Abstract
Purpose
The objective of the current study is to investigate the hypothesis that bioactive terpenoids and flavonoids of Ginkgo biloba extract (GBE) induce human hepatic drug metabolizing enzymes (DMEs) and transporters through the selective activation of pregnane X receptor (PXR), constitutive androstane receptor (CAR), and aryl hydrocarbon receptor (AhR).
Methods
Human primary hepatocyte (HPH), and HepG2 cells are used as in vitro models for enzyme induction and nuclear receptor activation studies. A combination of real-time RT-PCR, transient transfection, and cell-based reporter assays were employed.
Results
In human primary hepatocytes, real-time PCR analysis showed induction of CYP2B6, CYP3A4, UGT1A1, MDR1, and MRP2 by EGb 761, ginkgolide A (GA) and ginkgolide B (GB), but not by bilobalide (BB) or the flavonoids (quercetin, kaempferol and tamarixetin) of GBE. Cell-based reporter assays in HepG2 revealed that GA and GB are potent activators of PXR; quercetin and kaempferol activate PXR, CAR, and AhR, whereas BB exerts no effects on these xenobiotic receptors. Notably, the flavonoids induced the expression of UGT1A1 and CYP1A2 in HepG2 cells but not in HPH.
Conclusion
Our results indicate that terpenoids and flavonoids of GBE exhibit differential induction of DMEs through the selective activation of PXR, CAR, and AhR.
Keywords: EGb 761, Cytochrome P450, PXR, CAR, AhR
INTRODUCTION
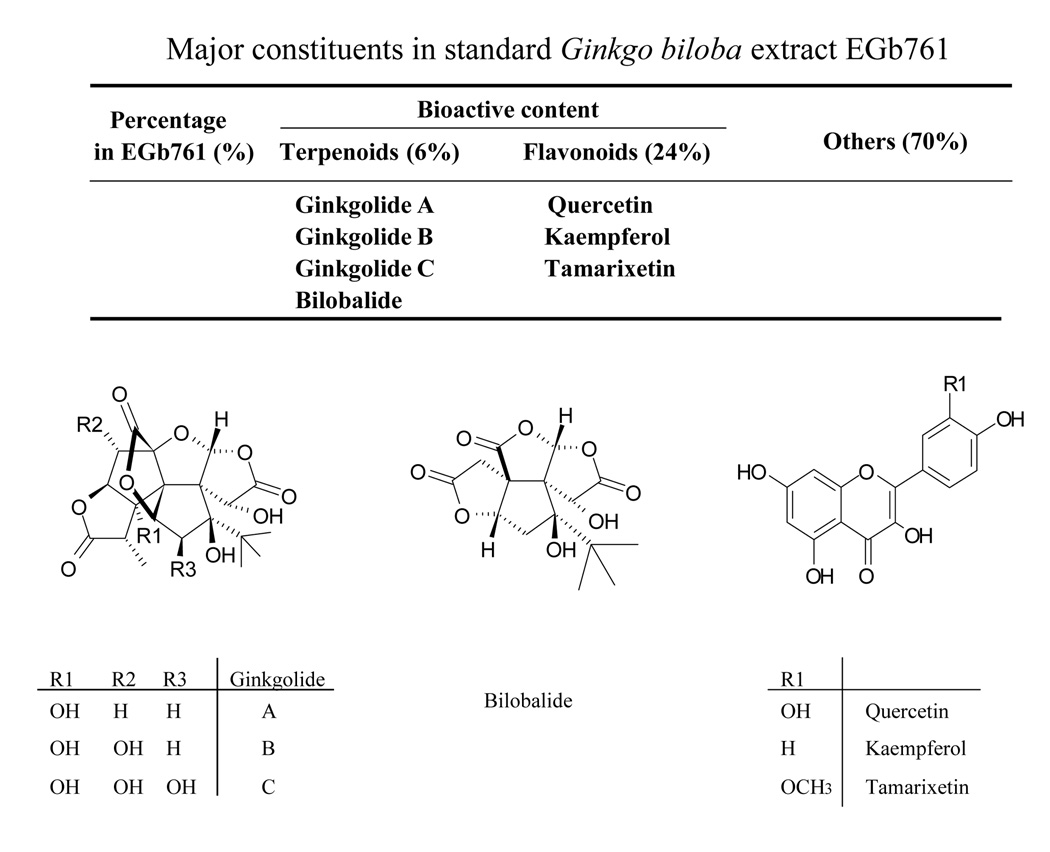
The use of Ginkgo biloba extract (GBE) as a therapeutic agent for a variety of ailments, particularly in the hopes of improving memory, has gained widespread popularity over the years. The standard GBE, EGb 761 is one of the most commonly prescribed drugs for dementia in many countries and a leading dietary supplement in the United States for memory enhancement(1). In addition, EGb 761 has also been used to treat asthma, bronchitis, fatigue, sexual dysfunctions, and other health conditions(2). EGb 761 contains two groups of bioactive constituents, the flavonoids (24%) and the terpenoids (6%), which have been actively investigated for their neuroprotective and neuromodulatory properties(3). The flavonoid fraction is primarily composed of quercetin (Que), kaempferol (Kae) and tamarixetin (Tam) (Figure 1). The terpenoid fraction primarily contains ginkgolides A (GA), B (GB), C, J and M, and bilobalide (BB), which are specific to the Ginkgo biloba tree(4). The GB is known as a potent antagonist of platelet activating factor receptor and the flavonoids impart antioxidative properties to EGb 761(5, 6).
Fig. 1.
Chemical structures and composition of bioactive terpenoids and flavonoids in EGb 761.
Despite the potentially beneficial effects of GBE, the widespread use of this herbal remedy, often as self-medication, may lead to undesired herbal-drug interactions with concurrent medications. Recently, a number of studies indicated that GBE may influence the pharmacokinetics of co-administered drugs through altering the expression and activity of major cytochrome P450 (CYP) enzymes in the liver(7, 8). Studies in rats demonstrated that administration of GBE significantly increased the expression and activity of CYP2B, CYP3A, and CYP1A(9–11). In rat primary hepatocyte cultures, GA and BB displayed distinct roles in the regulation of CYP2B1 and CYP3A23 gene expression; BB showed preferential induction of CYP2B1 over CYP3A23, while GA primarily induced the expression of CYP3A23 but not CYP2B1(12). Additionally, pretreatment with GBE potentiated acetaminophen-mediated toxicity in rat primary hepatocytes, which was deemed to have resulted from GA induced expression of CYP3A(13). Furthermore, a clinical study observed that after two weeks of GBE exposure, the AUC and Cmax of midazolam, a known CYP3A4 substrate, were reduced by 34% and 31%, respectively(7). In contrast, Curley et al.(8) reported that midazolam metabolism in 12 volunteers has no apparent changes after 28 days GBE exposure. Overall, current studies regarding GBE induction of CYPs contain contradictory results, and are far from conclusive. Data that pertain to GBE-mediated induction of human drug-metabolizing enzymes (DME) other than CYPs, in particular, are limited, and the molecular mechanisms underlying the observed inductions are largely unknown.
In recent years, transcriptional regulation of hepatic DMEs has been extensively investigated(14, 15). The pregnane X receptor (PXR; NR1I2) and the constitutive androstane receptor (CAR; NR1I3) are two closely related members of the nuclear receptor (NR) family that regulate a broad and overlapping set of genes involved in the detoxification and elimination of xenobiotics and endobiotics(16). Additionally, the aryl hydrocarbon receptor (AhR), a ligand-activated basic helix-loop-helix Per-Aant-Sim (PAS) transcription factor, regulates genes from CYP subfamily 1A and the phase II enzyme UDP-glucuronosyltransferase 1A1 (UGT1A1)(17, 18). Collectively, PXR, CAR, and AhR are considered promiscuous xenobiotic sensors that translate chemical activation into coordinated metabolism and transport in the liver. Therefore, understanding xenobiotic induction of metabolism and transport at the transcriptional level may lead to enhanced prediction of drug interactions, and xenobiotic-induced hepatotoxicity.
