Abstract
Tumor development or recurrence is always a matter of concern following radiofrequency thermal ablation (RFA) of tumors. To determine whether combining RFA with immunologically active cytokines might induce tumor-specific immune responses against mammary carcinoma and inhibit tumor development or metastasis, we evaluated intralesional injection of IL-7 and IL-15 in RFA-treated murine tumors. We used two different breast carcinoma models: neu-overexpressing mouse mammary carcinoma (MMC) in FVBN202 transgenic mouse and 4T1 tumors in Balb/c mouse. MMC tend to relapse even in the presence of neu-specific immune responses, and 4T1 is a weakly immunogenic, aggressive and highly metastatic transplantable tumor. In vivo growth of both of these tumors is also associated with increased numbers of CD11b+Gr1+ myeloid-derived suppressor cells (MDSC). We showed for the first time that unlike RFA alone, RFA combined with the administration of intralesional IL-7 and IL-15 (after RFA), induced immune responses to tumors, inhibited tumor development and lung metastasis, and reduced MDSC.
Keywords: radiofrequency thermal ablation (RFA), breast cancer, immunotherapy, myeloid-derived suppressor cells (MDSC), Interleukin-7 (IL-7), Interleukin 15 (IL-15)
Introduction
Radiofrequency thermal ablation (RFA) of solid tumors delivers a high frequency alternating current, which causes ionic agitation and frictional heating, resulting in the killing of tumor cells. At temperatures above 55°C, cellular proteins are denatured and cell membranes are destroyed, leaving a necrotic region surrounding the electrode. RFA was first reported as a treatment for breast cancer in 1999 by Jeffrey et al who reported tumor cell death in 5 out of 5 patients studied [1]. RFA has also been used for the treatment of renal cell carcinomas, primary and metastatic hepatic carcinomas, lung carcinomas, prostate cancer, metastatic bone lesions, and many other types of cancer [2]. In addition to the localized effects against the treated tumors, RFA may also stimulate T cell responses [3, 4]. It was reported that RFA can provide a source of tumor antigen for uptake and presentation by dendritic cells (DC) and can induce immune responses against B16 melanoma in mice [4].
RFA has been shown to be safe in breast cancer patients [5]. It was also suggested to be a potent activator of anti-tumor immune responses that might reduce the risk of tumor recurrence [6]. However, there is no single report on the efficacy of RFA in eliciting anti-tumor immune responses thereby reducing relapse and/or metastasis of breast cancer. On the other hand, RFA has been shown to induce anti-tumor immune responses against hepatocellular carcinoma [3]. However, such immune responses could not protect the patients from tumor recurrence [7]. These studies further indicate the need for additional immune manipulation in conjunction with RFA for the treatment of solid tumors. It has yet to be determined whether an adjuvant immunotherapy combined with RFA might improve the therapeutic efficacy of RFA by inducing anti-tumor immune responses and inhibiting tumor relapse and metastasis.
The gamma chain cytokines, IL-7 and IL-15, are attractive candidates for adjuvant immunotherapy because of their role in maintenance/proliferation of effector and memory T cells. IL-7 plays a critical role in lymphocyte development and homeostasis of naïve and memory CD8+ T cells [8]. It has been implicated as a pro-survival cytokine for early memory CD8 T cell selection and maintenance [9-11]. High IL-7 levels may lead to autoimmunity and ectopic lymphocyte infiltrates, suggesting its potential role in enhancing anti-tumor immune responses [12]. IL-15 is involved in maintenance and proliferation of memory T cells [13]. Moreover, unlike IL-2, IL-7 and IL-15 do not cause activation-induced cell death, nor are they needed for the maintenance of CD4+ CD25+ regulatory T cells (T regs) [13]. In fact, one characteristic of T regs is lack of expression of IL-7 receptor alpha, CD127 [14]. Therefore, we sought to determine whether addition of intralesional IL-7 and IL-15 after RFA treatment might improve induction of anti-tumor immune responses and result in the inhibition of tumor recurrence or metastasis. In order to perform these studies, we used two different breast tumor models. One model was a neu-overexpressing murine mammary carcinoma (MMC) isolated from spontaneous mammary tumors of FVBN202 transgenic mice [15, 16]. These mice over-express the rat neu oncogenic protein in their mammary gland tissue and develop spontaneous mammary carcinoma [17]. MMC tumors tend to relapse in the presence of specific types of anti-tumor immune responses [16, 18, 19]. The second model was the 4T1 mammary carcinoma, which closely resembles human breast cancer, with weak immunogenicity and aggressive growth and metastatic properties [20]. 4T1 tumors spontaneously metastasize primarily to the lungs (>95%), but also spread to the liver (>75%) and brain (40%) as well as the blood and lymph nodes. In both of these mammary carcinoma models, failure of the rejection of transplanted tumors is associated with increased numbers of CD11b+Gr1+ myeloid-derived suppressor cells (MDSC) in the spleens of tumor-bearing mice [19, 21]. In the present studies we determined for the first time that RFA combined with intralesional IL-7 and IL-15 induces tumor-specific immune responses, protects animals against tumor relapse or metastasis, and reduces MDSC.
