | frameshift | |
| 10 | Normal | None | |
| Invasive serous cancer | 715A>G | Asn to Asp | |
| p53 signature | 646G>A | Val to Met |
EIC = endometrial intraepithelial carcinoma
Discussion
The cause of uterine serous carcinoma is not clear but it has been associated with several pathologic entities, including a pre-existing endometrioid carcinoma, serous intraepithelial carcinoma and endometrial glandular dysplasia. 4 7 8 21 Endometrial glandular dysplasia is a strong candidate for a pre-existing (precursor) lesion that is not malignant but harbors the potential to progress to serous intraepithelial carcinoma. There is a growing body of morphologic and molecular evidence implicating endometrial glandular dysplasia as a putative precursor of endometrial serous carcinoma.5 6 21 Briefly, foci of endometrial glandular dysplasia are characterized by atypical cells exhibiting serous differentiation, with nuclear atypia that falls short of the degree seen in serous carcinoma. 21
Recent reports have linked the p53 signature to serous carcinogenesis in the fallopian tube, highlighting a series of properties that this process has in common with serous carcinoma of the fallopian tube, including location in the distal fallopian tube, involvement of the secretory cell phenotype, strong immunostaining for p53, p53 mutations, evidence of DNA damage (punctate staining for γ-H2AX), physical association with serous intraepithelial carcinoma and shared risk factors with ovarian cancer .15 16 17 18 Prior to this report, a similar entity had not been described in the endometrium.
This report describes foci of p53-positive benign epithelium in the endometrium raising the possibility that an entity similar to the p53 signature develops in the endometrial surface lining. The strongest case can be made for the p53-positive epithelia seen adjacent to serous intraepithelial carcinoma. First, these foci are typically immunopositive for γ-H2AX, defined as punctate immunostaining that presumably signifies a response to DNA damage. Second, γ-H2AX is invariably present in serous carcinoma. Third, based on the p53 sequence data, some p53 signatures are genetically related to their malignant counterparts. However, is important to emphasize that not all p53 signatures shared the same mutation with the corresponding malignancy, consistent with the fact that the two entities are not always biologically related. We have previously shown that multiple p53 signatures, each with a different p53 mutation, can be found adjacent to serous carcinomas, suggesting that multiple early events can occur, with only a subset progressing to malignancy.15
Based on the findings in this report and the existence of other pathways to uterine serous cancer, the p53 signature associates with, and probably accounts for, only a subset of uterine serous malignancies. It should be emphasized that this study focused on classic examples of early serous carcinoma, primarily serous intraepithelial carcinoma. Based on this report, a subset of serous malignancies appears to be related etiologically to a p53 signature. Not studied was the greater spectrum of these tumors, included those with mixed serous and endometrioid phenotypes including those with abrupt or gradual transitions from endometrioid to serous differentiation. These tumors appear much less likely to arise within p53 signatures. Nevertheless, all of these pathways appear to share a common feature, which is the emergence of a p53 mutation. The mutation may develop prior to morphologic atypia (p53 signature), associate with minor epithelial atypia (endometrial glandular dysplasia) or emerge from a pre-existing malignancy previously lacking a p53 mutation, resulting in biphasic tumor patterns. When serous intraepithelial carcinoma is encountered, the presence of endometrial glandular dysplasia, the p53 signature, or both, appears more likely, as shown in this report.
Although the p53 signature appears to be a common entity in the fallopian tube, its frequency in the endometrium remains unclear, as does the significance of the p53 staining seen in the benign polyps. In contrast to the p53 signatures described in the fallopian tube, the p53-positive foci in the endometrial polyps did not stain for γ-H2AX. Thus, the significance of such changes, when found in otherwise benign glands, remains to be determined.
Figure 3.