The objective of this study was to investigate the hypothesis that bioactive terpenoids and flavonoids of EGb 761 induce human hepatic DMEs and drug transporters through the activation of PXR, CAR, and AhR. Cell-based reporter assays and transfection experiments in HepG2 cells and human hepatocytes were used to determine the differential activation of NRs by terpenoids and flavonoids. Induction of CYP3A4, CYP2B6, CYP1A2, UGT1A1, MDR1, and MRP2 was compared between NR transfected hepatoma cells and human primary hepatocytes, which express physiologically relevant DMEs and drug transporters. Our results indicate that among the active components of EGb 761, GA and GB are potent activators of PXR and inducers of related DMEs; Que, Kae, and Tam activate PXR, CAR, and AhR, whereas BB displays no effects on these transcription factors. The flavonoids of EGb 761 induced the expression of UGT1A1 and CYP1A2 in HepG2 cells by activating the endogenous AhR. In contrast, this induction was absent in human hepatocytes presumably due to the metabolic instability of flavonoids in this physiologically relevant system.
MATERIALAND METHODS
Chemicals and Reagents
Rifampicin (RIF), 3-Methylcholanthrene (3-MC), GA, GB, and BB were purchased from Sigma-Aldrich (St. Louis, MO). CITCO was obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA). The standardized Ginkgo biloba leaf extract EGb 761 was from Schwabe Pharmaceuticals (Karlsruhe, Germany), which is currently being used in phase II clinical trials(19). The individual EGb 761 constituents Que, Kae and Tam were obtained from Dr. I. Khan of National Natural Product Research Center (Oxford, MS). Oligonucleotide primers and TaqMan fluorescent probes were synthesized by Sigma Genosys (The Woodlands, TX) and Applied Biosystems (Foster, CA), respectively. The Dual-Luciferase Reporter Assay System was purchased through Promega (Madison, WI). Matrigel, insulin and ITS+ were obtained from BD Biosciences (Bedford, MA). Other cell culture reagents were purchased from Invitrogen (Calsbad, CA) or Sigma-Aldrich.
Plasmid Constructs
The UGT1A1-gtPBREM containing a 290 base pairs (bp) sequence from UGT1A1 promoter (−3483/−3194), and the pCR3-hCAR expression vector were generously provided by Dr. Masahiko Negishi (National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC). The CMV2-hCAR3 vector was obtained from Dr. Curtis Omiecinski (Pennsylvania State University, University Park, Pennsylvania). The pSG5-hPXR expression vector was obtained from Dr. Steven Kliewer (University of Texas Southwestern Medical Center, Dallas, TX). The CYP2B6 reporter constructs, containing both PBREM and the distal XREM (CYP2B6-2.2kb) was generated as described previously(20). The pRL-TK Renilla luciferase plasmid used to normalize firefly luciferase activities was from Promega.
Induction Studies in Human Primary Hepatocyte Cultures
Liver tissues were acquired from qualified medical staff following donor consent and prior approval from the Institutional Review Board at the University of Maryland at Baltimore. Hepatocytes were isolated from human liver specimens by a modification of the two-step collagenase digestion method as previously described(21). Hepatocytes were seeded at 1.5 × 106 cells/well in six-well biocoat plates in DMEM supplemented with 5% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 4 µg/ml insulin, and 1 µM dexamethasone. After attachment at 37°C in a humidified atmosphere of 5% CO2, hepatocytes were cultured in complete William’s Medium E (WME) and overlaid with Matrigel (0.25mg/ml) as described previously(22). The hepatocytes were maintained for 36 hrs before the treatment with RIF (10 µM), CITCO (1 µM) 3-MC (5 µM), GA (50 µM), GB (50 µM), BB (50 µM), EGb 761 (100 µg/ml), Que (25 µg/ml), Kae (20 µg/ml) and Tam (10 µg/ml) for another 24 hrs.
Real-Time PCR Analysis
Total RNA was isolated using the RNeasy Mini Kit (Qiagen) and reverse transcribed using High Capacity cDNA Archive kit (Applied Biosystems, Foster, CA) following the manufacturers’ instructions. Primers and probes for CYP2B6, CYP3A4, UGT1A1, MDR1, and MRP2 genes were designed using Primer Express Version 2.0 and entered into the NCBI Blast to ensure specificity as described previously (Table 1)(23–26). A mixture of CYP1A2 primers and probe (Assay ID: Hs00167927_ml) was ordered from Applied Biosystems (Foster, CA). The mRNA expression of CYP2B6, CYP3A4, CYP1A2, UGT1A1, MDR1, or MRP2 was normalized against that of human β-actin, which was detected using a pre-developed primer/probe mixture (Applied Biosystems, Foster, CA). TaqMan PCR assays were performed in 96-well optical plates on an ABI Prism 7000 Sequence Detection System (Applied Biosystems). Fold induction values were calculated according to the equation 2ΔΔCt, where ΔCt represents the differences in cycle threshold numbers between the target gene and β-actin, and ΔΔCt represents the relative change in these differences between control and treatment groups.
Table 1.
Primers and probes used for real time PCR analysis
| Gene | Sequence | Reference | |
|---|---|---|---|
| CYP2B6 | forward primer | 5-AAGCGGATTTGTCTTGGTGAA-3 | (26) |
| reverse primer | 5-TGGAGGATGGTGGTGAAGAAG-3 | ||
| probe | 6-FAM-CATCGCCCGTGCGGAATTGTTC-TAMRA | ||
| CYP3A4 | forward primer | 5-TCAGCCTGGTGCTCCTCTATCTAT-3 | (26) |
| reverse primer | 5-AAGCCCTTATGGTAGGACAAAATATTT-3 | ||
| probe | 6-FAM-TCCAGGGCCCACACCTCTGCCT-TAMRA | ||
| UGT1A1 | forward primer | 5-GGCCCATCATGCCCAATAT-3 | (23) |
| reverse primer | 5-TTCAAATTCCTGGGATAGTGGATT-3 | ||
| probe | 6-FAM-TTTTTGTTGGTGGAATCAACTGCCTTCAC-TAMRA | ||
| MDR1 | forward primer | 5-GTCCCAGGAGCCCATCCT-3 | (25) |
| reverse primer | 5-CCCGGCTGTTGTCTCCAT-3 | ||
| probe | 6-FAM-TGACTGCAGCATTGCTGAGAACATTGC-TAMRA | ||
| MRP2 | forward primer | 5-ACGGACAGCTATCATGGCTTCT-3 | (24) |
| reverse primer | 5-TGGTCACATCCATGAGCTTCT-3 | ||
| probe | 6-FAM-ACCCTATCCAACTTGGCCAGGAAGGAGT-TAMRA |
Transient Transfection in HepG2 Cells
HepG2 cells in 24-well plates were transfected with CYP2B6-2.2kb reporter construct in the presence or absence of hPXR, hCAR or hCAR3 expression vector; or with UGT1A1-gtPBREM reporter construct in the absence of exogenous NRs using Fugene 6 Transfection Kit following the manufacturer’s instruction. Twenty four hours after transfection, cells were treated with solvent (0.1% DMSO) or test compounds at the concentration of RIF (10 µM), CITCO (1 µM), 3-MC (5 µM), GA (50 µM), GB (50 µM), BB (50 µM), EGb 761 (100 µg/ml), Que (25 µg/ml), Kae (20 µg/ml) or Tam (10 µg/ml) for 24 hrs. Subsequently, cell lysates were assayed for firefly activities normalized against the activities of cotransfected Renilla luciferase using Dual-Luciferase Kit (Promega, WI). Data were represented as mean ± S.D. of three individual transfections. To analyze PXR- and CAR-mediated induction of CYP3A4 in hepatoma cells, HepG2 cells seeded in 12-well plates were transfected with hCAR or hPXR expression vector, then treated for 24 hrs with RIF (10 µM), CITCO (1 µM), EGb 761 (100 µg/ml), Que (25 µg/ml), Kae (20 µg/ml) or Tam (10 µg/ml). Total RNA was isolated using RNeasy Mini Kit (Qiagen), and subjected to real-time RT-PCR analysis as described above.