Materials and Methods
Animals
Female FVBN202 transgenic (Charles River Laboratories) and female Balb/c mice (Jackason Laboratories) were used throughout these studies. FVBN202 is the rat neu transgenic mouse model in which 100% of females develop spontaneous mammary tumors by 6−10 months of age, with many features similar to human breast cancer. These mice overexpress an unactivated rat neu transgene, as self antigen, under the regulation of the MMTV promoter [17]. These mice are tolerant to neu protein prior to the development of atypical hyperplasia in their mammary glands [19, 22]. The studies have been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Virginia Commonwealth University. Animals were between 8−10 weeks of age unless otherwise stated.
Tumor cell lines
MMC cell line was established from a spontaneous tumor harvested from FVBN202 transgenic mice as previously described with minor modifications [16, 23]. Neu antigen negative variant (ANV) was established from recurrence of MMC under immune pressure in vivo [16, 18]. 4T1 mammary tumor cells were kindly provided by Dr. Jane Tsai at the Michigan Cancer Foundation, Detroit, Michigan. Cells were maintained in Dulbecco's Modified Essential Medium (DMEM) with 10% heat-inactivated fetal calf serum (Hyclone, Logan, UT), 1 mM sodium pyruvate, 100 μ/ml penicillin and 100 μg/ml streptomycin (Sigma, St. Louis MO). The 4T1 tumor is a weakly immunogenic and highly metastatic breast carcinoma line established in Balb/c mice. Animals were inoculated with 5×106 MMC or 5×104 4T1 tumors unless otherwise stated.
RFA treatment
Animals were anesthetized with intraperitoneal injection of 200 μl of Ketamine/Xylazine (80 mg/kg of Ketamine and 10 mg/kg of Xylazine) solution. After shaving the area, the XL probe and RF generator (Model Rita 1500X RF generator, Angiodynamics, Queensbury, NY) were used to ablate the tumor, by raising the temperature up to 110 °C. Ten-fifteen min after RFA, 1 μg of cytokines (GM-CSF or IL-7 and IL-15) were injected intralesionally. The animals were observed for two hours after the procedure until full recovery.
In vivo tumor challenge studies
Animals were inoculated s.c. with MMC (5×106 cells/mouse) or 4T1 (5×104/mouse). Animals were inspected twice every week for the development of tumors. Masses were measured with calipers along the two perpendicular diameters. Tumor volume was calculated by: V(volume) = L(length) × W(width)2 ÷ 2. Mice were sacrificed before tumor volume exceeded 2000 mm3.
IFN-γELISA
Splenocytes were cultured in the presence of IL-2 (40 U/ml) for 5 days in order to enrich for CD25+ effector T cells and to reduce MDSC and eliminate background IFN-γsecretion by MDSC. Such in vitro cultures dramatically reduced the number of MDSC since these cells undergo apoptosis starting immediately after in vitro culture. Splenocytes were then cultured with complete medium (RPMI1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin) in the presence or absence of irradiated MMC or 4T1 (15000 rad, in a E:T ratio of 10:1). Supernatants were collected after 20 hrs and IFN-γ was detected using a Mouse IFN-γ ELISA kit (BD Pharmingen, San Diego, CA) according to manufacture's protocol. Results were reported as the mean values of duplicate wells.
Flow Cytometry
All staining procedures were conducted on ice, and washing steps were performed with a PBS-0.1% sodium azide to avoid internalization/recycling of the receptors. Anti-mouse CD16/CD32 antibody (0.5 μg/200 μl/2−5×10 cells; BD PharMingen) was used prior to the specific antibody staining in order to block the cell surface Fc receptors. FITC-conjugated anti-mouse CD11b and PE-conjugated anti-mouse Gr1 antibodies were used according to the manufacturer's instructions (Biolegend, San Diego, CA). Cells were finally washed and fixed with 1% ultra pure formaldehyde and assayed on a Beckman Coulter Epics XL and data were analyzed using Expo 32 software.