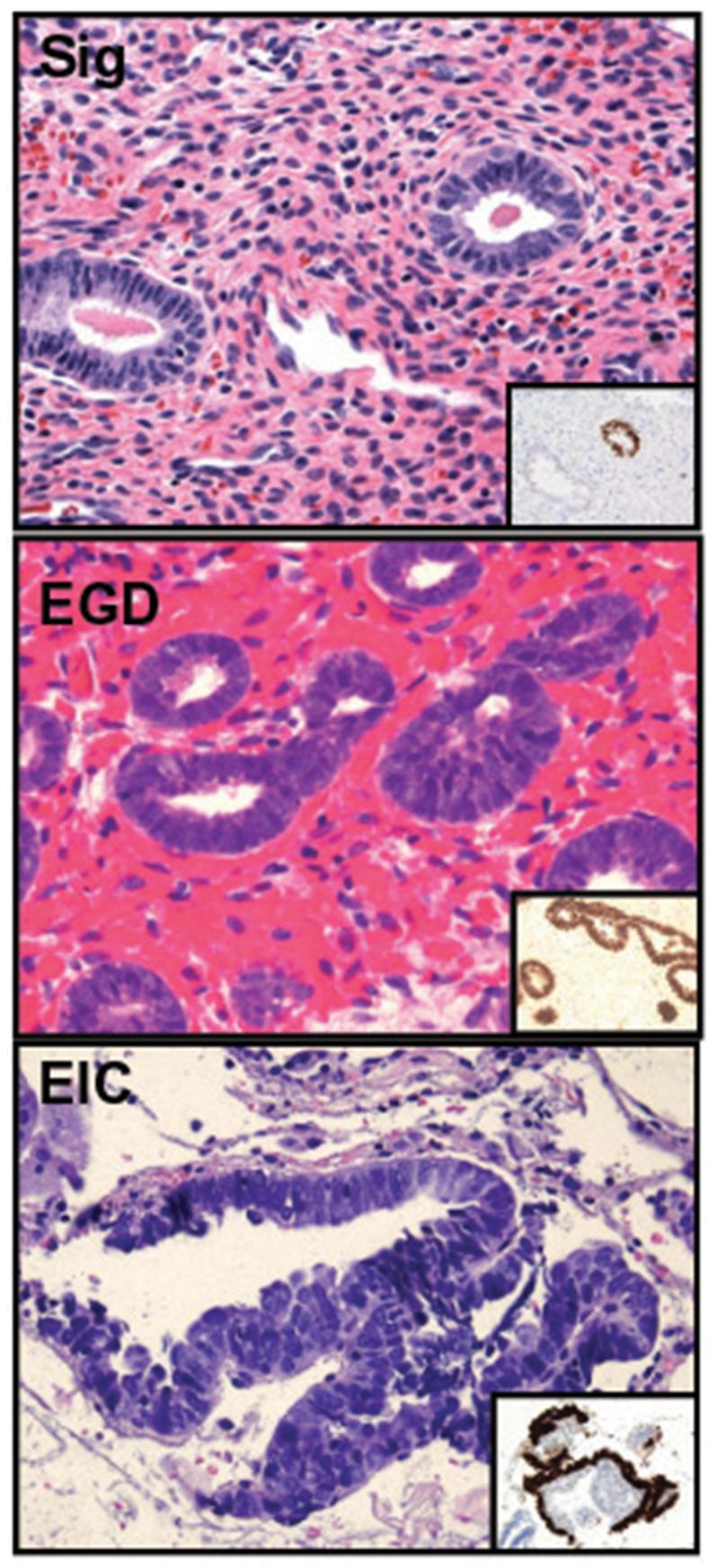
Three components of a possible serous carcinogenic sequence in the endometrium, including the p53 signature (Sig), endometrial glandular dysplasia (EGD), and serous endometrial intraepithelial carcinoma (EIC).
Acknowledgments
This work was supported by grants from the NCI (P50 CA105009 [SPORE]: D. Cramer, PI), NCI KO8 CA108748 (R Drapkin, PI), NCI 1R21CA124688-01A1 (CP Crum, PI), The Columbia Hospital For Women Research Foundation (CP Crum, PI), and the Francis Ward Paine and TSA Pemberton Funds from the Division of Women’s and Perinatal Pathology, Brigham and Women’s Hospital.