Transfection of Human Primary Hepatocytes
Human primary hepatocytes in 24-well Biocoat plates were transfected 12 hrs after seeding using Effectene® transfection reagent (Qiagen) following the manufacturer’s instruction. Briefly, the transfection mixes contained 200 ng of CYP2B6 reporter construct (CYP2B6-2.2kb) and 25 ng of internal control plasmid (pRL-TK Renilla luciferase). Transfected cells were treated with solvent (0.1% DMSO) or test compounds at the concentration of RIF (10 µM), CITCO (1 µM), GA (50 µM), GB (50 µM), BB (50 µM), EGb 761 (100 µg/ml), Que (25 µg/ml), Kae (20 µg/ml) or Tam (10 µg/ml) for 24 hrs. Subsequently, luciferase activities were measured with hepatocyte lysates using the Dual Luciferase Reporter reagents according to the manufacturer’s instructions (Promega).
Statistical Analysis
All data represent three independent experiments and are expressed as the mean ± S.D. Statistical comparisons were made using Students’t-test. Statistical significance was set at p values <0.05 (* or #), or <0.01 (** or ##).
RESULTS
Induction of DME and drug transporter gene expression in human primary hepatocytes
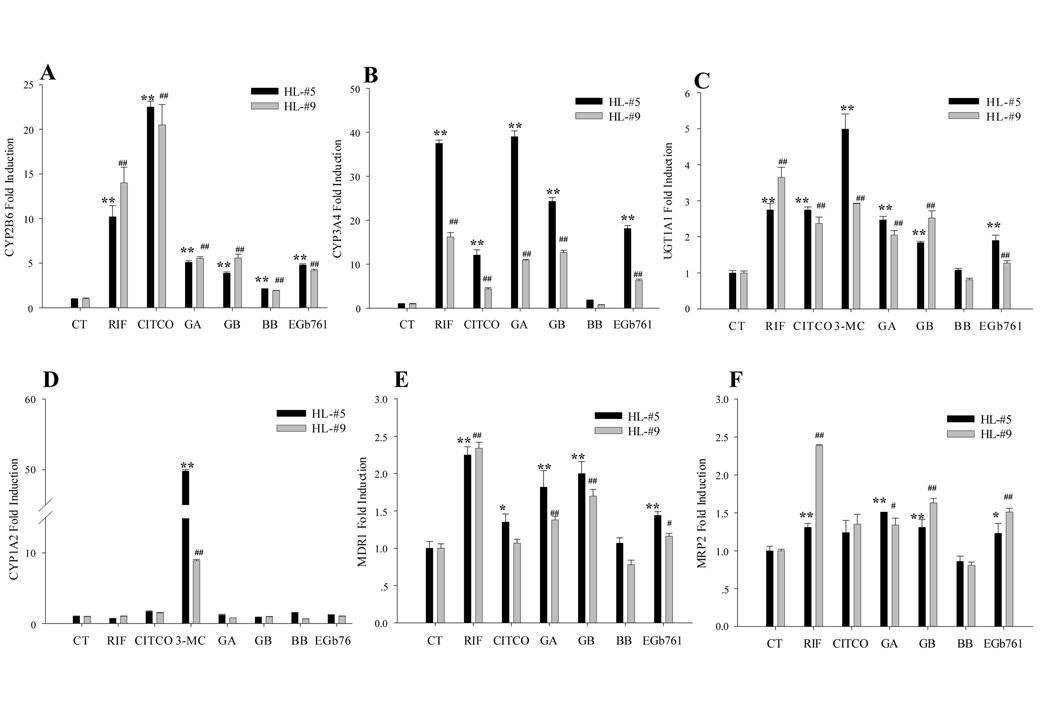
Due to the lack of phenotypic DME expression in nearly all immortalized cell lines, human primary hepatocyte cultures have been considered as the ‘gold standard’ in vitro model for studying drug metabolism(27). In the current study, we evaluated the effects of EGb 761 and its active constituents on the expression of CYP2B6, CYP3A4, CYP1A2, UGT1A1, MDR1, and MRP2 genes in human primary hepatocytes using real-time RT-PCR assays. As expected, the positive control RIF and CITCO efficiently induced CYP2B6, CYP3A4, MDR1, and MRP2, while 3-MC exhibited marked induction of both UGT1A1 and CYP1A2 (Figure 2). In human hepatocytes, EGb 761 (100 µg/ml), GA, and GB at 50 µM significantly induced the expressions of a series of PXR and CAR target genes including CYP2B6, CYP3A4, UGT1A1, MDR1, and MRP2, whereas BB (50 µM), exhibited only modest effects on the expression of the tested DMEs and drug transporters (Fig. 2).
Fig. 2.
Induction of DMEs and transporters in human primary hepatocytes. Human hepatocytes (HL-#5 and HL-#9) cultured in WME were treated for 24 hrs with RIF (10 µM), CITCO (1 µM), 3-MC (5 µM), GA (50 µM), GB (50 µM), BB (50µM), and EGb 761 (100 µg/ml), respectively. Total RNA was isolated, reverse transcribed, and subjected to TaqMan real-time PCR. The expression level of mRNA for CYP2B6 (A), CYP3A4 (B), UGT1A1 (C), CYP1A2 (D), MDR1 (E), and MRP2 (F) were normalized against β-actin. Inductions of these enzymes relative to vehicle control were calculated as described under “Materials and Methods”. All data are expressed as mean ± S.D. **, ## p<0.01; *, # p<0.05.