Statistical analysis
Results of tumor growth were analyzed using Student's t test. A value of P < 0.05 was considered significant.
Results
RFA alone failed to break tolerance against MMC while post-RFA intralesional injection of IL-7 and IL-15 following RFA treatment induced MMC-specific immune responses
We sought to determine whether RFA treatment of MMC would break tolerance in MMC tumor-bearing FVBN202 mice. Animals that did not have Ab responses against the neu protein were selected and inoculated s.c. with MMC. Two weeks later, solid tumors were completely ablated by RFA. A group of mice were injected intralesionally with IL-7 (1 μg/mouse) and IL-15 (1 μg/mouse) 10−15 minutes after RFA treatment. Control group mice remained untreated following MMC challenge. Animals were sacrificed 5 weeks after the treatment and their splenocytes were cultured in the presence of IL-2 (40 U/ml) for 5 days followed by culture in the presence or absence of irradiated MMC (15,000 rad; E:T ratio of 10:1) for 20 hr. Supernatants were collected and tested for IFN-γ by ELISA. As shown in Fig. 1A, splenocytes of control animals did not secrete appreciable IFN-γ above the background levels nor did splenocytes of animals whose tumors were treated with RFA. However, splenocytes of animals who were treated with RFA followed by intralesional injection of IL-7 and IL-15 secreted high levels of IFN-γ (1100 pg/ml) in response to MMC cells (P= 0.007). No IFN-γ was detected against ANV tumor cells (data not shown).
Figure 1. RFA combined with IL-7 and IL-15 induced tumor-specific T cell.
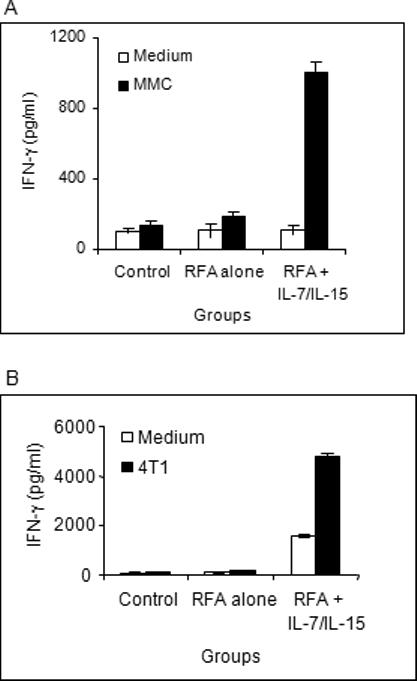
A) FVBN202 mice (n=4) that did not have Ab responses against the neu protein were selected and inoculated s.c. with MMC. Two weeks later, solid tumors were completely ablated by RFA. A group of mice (n=4) were injected intralesionally with IL-7 (1 γg/mouse) and IL-15 (1 γg/mouse) 10−15 minutes after RFA treatment. Control group mice (n=4) remained untreated following MMC challenge. Animals were sacrificed 5 weeks after the treatment and their splenocytes were subjected to IFN-γ by ELISA in the presence or absence of irradiated MMC for 24 hrs. B) Balb/c female mice were inoculated with 4T1 tumors. Control groups (n=4) remained untreated following challenge with 4T1 tumors. Test groups were treated with RFA alone (n=4) or RFA + intralesional injection of IL-7 and IL-15 (n=3). Animals were sacrificed 2−3 weeks after the challenge and their splenocytes were cultured in the presence of IL-2 (40 U/ml) for 5 days and then subjected to IFN-γ ELISA assay in the presence or absence of irradiated 4T1 cells for 20 hrs.
Intralesional injection of IL-7 and IL-15 following RFA treatment induced 4T1 tumor-specific immune responses
In order to determine whether RFA alone or in combination with IL-7 and IL-15 would induce immune responses against 4T1 tumors, we inoculated Balb/c mice with 4T1 tumors. Control groups remained untreated following challenge with 4T1 tumors. Test groups were treated with RFA alone or RFA + intralesional injection of IL-7 and IL-15. Animals were sacrificed 2−3 week after the challenge and their splenocytes were cultured in the presence of IL-2 (40 U/ml) for 5 days and then subjected to IFN-γ ELISA assay in the presence or absence of irradiated 4T1 cells for 20 hrs. Supernatants were then harvested for IFN-γ assay. As shown in Fig. 1B, lymphocytes from neither control mice nor RFA-treated mice produced IFN-γ upon in vitro stimulation with irradiated 4T1 cells. On the other hand, lymphocytes from animals who received combined treatment with RFA and IL-7 + IL-15 showed appreciable secretion of IFN-γ even in the absence of tumor but this was markedly increased in the presence of 4T1 tumor cells (from 600 pg/ml to 5000 pg/ml; P= 0.004).