Footnotes
Disclosure/Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008r;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Chambers JT, Merino M, Kohorn EI, Peschel RE, Schwartz PE. Uterine papillary serous carcinoma. Obstet Gynecol. 1987;69:109–13. [PubMed] [Google Scholar]
- 4.Ambros RA, Sherman ME, Zahn CM, Bitterman P, Kurman RJ. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol. 1995;26:1260–7. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 5.Jia L, Liu Y, Yi X, Miron A, Crum CP, Kong B, Zheng W. Endometrial glandular dysplasia with frequent p53 gene mutation: a genetic evidence supporting its precancer nature for endometrial serous carcinoma. Clin Cancer Res. 2008;14:2263–9. doi: 10.1158/1078-0432.CCR-07-4837. [DOI] [PubMed] [Google Scholar]
- 6.Fadare O, Zheng W. Endometrial Glandular Dysplasia (EmGD): morphologically and biologically distinctive putative precursor lesions of Type II endometrial cancers. Diagn Pathol. 2008;8:3–6. doi: 10.1186/1746-1596-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman ME, Bur ME, Kurman RJ. p53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995;26:1268–74. doi: 10.1016/0046-8177(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 8.Lomo L, Nucci MR, Lee KR, Lin MC, Hirsch MS, Crum CP, Mutter GL. Histologic and immunohistochemical decision-making in endometrial adenocarcinoma. Mod Pathol. 2008;21:937–42. doi: 10.1038/modpathol.2008.97. [DOI] [PubMed] [Google Scholar]
- 9.Cass I, Holschneider C, Datta N, Barbuto D, Walts AE, Karlan BY. BRCA-mutation-associated fallopian tube carcinoma: a distinct clinical phenotype? Obstet Gynecol. 2005;106:1327–34. doi: 10.1097/01.AOG.0000187892.78392.3f. [DOI] [PubMed] [Google Scholar]
- 10.Beiner ME, Finch A, Rosen B, Lubinski J, Moller P, Ghadirian P, Lynch HT, Friedman E, Sun P, Narod SA. Hereditary Ovarian Cancer Clinical Study Group.The risk of endometrial cancer in women with BRCA1 and BRCA2 mutations. A prospective study. Gynecol Oncol. 2007;104:7–10. doi: 10.1016/j.ygyno.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, Garber JE, Cramer DW, Crum CP. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;3:230–6. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 12.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–90. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 13.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 14.Carlson J, Miron A, Jarboe E, Parast MM, Hirsch MS, Lee Y, Muto MG, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma (STIC): Its potential role in primary peritoneal serous cancer (PPSC) and serous cancer prevention. 2008;26:4160–5. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. Erratum in: J Pathol. 2007;213:116. [DOI] [PubMed] [Google Scholar]
- 16.Jarboe E, Folkins A, Nucci MR, Kindelberger D, Drapkin R, Miron A, Lee Y, Crum CP. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 17.Folkins AK, Jarboe EA, Saleemuddin A, Lee Y, Callahan MJ, Drapkin R, Garber JE, Muto MG, Tworoger S, Crum CP. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol. 2008;109:168–73. doi: 10.1016/j.ygyno.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleemuddin A, Folkins AK, Garrett L, Garber J, Muto MG, Crum CP, Tworoger SS. Risk Factors for a Serous Cancer Precursor (“p53 signature”) in Women with Inherited BRCA Mutations. Gynecol Oncol. 2008 doi: 10.1016/j.ygyno.2008.07.018. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiriet C, Hayes JJ. Chromatin in need of a fix: phosphorylation of H2AX connects chromatin to DNA repair. Mol Cell. 2005;18:617–22. doi: 10.1016/j.molcel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Williams C, Ponten F, Moberg C, Soderkvist P, Uhlen M, Ponten J, Sitbon G, Lundeberg J. A high frequency of sequence alterations is due to formalin fixation of archival specimens. Am J Pathol. 1999;155:1467–71. doi: 10.1016/S0002-9440(10)65461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang SX, Chambers SK, Cheng L, Zhang S, Zhou Y, Zheng W. Endometrial glandular dysplasia: a putative precursor lesion of uterine papillary serous carcinoma. Part II: molecular features. Int J Surg Pathol. 2004;12:319–31. doi: 10.1177/106689690401200405. [DOI] [PubMed] [Google Scholar]