Activation of human PXR, CAR and AhR by EGb 761 and its bioactive components
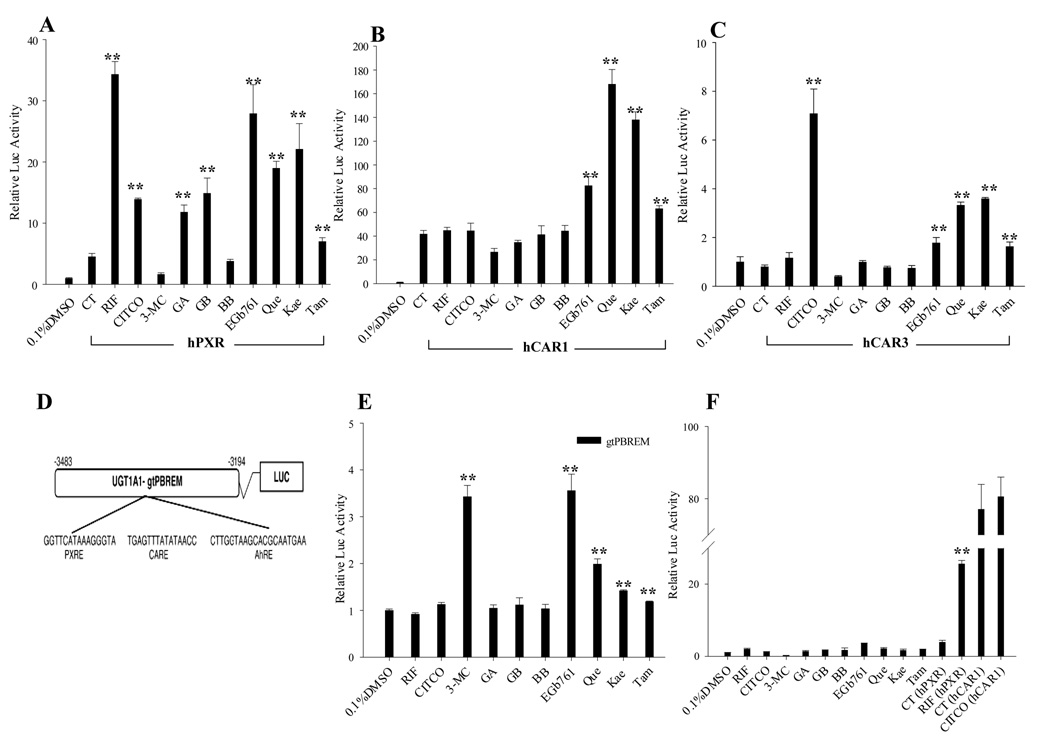
Given that CYP2B6 and CYP3A4 are typical target genes for hCAR and hPXR, and UGT1A1 can be induced by the activation of hCAR, hPXR and hAhR, we further investigated the ability of EGb 761 and its bioactive components to activate these receptors in cell-based reporter experiments. As shown in Fig. 3A, EGb 761, GA, GB, Que, Kae, and Tam showed significant increases of hPXR-mediated CYP2B6 reporter activities. In contrast, only marginal activation of hPXR was observed after the treatment of BB, which was in agreement with the negligible induction of CYP2B6 and CYP3A4 by BB in human hepatocytes. Intriguingly, EGb 761 and its flavonoids, especially Que and Kae, increased the activity of hCAR to an extent that exceeds that of CITCO treatment in transfected HepG2 cells (Fig. 3B). Utilizing a hCAR splicing variant (hCAR3) expression vector, our reporter assay also revealed significant activation of hCAR3 by EGb 761, Que, Kae, and Tam (Fig. 3C). Furthermore, transfection of CYP2B6-2.2kb in the absence of exogenous NRs attest that endogenous PXR and CAR in HepG2 cells only exhibit negligible activation of the reporter compared with the potent response when exogenous PXR or CAR was included (Fig. 3F). To determine whether the induction of UGT1A1 in human hepatocytes is correlated with the activation of AhR, HepG2 cells, which endogenously express abundant AhR, were transfected with UGT1A1-gtPBREM reporter construct alone(28). Notably, only EGb 761 and its flavonoids increased the expression of UGT1A1-gtPBREM reporter gene, whereas all the terpenoids tested exhibit no effects on AhR activity (Fig. 3E).
Fig. 3.
EGb 761 and its active constituents increase the activities of hPXR, hCAR, and hAhR. HepG2 cells were transfected with hPXR (A), hCAR (B), hCAR3 (C) expression vectors or without exogenous NRs (F) in the presence of CYP2B6-2.2kb reporter construct; or with the UGT1A1-gtPBREM reporter construct in the absence of exogenous NRs (E). Transfected cells were then treated with GA (50 µM), GB (50 µM), BB (50 µM), EGb 761 (100 µg/ml), Que (25 µg/ml), Kae (20 µg/ml) or Tam (10 µg/ml) for 24 hrs. CITCO (1 µM), RIF (10 µM), and 3-MC (5 µM) were used as positive control for hCAR, hPXR and hAhR, respectively. Luciferase activities were determined and expressed relative to vehicle control. Data represent the mean ± S.D. (n=3).
Flavonoids of EGb 761 failed to induce the expression of DMEs and transporters in human primary hepatocytes
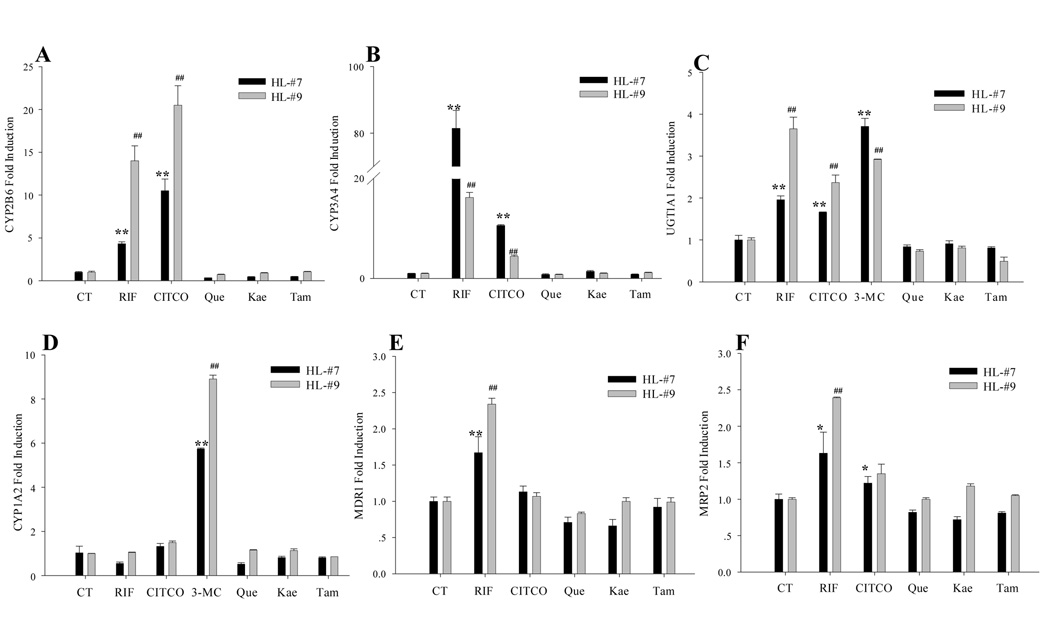
Results obtained from cell-based reporter assays indicated that flavonoids of EGb 761 activate multiple xenobiotic receptors including PXR, CAR, and AhR. To determine if the flavonoids contributed to the observed induction by EGb 761 in human hepatocytes, cultured human primary hepatocytes were treated for 24 hrs with Que (25 µg/ml), Kae (20 µg/ml), Tam (10 µg/ml), or vehicle and positive controls. Surprisingly, all flavonoids of EGb 761 failed to induce any tested DMEs or drug transporters in human hepatocytes as showed in Fig. 4, revealing a discrepancy between the potent activation of NRs in HepG2 cells and the lack of induction of their target genes in human hepatocytes.
Fig. 4.
Effects of flavonoids of EGb 761 on the expression of DMEs and transporters in human primary hepatocytes. Human hepatocytes (HL-#7 and HL-#9) cultured in WME were treated for 24 hrs with RIF (10 µM), CITCO (1 µM ), 3-MC (5 µM), Que (25 µg/ml), Kae (20 µg/ml) or Tam (10 µg/ml), respectively. Total RNA was isolated, and real-time PCR were performed as described under “Materials and Methods”. CYP2B6 (A), CYP3A4 (B), UGT1A1 (C), CYP1A2 (D), MDR1 (E), and MRP2 (F) expression levels were normalized against β-actin. Inductions of these enzymes relative to vehicle control were calculated. All data are expressed as mean ± S.D. **, ## p<0.01; *, # p<0.05.