Intralesional injection of IL-7 and IL-15 in RFA-treated MMC resulted in a relapse-free survival
FVBN202 transgenic mice (10 mice per group) or Balb/c mice (4 mice per group) were inoculated with neu overexpressing MMC or 4T1 mammary tumors, respectively. Two weeks after the tumor challenge, tumors (MMC in FVBN202: ∼270 mm3 or 4T1 in Balb/c: ∼60 mm3) were either treated with RFA alone or RFA followed by intralesional injection of recombinant IL-7 and IL-15. Control groups remained untreated following tumor challenge. A group of FVBN202 transgenic mice was also inoculated with MMC followed by intralesional injection of IL-7 and IL-15 alone, without prior RFA (n=4). As shown in Fig. 2A, all control groups and FVBN202 transgenic mice who received IL-7 and IL-15 in the absence of RFA treatment developed progressive tumor growth. RFA alone temporarily slowed the growth of primary tumors so that no tumor was visible after RFA (Fig. 2A; 2 weeks after the challenge, P= 0.000002). However, tumors recurred at the periphery of the RFA area and re-grew at a slower rate than those in control groups (week 3, P= 0.0000006, week 4, P= 0.00003, week 5, P= 0.0005). Similar observations were made in Balb/c mice inoculated with 4T1 tumors (Fig. 2B). There was no tumor detectable after RFA treatment and RFA-treated tumors grew at a significantly slower rate than control tumors (P= 0.02 at week 2; P= 0.009 at week 4 and P= 0.001 at week 5). Three weeks after RFA treatment, recurrent MMC tumors reached 800−1100 mm3 in FVBN202 transgenic mice (Fig 2A; P= 0.0007) and recurrent 4T1 tumors reached 100−250 mm3 in Balb/c mice (Fig. 2B, P= 0.006). Interestingly, animals who had received RFA followed by intralesional injection of IL-7 and IL-15 remained tumor-free until the end of trial.
Figure 2. RFA combined with IL-7 and IL-15 protected animals against tumor recurrence.
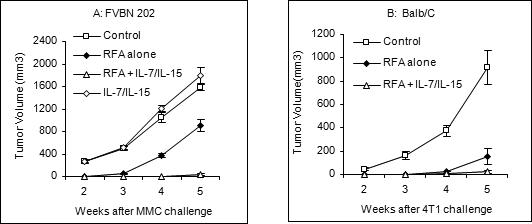
FVBN202 transgenic mice (A; 10 mice per group) or Balb/c mice (B; 4 mice per group) were inoculated with neu overexpressing MMC or 4T1 mammary tumors, respectively. Two weeks after the tumor challenge, tumors were either treated with RFA alone or RFA followed by intralesional injection of recombinant IL-7 and IL-15. Control groups remained untreated following tumor challenge. A group of FVBN202 transgenic mice was also inoculated with MMC followed by intralesional injection of IL-7 and IL-15 alone, without prior RFA.
Intralesional injection with IL-7 and IL-15 in RFA-treated tumors resulted in the inhibition of lung metastasis of 4T1 mammary tumors
Because 4T1 exhibits a high rate of lung metastasis soon after inoculation, we sought to determine whether induction of anti-tumor immune responses by RFA plus intralesional IL-7 and IL-15 would inhibit tumor metastasis in Balb/c mice. Animals inoculated with 4T1 cells were treated with RFA alone or RFA and intralesional IL-7 + IL-15 10 days after tumor inoculation. Control groups remained untreated. Mice were sacrificed 3−4 weeks after tumor inoculation, and lungs were removed and evaluated for metastasis. As shown in Fig. 3, both RFA-treated (A) and untreated mice (B) showed marked development of lung metastasis, while animals who had received IL-7 and IL-15 after RFA treatment (C) showed inhibition of metastatic nodules in their lungs compared to other groups. Healthy lung from naïve mice was used as negative control for metastasis (D).
Figure 3. Intralesional injection with IL-7 and IL-15 in RFA-treated tumors resulted in the inhibition of lung metastasis of 4T1 mammary tumors.
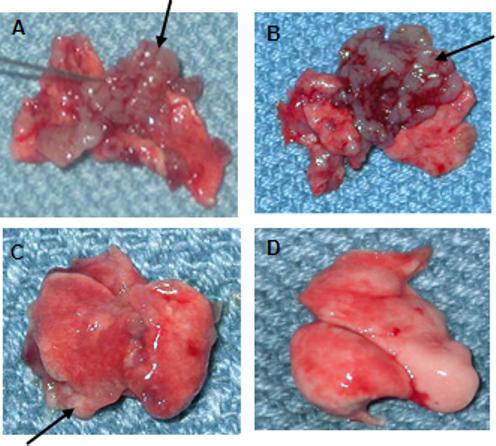
Balb/c female mice inoculated with 4T1 cells were treated with RFA alone (n=4) or RFA and intralesional IL-7 + IL-15 (n=3) 10 days after tumor inoculation. Control groups remained untreated (n=4). Mice were sacrificed 3−4 weeks after tumor inoculation, and lungs were removed and evaluated for metastasis using a light microscope. Tumors are indicated by arrows.
Intralesional injection of MMC with GM-CSF leads to increased MDSC in the spleen while IL-7 and IL-15 did not show such effects
We compared the effects of GM-CSF and IL-7/IL-15 on the accumulation of MDSC in the spleen of tumor-bearing animals. FVBN202 transgenic mice were inoculated with MMC and received intralesional injection of GM-CSF or IL-7 and IL-15 after 2 weeks. Control groups received no cytokine treatment. Animals were then sacrificed 4 weeks after the tumor challenge, and presence of MDSC in their spleens was assessed. As shown in Fig. 4, the number of CD11b+Gr1+ MDSC in gated granulocyte regions increased from 84% in control tumor-bearing mice (A) to 92% in GM-CSF-treated mice (B), while injection of IL-7 and IL-15 actually decreased MDSC to 61% (C). The normal proportion of MDSC in FVBN202 mice prior to tumor development was ∼15−20%. Absolute number of MDSC in spleen showed similar trend (data not shown).
Figure 4. Intralesional injection of MMC with GM-CSF leads to increased MDSC in the spleen while IL-7 and IL-15 did not show such effects.
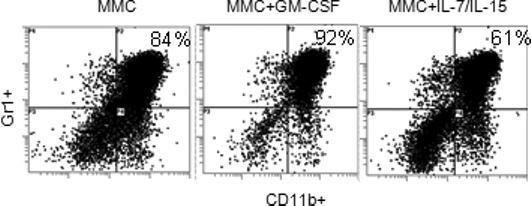
FVBN202 transgenic mice were inoculated with MMC (4×106 cells/mouse) and received intralesional injection of GM-CSF or IL-7 and IL-15 (1 μg/mouse) after 2 weeks. Control groups received no cytokine treatment. Animals were then sacrificed 4 weeks after the tumor challenge, and presence of CD11b+Gr1+ MDSC in their spleens was assessed by flow cytometry. Representative data are presented from 3 mice per group.
Intralesional injection with IL-7 and IL-15 in RFA-treated animals resulted in reduced numbers of CD11b+Gr1+ MDCS compared to control tumor-bearing mice
Since failure in the rejection of MMC and 4T1 tumors was associated with increased numbers of CD11b+Gr1+ MDSC, we sought to determine whether the anti-tumor effect of combined RFA/IL-7/IL-15 treatment was associated with reduced numbers of MDSC. FVBN202 transgenic mice were challenged with MMC and Balb/c mice were challenged with 4T1 tumors. Ten-fourteen days later, animals were treated with RFA, RFA + IL-7 and IL-15, or remained untreated. Animals were sacrificed 1−2 weeks later and their splenocytes were stained with FITC-CD11b and PE-Gr1 Abs. Percent double positive cells were analyzed on gated granulocyte regions. As shown in Fig. 5, control tumor-bearing mice showed increased levels of MDSC (84% in FVBN202 and 92% in Balb/c) compared to naïve mice (21% in FVBN202 and 33% in Balb/c). While RFA-treated groups showed a slight decrease in the number of MDSC (55% in FVBN202 and 71% in Balb/c), RFA + IL-7/IL-15-treated groups showed a marked decrease in splenic MDSC (38% in FVBN202 and 56% in Balb/c) compared to control tumor-bearing mice (84% in FVBN202 and 92% in Balb/c).