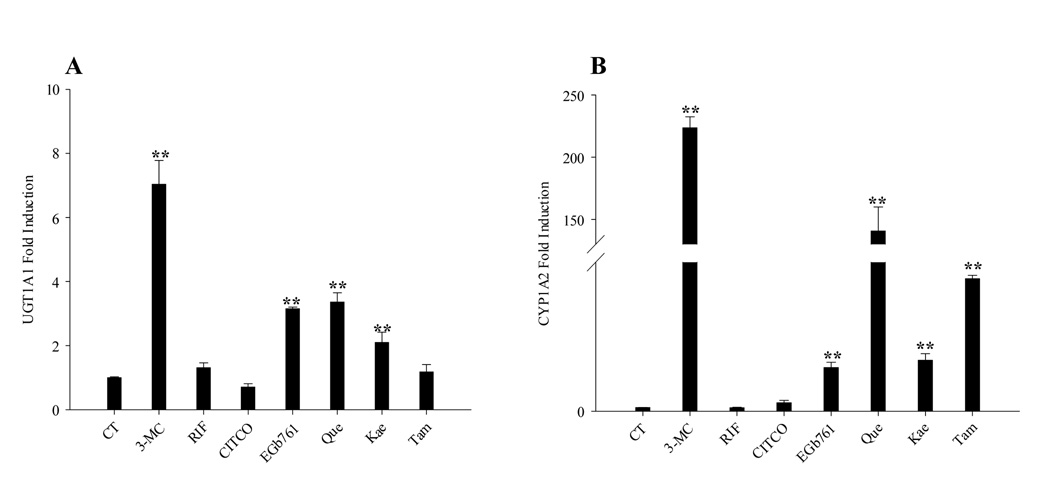
EGb 761 and its flavonoids induce the expression of UGT1A1 and CYP1A2 in HepG2 cells
Since endogenous AhR is expressed in HepG2 cells, the induction of UGT1A1 and a typical AhR target gene CYP1A2 was further examined in HepG2 cells following the treatment with EGb 761 and its flavonoids for 48 hrs. In contrast to the results from human hepatocytes, flavonoids of EGb 761 are associated with potent induction of UGT1A1 and CYP1A2 mRNA expression in treated HepG2 cells (Fig. 5, A and B), whereas typical activators of PXR (RIF 10 µM) and CAR (CITCO 1 µM) demonstrated no effects on UGT1A1 induction due to the negligible expression of these receptors in HepG2 cells(29, 30).
Fig. 5.
EGb 761 and its flavonoids induce the expression of CYP1A2 and UGT1A1 in HepG2 cells through the activation of endogenous AhR. HepG2 cells were treated for 48 hrs with RIF (10 µM), CITCO (1 µM), 3-MC (5 µM), EGb 761 (100 µg/ml), Que (25 µg/ml), Kae (20 µg/ml) or Tam (10 µg/ml), respectively. UGT1A1 (A), and CYP1A2 (B) expression levels were determined using TaqMan real-time PCR as detailed in “Material and Methods”. Inductions relative to vehicle control were calculated, and all data are expressed as mean ± S.D. (n=3). **, p<0.01.
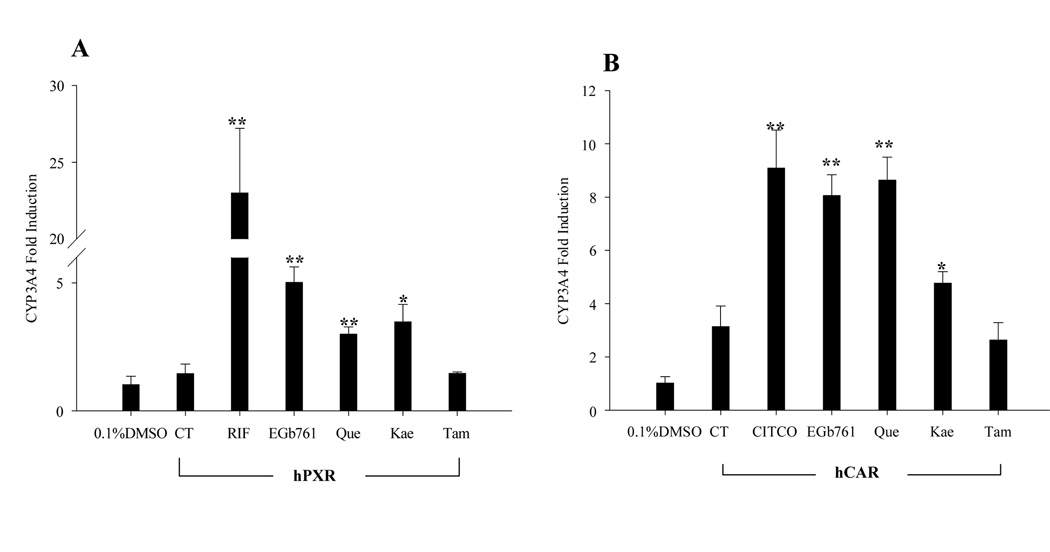
EGb 761 and its flavonoids increased hCAR- and hPXR-mediated induction of CYP3A4 expression in HepG2 cells
To determine whether the lack of CYP3A4 induction in HepG2 cells by the flavonoids of EGb 761 was due to the lack of PXR and CAR expression, HepG2 cells transfected with hCAR or hPXR expression vector were exposed to EGb 761, Que, Kae and Tam. Consistent with the results from cell-based reporter assays (Fig. 3) and the fact that both CAR and PXR regulate drug-induced CYP3A4 gene expression(31, 32), our results showed that EGb 761, Que and Kae induced CYP3A4 mRNA expression in hCAR- and hPXR-transfected HepG2 cells through activation of these receptors (Fig. 6).
Fig. 6.
Flavonoids of EGb 761 induce the expression of CYP3A4 in HepG2 cells transfected with PXR, or CAR. HepG2 cells were transfected with hPXR (A), or hCAR (B) expression vector, and treated with RIF (10 µM), CITCO (1 µM), EGb 761 (100 µg/ml), Que (25 µg/ml), Kae (20 µg/ml) or Tam (10 µg/ml) for 24 hrs. Total RNA was collected, reverse transcribed, and subjected to TaqMan real-time PCR as described in “Materials and Methods”. All data are expressed as mean ± S.D. (n=3), (*, p<0.05; **, p<0.01).
Flavonoids of EGb 761 were unable to transactivate CYP2B6 reporter gene expression in human primary hepatocytes
In contrast to immortalized cell lines, cultured primary hepatocytes retain the expression of most endogenous transcription factors such as nuclear receptors(33). To determine if EGb 761 and its active components could transactivate CYP2B6 reporter gene expression through endogenous transcription factors, CYP2B6 reporter gene construct was transfected into human primary hepatocytes without the co-transfection of exogenous nuclear receptors. As indicated in Fig. 7, EGb 761, GA and GB were associated with enhanced expression of CYP2B6-2.2kb reporter gene, presumably through the endogenous PXR and CAR, whereas no activation was observed after the treatment with Que, Kae, and Tam. These results are consistent with the actual induction profiles of terpenoids and flavonoids in human primary hepatocytes.
Fig. 7.