Figure 5. Intralesional injection with IL-7 and IL-15 in RFA-treated animals reduced CD11b+Gr1+ MDCS compared to untreated tumor-bearing mice.
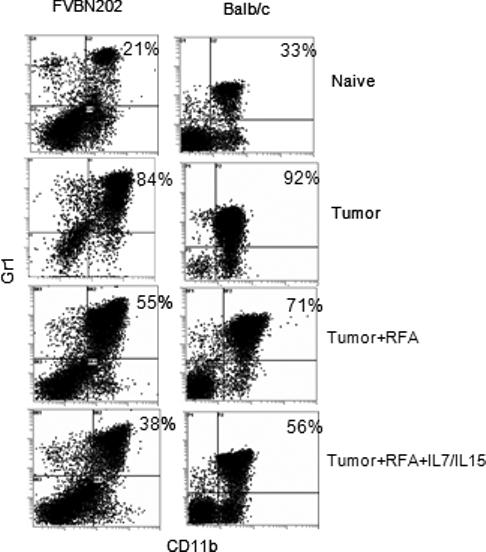
FVBN202 transgenic mice were challenged with MMC and Balb/c mice were challenged with 4T1 tumors. Ten-fourteen days later, animals were treated with RFA, RFA + IL-7 and IL-15, or remained untreated. Naïve mice were used as controls. Animals were sacrificed 1−2 weeks later and their splenocytes were stained with FITC-CD11b and PEGr1 Abs. Percent double positive cells were analyzed on gated granulocyte regions. Representative data are presented from four mice per group.
Discussion
RFA is a clinically approved treatment for the destruction of tumor tissues, particularly unresectable primary and metastatic hepatic tumors. RFA generates local necrosis followed by a marked inflammatory response and infiltration of T cells at the site of treatment [24-26]. Necrotic tumors may then provide a unique source of antigens for the induction of anti-tumor immune responses. RFA has been evaluated for metastatic and primary breast, lung, kidney and bone cancers [3]. In addition, it has been shown that small molecules or cytokines could be retained and gradually released from RFA-treated tumors for a prolonged period of time [27]. Therefore, intralesional injection of cytokines in RFA-treated tumor tissues should allow a continuous supply of tumor antigens and cytokines for the retention, activation, proliferation, and survival of the tumor-infiltrating T cells following RFA.
Initial animal studies and subsequent human studies have demonstrated that RFA alone can induce tumor-specific immune responses against hepatocellular carcinoma. The use of RFA on implanted VX2 hepatoma was shown to cause increased antigen-specific proliferation of lymphocytes from the peripheral blood of RFA treated animals over that of control animals. The tumor sites of these mice also showed an increased infiltration of CD3+ cells after treatment with RFA [4]. RFA has also been reported to enhance anti-tumor immune responses in a fraction of patients with hepatocellular carcinoma [3, 28]. However, these T cell responses did not offer any protection against tumor recurrence, therefore suggesting the need for additional immune manipulation in conjunction with RFA treatment. Using two animal models of mammary carcinoma, we have demonstrated that RFA alone failed to induce immune responses against tumors, while intralesional injection of a single dose of IL-7 and IL-15 following RFA resulted in the induction of marked IFN-γ production by splenocytes in response to MMC and 4T1 tumor cells in vitro, as well as inhibition of tumor recurrence and lung metastasis in vivo. Since it is difficult to quantify complete ablation of microscopic tumors, it is also likely that IL-7 and IL-15 could cause a more complete ablation of the primary tumor rather than inhibition of tumor recurrence. In any case, IL-7 and IL-15 caused inhibition of tumor development following RFA treatment. Failure of RFA alone to induce tumor-specific immune responses may result, in part, from the presence of increased levels of MDSC in both MMC and 4T1 tumor-bearing mice, in contrast to hepatocellular carcinoma model. Levels of MDSC were greater in 4T1-bearing mice than MMC-bearing animals. Higher background IFN-γ secretion by splenocytes of 4T1-bearing mice even in the absence of in vitro stimulation with the tumor may be due to the presence of residual metastatic nodules that provide the source of antigen for continuous stimulation of T cells. This needs to be confirmed by using imaging technology.
Intratumoral injection of cytokines, GM-CSF in particular, has been shown to have a marked paracrine effect (high concentration of a substance in tumor microenvironment) for the induction of anti-tumor immune responses. However, it requires daily or twice daily injections because of the rapid clearance of GM-CSF by the tumor's rich neovasculature. On the other hand, intralesional injection of cytokines in RFA-treated tissues will result in the retention and grandual release of cytokine at the tumor site. Therefore, a single injection would be sufficient for the induction and/or retention of local immune responses. Having detected a marked increase in numbers of MDSC due to the presence of MMC or 4T1 tumors in FVBN202 and Balb/c mice, we decided not to use GM-CSF in order to avoid a further increase in the recruitment of MDSC. We determined that RFA followed by intralesional injection of GM-CSF resulted in an increased MDSC burden compared to MMC-bearing animals who received no treatment while injection of IL-7 and IL-15 moderately reduced MDSC, even in tumor-bearing mice. Therefore, we decided to test IL-7 and IL-15 instead of GM-CSF intralesionally after RFA. It has been shown that GM-CSF promotes bone metastasis of breast tumors [29, 30] and expansion of MDSC in melanoma patients [31]. Although in tumor models where MDSC do not appear to be the major suppressor cells, GM-CSF has been shown to be a potent inducer of anti-tumor immune responses [32]. On the other hand, gamma chain cytokines IL-7 and IL-15 have been shown to play a critical role in augmenting anti-tumor activity of T cells [33, 34]. It was reported that proliferation, persistence, and anti-tumor efficacy of melanoma specific T cells depended on the presence of IL-7 or IL-15 [35]. It was also reported that administration of IL-7 and IL-15 in an adjuvant setting augmented immune responses to subdominant epitopes and improved survival of antigen-specific T memory cells [36]. Therefore, it is likely that RFA treatment provided an antigenic pool for activation of T cells, and triggering of anti-tumor immune responses was facilitated by intralesional gamma chain cytokines IL-7 and IL-15. Prolonged release of intralesionally administered cytokines is also a unique phenomenon which can be utilized in this setting to avoid the need for frequent administration of cytokines and systemic toxicity.
We have shown here for the first time that intralesional injection of IL-7 and IL-15 can elicit protective immunity against tumors following RFA treatment. This approach needs to be evaluated in other tumor models, and its efficacy in long term tumor-free survival need to be tested in follow-up studies on the MMC and 4T1 models. In addition, combination of IL-7 and IL-15 with other treatment options such as cryotherapy and electroporation remain to be evaluated. Unlike RFA, cryotherapy will not denature proteins yet causes tumor antigen release. Cryo ablation was also shown to be superior to RFA in facilitating tumor-antigen presentation in vivo [37]. Combination of cryo ablation with CpG was also shown to induce enhanced anti-tumor responses compared to each treatment alone [38].
Acknowledgement
This work was supported by the National Institute of Health R01 CA104757 grant (M.H. Manjili) and flow cytometry shared resources facility supported in part by the NIH grant P30CA16059. We gratefully acknowledge the support of VCU Massey Cancer Center and the Commonwealth Foundation for Cancer Research. The authors would like to thank the support of Mr. Robert Tucker from Angiodynamics corp. for assisting the study by providing the RF generator and probes.
References
- 1.Jeffrey SS, Birdwell RL, Ikeda DM, Daniel BL, Nowels KW, Dirbas FM, Griffey SM. Radiofrequency ablation of breast cancer: first report of an emerging technology. Arch Surg. 1999;134:1064–1068. doi: 10.1001/archsurg.134.10.1064. [DOI] [PubMed] [Google Scholar]
- 2.Vlastos G, Verkooijen HM. Minimally invasive approaches for diagnosis and treatment of early-stage breast cancer. The oncologist. 2007;12:1–10. doi: 10.1634/theoncologist.12-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139–1146. doi: 10.1158/0008-5472.CAN-05-2244. [DOI] [PubMed] [Google Scholar]
- 4.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 5.Fornage BD, Sneige N, Ross MI, Mirza AN, Kuerer HM, Edeiken BS, Ames FC, Newman LA, Babiera GV, Singletary SE. Small (< or = 2-cm) breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology. 2004;231:215–124. doi: 10.1148/radiol.2311030651. [DOI] [PubMed] [Google Scholar]
- 6.Sabel MS, Edge SB. In-situ ablation of breast cancer. Breast Dis. 2001;12:131–140. doi: 10.3233/bd-2001-12113. [DOI] [PubMed] [Google Scholar]
- 7.Machi J, Bueno RS, Wong LL. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005;29:1364–1373. doi: 10.1007/s00268-005-7829-6. [DOI] [PubMed] [Google Scholar]