Flavonoids of EGb 761 failed to transactivate CYP2B6 reporter gene expression in human primary hepatocytes. Human primary hepatocytes (HL-#10 and HL-#13) in 24-well Biacoat plates were transfected with CYP2B6 reporter vector (CYP2B6-2.2kb) then treated with RIF (10 µM), CITCO (1 µM), 3-MC (5 µM), GA (50 uM), GB (50 uM), BB (50 uM), EGb 761 (100 µg/ml), Que (25 µg/ml), Kae (20 µg/ml), or Tam (10 µg/ml) for 24 hrs. Dual luciferase activities were measured following the manufacturer’s protocol. Data represent the mean ± S.D. for each treatment group (n=3), (*, p<0.05; **, p<0.01).
DISCUSSION
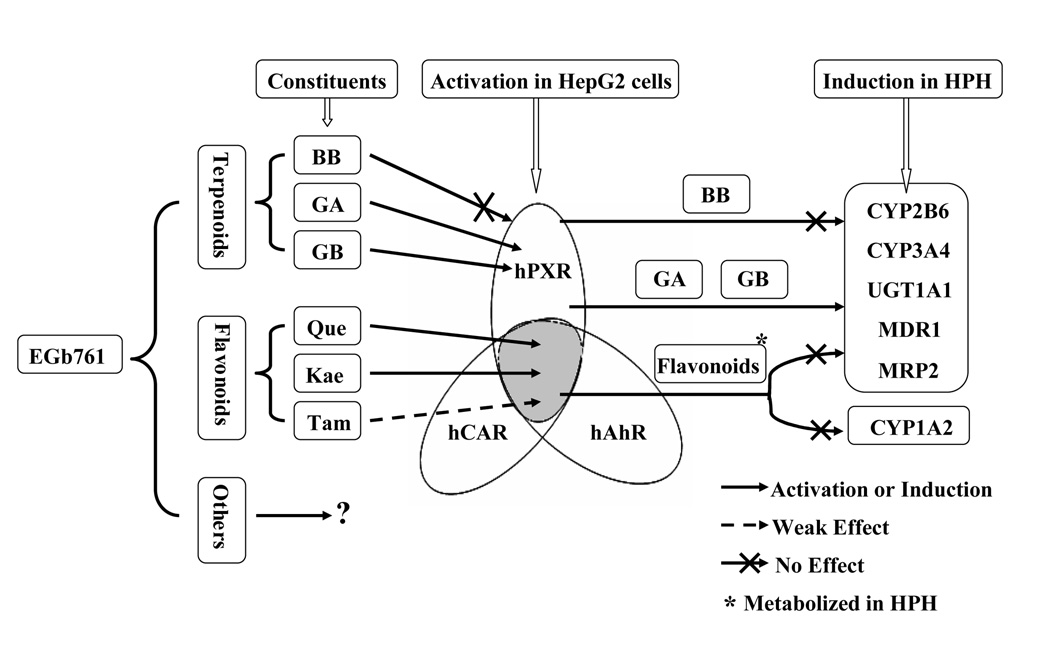
Due to the widespread use of Ginkgo biloba extract as an alternative medication, its beneficial and detrimental effects have generated a heightened awareness. Recently, a number of reports revealed that GBE increased the expression of several major CYP enzymes in the liver thus may exert pharmacokinetic-based herbal-drug interactions with concurrent remedies(7, 9). However, the molecular mechanisms underlying the effects of GBE, especially its bioactive components, are largely unexplored. We have demonstrated that terpenoids and flavonoids of EGb 761 differentially induce the expression of hepatic CYP2B6, CYP3A4, UGT1A1, MDR1, and MRP2 through selective activation of PXR, CAR, and AhR. Among the bioactive constituents of EGb 761, terpenoids GA and GB activated PXR and induced PXR target genes in human primary hepatocytes; while the flavonoids exhibited significant activation of PXR, CAR, and AhR along with the induction of related target genes in transfected HepG2 cells, but not in human hepatocyte cultures. This evidence suggests that EGb 761 and its bioactive terpenoids and flavonoids reciprocally activate major xenobiotic receptors and induce the expression of their target genes thereafter. In human hepatocytes, the observed induction by EGb 761 is primarily attributed to the active terpenoids not to the flavonoids (Fig. 8).
Fig. 8.
Schematic representation of EGb 761-mediated NR activation and DME induction. This figure represents major bioactive terpenoids and flavonoids of EGb 761 in activating xenobiotic receptors PXR, CAR, and AhR in cell line, and inducing key DMEs and transporters in human primary hepatocytes. *: flavonoids are metabolically unstable in human primary hepatocytes.
The liver is endowed with the metabolic capability of detoxifying natural and synthetic compounds including therapeutic drugs. Cultured human primary hepatocytes, which closely resemble the physiological gene expression of human liver, have become a standard in vitro model for studying drug metabolism(27). Using human hepatocytes, we initially tested the effects of EGb 761 and its terpenoids on the expression of DMEs and transporters, including CYP2B6, CYP3A4, CYP1A2, UGT1A1, MDR1 and MRP2. Our data revealed that the expression of UGT1A1, MDR1, and MRP2 were induced by the treatment of EGb 761, GA, or GB. To our knowledge, this is the first study to demonstrate that EGb 761 and its terpenoids increased the expression of key phase II DME, and drug transporters in human primary hepatocyte cultures. In agreement with a previous study, our results also demonstrated EGb 761, GA and GB induced the expression of CYP3A4 and CYP2B6(34). However, BB at 50 µM, failed to induce the tested DMEs and transporters, which is in contrast to a recent report by Deng et al.(35). Although different drug concentrations have been used in Deng’s and our studies, the various sources of BB used for these two studies may also contribute to the differential results. These results indicate that GBE may affect the pharmacokinetic profile of co-administered drugs by altering their hepatic metabolism as well as efflux. Given that all these tested DMEs and transporters are typical target genes for xenobiotic receptor PXR , CAR or AhR(16, 17, 36), it would be of interest to determine whether bioactive components of EGb 761 induce hepatic drug metabolism and transport through the activation of these xenobiotic receptors.
In vitro cell-based reporter assays have been used extensively for identifying activators of various nuclear receptors, and often significant correlation between the activation of NR in reporter assays and the induction of enzymes in primary hepatocytes has been achieved. Our data obtained from reporter assays showed that treatment of EGb 761 or its flavonoids resulted in robust activation of PXR, CAR, and AhR in transfected HepG2 cells. Interestingly, treatment with Que and Kae led to an unexpectedly potent activation of hCAR in HepG2 cells. Since hCAR is constantly activated in immortalized cells, and the mechanisms underlying CAR activation are yet elusive, additional experiments are required in order to properly explain this observed activation of hCAR. Recently, one of the human CAR splicing variants, hCAR3, has been characterized with low basal activity, but efficient ligand activation in several cell lines(26, 37). Our current study showed that similar to the known hCAR ligand CITCO, the flavonoids and EGb 761 significantly increased the hCAR3 activation, indicating their potential agonistic roles to hCAR. Because HepG2 cells express sufficient endogenous AhR, but negligible level of CAR and PXR proteins(28–30), experiments were conducted to determine the activation of UGT1A1-gtPBREM reporter gene through the endogenous AhR. Our reporter assay demonstrated that EGb 761 and its flavonoids significantly enhanced the gt-BBREM luciferase expression through the activation of endogenous AhR, whereas all the terpenoids exhibited no effects on AhR activation, suggesting flavonoids but not terpenoids contribute to the EGb 761 activation of AhR. Additionally, we also demonstrated that GA and GB selectively activate hPXR- but not CAR- or AhR-mediated luciferase reporter; this is in agreement with two recent reports where GA and GB at 10–100 µM, and GBE at 200–800 µg/ml demonstrated potent activation of PXR in cell-based reporter assays(38, 39).
Contradictory results have been published in the literature regarding GBE-mediated induction of CYP1A2, a typical target gene of AhR. These reports demonstrate various effects of GBE on the CYP1A2 expression, including induction, non-effects, and repression(10, 40, 41). Our studies in human hepatocytes showed no induction of CYP1A2 by EGb 761 or its terpenoids. Moreover, in contrast to our reporter assay data obtained from hepatoma cell line, treatment of human primary hepatocytes with all three flavonoids resulted in no induction of the tested DMEs and transporters. Because human primary hepatocytes maintain physiologically relevant amount of DMEs, and are capable of eliminating drugs more efficiently compared with immortalized cell lines(27), one speculation for the observed discrepancy is that flavonoids of EGb 761 were quickly metabolized in human primary hepatocyte cultures.
To explore this possibility, we took the advantage of HepG2 cells, in which AhR, but not CAR or PXR is abundantly expressed, and limited overall metabolic capability has been reported(28–30). In agreement with our cell-based reporter results, increased expression of UGT1A1 and CYP1A2 mRNA has been observed in HepG2 cells following the treatment of EGb 761 and its flavonoids. Moreover, transient transfection of hPXR or hCAR in HepG2 cells resulted in EGb 761, Que, and Kae mediated induction of endogenous CYP3A4. Indeed, several previous studies have demonstrated that intact flavonoids of EGb 761 are not present in the systemic circulation, due to the intensive metabolic activity of the gastrointestinal tract and liver(42, 43). Our reporter assay in human primary hepatocytes further confirmed that EGb 761 and its terpenoids significantly increased the expression of CYP2B6 reporter gene through the endogenous nuclear receptors, while the flavonoids failed to do so. Although detailed analysis of the flavonoids and their metabolites are required in order to define the role of flavonoids in EGb 761-mediated enzyme induction, current results support the opinion that flavonoids of EGb 761 are metabolically unstable.
In summary, our data suggest that EGb 761 induced the expression of multiple hepatic DMEs and transporters including CYP2B6, CYP3A4, UGT1A1, MDR1, and MRP2 through the activation of xenobiotic receptors PXR, CAR, and AhR. In the bioactive ingredients of EGb 761, terpenoids GA and GB activated PXR and induced related target genes in human hepatocytes; the flavonoids exhibited contradictory results between immortalized cell line and human primary hepatocytes, where significant activation of PXR, CAR, and AhR, along with induction of their target genes were observed in the former but not the later in vitro model. Therefore, EGb 761-induced expression of DMEs and transporters is predominantly attributed to the active terpenoids but not to the flavonoids.
Acknowledgments
The authors are grateful to Dr. Masahiko Negishi (National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC) for kindly providing the pCR3-hCAR, and UGT1A1-gtPBREM vectors; Dr. Curtis Omiecinski (Pennsylvania State University, University Park, Pennsylvania) for the CMV2-hCAR expression vector; and Dr. Steve Kliewer (University of Texas, Southwestern Medical Center, Dallas, TX) for the pSG5-hPXR expression vector. Human liver tissues were procured with the assistance of John Cottrell from the University of Maryland Medical Center (Baltimore, MD). This research was supported by National Institute of Health Grant (R01, DK061652).
ABBREVIATIONS
- GBE
ginkgo biloba extract
- NR
nuclear receptor
- CAR
constitutive androstane receptor
- PXR
pregnane X receptor
- AhR
aryl hydrocarbon receptor
- CYP
cytochrome P450
- UGT
UDP-glucuronosyltranferases
- CITCO
6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichloro-benzyl)-oxime
- RIF
rifampicin
- 3-MC
3-methylcholanthrene
- GA
ginkgolide A
- GB
ginkgolide B
- BB
bilobalide
- Que
quercetin
- Kae
kaempferol
- Tam
tamarixetin
- gtPBREM
UGT1A1 phenobarbital-responsive enhancer module.
References
- 1.Elovicand EP, Zafonte RD. Ginkgo biloba: applications in traumatic brain injury. J Head Trauma Rehabil. 2001;16:603–607. doi: 10.1097/00001199-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 2.DeFeudis FV. Ginkgo biloba extract (EGb 761): from chemistry to clinic. Publi Ullstein Med. 1998 [Google Scholar]
- 3.Smithand JV, Luo Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol. 2004;64:465–472. doi: 10.1007/s00253-003-1527-9. [DOI] [PubMed] [Google Scholar]
- 4.Jaracz S, Malik S, Nakanishi K. Isolation of ginkgolides A, B, C, J and bilobalide from G. biloba extracts. Phytochemistry. 2004;65:2897–2902. doi: 10.1016/j.phytochem.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Smith PF, Maclennan K, Darlington CL. The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF) J Ethnopharmacol. 1996;50:131–139. doi: 10.1016/0378-8741(96)01379-7. [DOI] [PubMed] [Google Scholar]
- 6.Smithand JV, Luo Y. Elevation of oxidative free radicals in Alzheimer's disease models can be attenuated by Ginkgo biloba extract EGb 761. J Alzheimers Dis. 2003;5:287–300. doi: 10.3233/jad-2003-5404. [DOI] [PubMed] [Google Scholar]
- 7.Robertson SM, Davey RT, Voell J, Formentini E, Alfaro RM, Penzak SR. Effect of Ginkgo biloba extract on lopinavir, midazolam and fexofenadine pharmacokinetics in healthy subjects. Curr Med Res Opin. 2008;24:591–599. doi: 10.1185/030079908x260871. [DOI] [PubMed] [Google Scholar]
- 8.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CY. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clinical pharmacology and therapeutics. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 9.Shinozuka K, Umegaki K, Kubota Y, Tanaka N, Mizuno H, Yamauchi J, Nakamura K, Kunitomo M. Feeding of Ginkgo biloba extract (GBE) enhances gene expression of hepatic cytochrome P-450 and attenuates the hypotensive effect of nicardipine in rats. Life sciences. 2002;70:2783–2792. doi: 10.1016/s0024-3205(02)01530-8. [DOI] [PubMed] [Google Scholar]
- 10.Deng Y, Bi HC, Zhao LZ, He F, Liu YQ, Yu JJ, Ou ZM, Ding L, Chen X, Huang ZY, Huang M, Zhou SF. Induction of cytochrome P450s by terpene trilactones and flavonoids of the Ginkgo biloba extract EGb 761 in rats. Xenobiotica. 2008;38:465–481. doi: 10.1080/00498250701883233. [DOI] [PubMed] [Google Scholar]
- 11.Chang TK, Chen J, Yeung EY. Effect of Ginkgo biloba extract on procarcinogen-bioactivating human CYP1 enzymes: identification of isorhamnetin, kaempferol, and quercetin as potent inhibitors of CYP1B1. Toxicology and applied pharmacology. 2006;213:18–26. doi: 10.1016/j.taap.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Chang TK, Chen J, Teng XW. Distinct role of bilobalide and ginkgolide A in the modulation of rat CYP2B1 and CYP3A23 gene expression by Ginkgo biloba extract in cultured hepatocytes. Drug metabolism and disposition: the biological fate of chemicals. 2006;34:234–242. doi: 10.1124/dmd.105.005751. [DOI] [PubMed] [Google Scholar]
- 13.Rajaraman G, Chen J, Chang TK. Ginkgolide A contributes to the potentiation of acetaminophen toxicity by Ginkgo biloba extract in primary cultures of rat hepatocytes. Toxicol Appl Pharmacol. 2006;217:225–233. doi: 10.1016/j.taap.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 15.LeCluyse EL. Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics. Chem Biol Interact. 2001;134:283–289. doi: 10.1016/s0009-2797(01)00163-6. [DOI] [PubMed] [Google Scholar]