- 8.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 9.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7R on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 11.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 12.Costello R, Duffaud F. The pleiotropic effects of interleukin 7 and their pathologic and therapeutic implications. Eur J Med. 1992;1:119–121. [PubMed] [Google Scholar]
- 13.Waldmann TA. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manjili MH, Arnouk H, Knutson KL, Kmieciak M, Disis ML, Subjeck JR, Kazim AL. Emergence of immune escape variant of mammary tumors that has distinct proteomic profile and a reduced ability to induce “danger signals”. Breast Cancer Res Treat. 2006;96:233–241. doi: 10.1007/s10549-005-9044-4. [DOI] [PubMed] [Google Scholar]
- 16.Kmieciak M, Knutson KL, Dumur CI, Manjili MH. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur J Immunol. 2007;37:675–685. doi: 10.1002/eji.200636639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knutson KL, Almand B, Dang Y, Disis ML. Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice. Cancer Res. 2004;64:1146–1151. doi: 10.1158/0008-5472.can-03-0173. [DOI] [PubMed] [Google Scholar]
- 19.Kmieciak M, Morales JK, Morales J, Bolesta E, Grimes M, Manjili MH. Danger signals and nonself entity of tumor antigen are both required for eliciting effective immune responses against HER-2/neu positive mammary carcinoma: implications for vaccine design. Cancer Immunol Immunother. 2008 Feb 16; doi: 10.1007/s00262-008-0475-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58:1486–1493. [PubMed] [Google Scholar]
- 21.Worschech A, Kmieciak M, Knutson KL, Bear HD, Szalay AA, Wang E, Marincola FM, Manjili MH. Signatures associated with rejection or recurrence in HER-2/neu positive mammary tumors. Cancer Res. 2008 doi: 10.1158/0008-5472.CAN-07-6822. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi N, Hiraoka S, Zhou XY, Nagafuku M, Ono S, Tsujimura T, Nakazawa M, Yura Y, Hamaoka T, Fujiwara H. Anti-HER-2/neu immune responses are induced before the development of clinical tumors but declined following tumorigenesis in HER-2/neu transgenic mice. Cancer Res. 2004;64:7588–7595. doi: 10.1158/0008-5472.CAN-04-1081. [DOI] [PubMed] [Google Scholar]
- 23.Knutson KL, Lu H, Stone B, Reiman JM, Behrens MD, Prosperi CM, Gad EA, Smorlesi A, Disis ML. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–1533. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 24.Le Veen RF. Laser hypertermia and radiofrequency ablation of hepatic lesions. Semin Intervent Radiol. 1997;14:313–324. [Google Scholar]
- 25.Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 26.Hansler J, Neureiter D, Strobel D, et al. Cellular and vascular reactions in the liver to radio-frequency thermo-ablation with wet needle applicators. Study on juvenile domestic pigs. Eur Surg Res. 2002;34:357–363. doi: 10.1159/000064000. [DOI] [PubMed] [Google Scholar]
- 27.Riley LB, Maggitti A, Habibi M, Desai DC. A new approach to paracrine-based immunotherapy using radiofrequency ablated tissue with fluorescein as a surrogate marker. Ann Surg Oncol. 2004;11(Supplement 2):S90. [Google Scholar]
- 28.Rughetti A, Rahimi H, Rossi P, Frati L, Nuti M, Gaspari A, Danza FM, Ercoli L. Modulation of blood circulating immune cells by radiofrequency tumor ablation. J Exp Clin Cancer Res. 2003;22(4 Suppl):247–250. [PubMed] [Google Scholar]
- 29.Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, McCauley L, Shi S, Chen S, Wang CY. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13:62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 30.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2007;18:226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 31.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 32.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I Clinical Trial of Autologous Ascites-derived Exosomes Combined With GM-CSF for Colorectal Cancer. Mol Ther. 2008 Feb 5; doi: 10.1038/mt.2008.1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, Proietti E. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13(2 Pt 1):644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 34.Leonhartsberger N, Ramoner R, Putz T, Gander H, Rahm A, Falkensammer C, Bartsch G, Thurnher M. Antigen-independent immune responses after dendritic cell vaccination. Cancer Immunol Immunother. 2007;56:897–903. doi: 10.1007/s00262-006-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang LX, Li R, Yang G, Lim M, O'Hara A, Chu Y, Fox BA, Restifo NP, Urba WJ, Hu HM. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–10577. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95(7):896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Toonen LW, Figdor CG, Ruers TJ, Adema GJ. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66(14):7285–7292. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]