- 16.Wangand H, LeCluyse EL. Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet. 2003;42:1331–1357. doi: 10.2165/00003088-200342150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Sugatani J, Kojima H, Ueda A, Kakizaki S, Yoshinari K, Gong QH, Owens IS, Negishi M, Sueyoshi T. Hepatology (Baltimore, Md. Vol. 33. 2001. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR; pp. 1232–1238. [DOI] [PubMed] [Google Scholar]
- 18.Yueh MF, Huang YH, Hiller A, Chen S, Nguyen N, Tukey RH. Involvement of the xenobiotic response element (XRE) in Ah receptor-mediated induction of human UDP-glucuronosyltransferase 1A1. J Biol Chem. 2003;278:15001–15006. doi: 10.1074/jbc.M300645200. [DOI] [PubMed] [Google Scholar]
- 19.DeKosky ST, Fitzpatrick A, Ivesv DG, Saxton J, Williamson J, Lopez OL, Burke G, Fried L, Kuller LH, Robbins J, Tracy R, Woolard N, Dunn L, Kronmal R, Nahin R, Furberg C. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemporary clinical trials. 2006;27:238–253. doi: 10.1016/j.cct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, LeCluyse EL. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem. 2003;278:14146–14152. doi: 10.1074/jbc.M212482200. [DOI] [PubMed] [Google Scholar]
- 21.LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, Richert L. Isolation and culture of primary human hepatocytes. Methods Mol Biol. 2005;290:207–229. doi: 10.1385/1-59259-838-2:207. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Chen T, Stanton JD, Sueyoshi T, Negishi M, Wang H. The peripheral benzodiazepine receptor ligand 1-(2-Chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol Pharmacol. 2008;74:443–453. doi: 10.1124/mol.108.046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith CM, Graham RA, Krol WL, Silver IS, Negishi M, Wang H, Lecluyse EL. Differential UGT1A1 induction by chrysin in primary human hepatocytes and HepG2 Cells. The Journal of pharmacology and experimental therapeutics. 2005;315:1256–1264. doi: 10.1124/jpet.105.090795. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura M, Yoshitsugu H, Naito S, Hiraoka I. Evaluation of gene induction of drug-metabolizing enzymes and transporters in primary culture of human hepatocytes using high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi. 2002;122:339–361. doi: 10.1248/yakushi.122.339. [DOI] [PubMed] [Google Scholar]
- 25.Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane X receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 26.Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, Negishi M, Wang H. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320:72–80. doi: 10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13:343–368. doi: 10.1016/s0928-0987(01)00135-x. [DOI] [PubMed] [Google Scholar]
- 28.Roberts EA, Johnson KC, Harper PA, Okey AB. Characterization of the Ah receptor mediating aryl hydrocarbon hydroxylase induction in the human liver cell line Hep G2. Arch Biochem Biophys. 1990;276:442–450. doi: 10.1016/0003-9861(90)90743-i. [DOI] [PubMed] [Google Scholar]
- 29.Rencurel F, Stenhouse A, Hawley SA, Friedberg T, Hardie DG, Sutherland C, Wolf CR. AMP-activated protein kinase mediates phenobarbital induction of CYP2B gene expression in hepatocytes and a newly derived human hepatoma cell line. J Biol Chem. 2005;280:4367–4373. doi: 10.1074/jbc.M412711200. [DOI] [PubMed] [Google Scholar]
- 30.Lemaire G, de Sousa G, Rahmani R. A PXR reporter gene assay in a stable cell culture system: CYP3A4 and CYP2B6 induction by pesticides. Biochem Pharmacol. 2004;68:2347–2358. doi: 10.1016/j.bcp.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 31.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. The Journal of clinical investigation. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodwin B, Hodgson E, D'Costa DJ, Robertson GR, Liddle C. Transcriptional regulation of the human CYP3A4 gene by the constitutive androstane receptor. Molecular pharmacology. 2002;62:359–365. doi: 10.1124/mol.62.2.359. [DOI] [PubMed] [Google Scholar]
- 33.Li AP, Maurel P, Gomez-Lechon MJ, Cheng LC, Jurima-Romet M. Preclinical evaluation of drug-drug interaction potential: present status of the application of primary human hepatocytes in the evaluation of cytochrome P450 induction. Chem Biol Interact. 1997;107:5–16. doi: 10.1016/s0009-2797(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 34.He N, Cai HB, Xie HG, Collins X, Edeki TI, Strom SC. Induction of cyp3a in primary cultures of human hepatocytes by ginkgolides a and B. Clinical and experimental pharmacology & physiology. 2007;34:632–635. doi: 10.1111/j.1440-1681.2007.04630.x. [DOI] [PubMed] [Google Scholar]
- 35.Deng Y, Bi H, Zhao L, Wang X, Chen J, Ou Z, Ding L, Xu L, Guan S, Chen X, Zhou S, Huang M. Induction of Cytochrome P450 3A by the Ginkgo biloba Extract and Bilobalides in Human and Rat Primary Hepatocytes. Drug Metabolism Letters. 2008;2:60–66. doi: 10.2174/187231208783478489. [DOI] [PubMed] [Google Scholar]
- 36.Xie W, Yeuh MF, Radominska-Pandya A, Saini SP, Negishi Y, Bottroff BS, Cabrera GY, Tukey RH, Evans RM. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci U S A. 2003;100:4150–4155. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auerbach SS, Stoner MA, Su S, Omiecinski CJ. Retinoid X receptor-alpha-dependent transactivation by a naturally occurring structural variant of human constitutive androstane receptor (NR1I3) Mol Pharmacol. 2005;68:1239–1253. doi: 10.1124/mol.105.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satsu H, Hiura Y, Mochizuki K, Hamada M, Shimizu M. Activation of pregnane X receptor and induction of MDR1 by dietary phytochemicals. Journal of agricultural and food chemistry. 2008;56:5366–5373. doi: 10.1021/jf073350e. [DOI] [PubMed] [Google Scholar]
- 39.Yeung EY, Sueyoshi T, Negishi M, Chang TK. Identification of Ginkgo biloba as a Novel Activator of Pregnane X Receptor. Drug metabolism and disposition: the biological fate of chemicals. 2008 doi: 10.1124/dmd.108.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umegaki K, Taki Y, Endoh K, Taku K, Tanabe H, Shinozuka K, Sugiyama T. Bilobalide in Ginkgo biloba extract is a major substance inducing hepatic CYPs. J Pharm Pharmacol. 2007;59:871–877. doi: 10.1211/jpp.59.6.0014. [DOI] [PubMed] [Google Scholar]
- 41.Hellum BH, Hu Z, Nilsen OG. The induction of CYP1A2, CYP2D6 and CYP3A4 by six trade herbal products in cultured primary human hepatocytes. Basic Clin Pharmacol Toxicol. 2007;100:23–30. doi: 10.1111/j.1742-7843.2007.00011.x. [DOI] [PubMed] [Google Scholar]
- 42.Sesink AL, O'Leary KA, Hollman PC. Quercetin glucuronides but not glucosides are present in human plasma after consumption of quercetin-3-glucoside or quercetin-4'-glucoside. The Journal of nutrition. 2001;131:1938–1941. doi: 10.1093/jn/131.7.1938. [DOI] [PubMed] [Google Scholar]
- 43.Graefe EU, Wittig J, Mueller S, Riethling AK, Uehleke B, Drewelow B, Pforte H, Jacobasch G, Derendorf H, Veit M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. Journal of clinical pharmacology. 2001;41:492–499. doi: 10.1177/00912700122010366. [DOI] [PubMed] [Google Scholar]