Abstract
DNA vaccines that encode encephalitogenic sequences in tandem can protect from subsequent experimental autoimmune encephalomyelitis induced with the corresponding peptide. The mechanism for this protection and, in particular, if it is specific for the amino acid sequence encoding the vaccine are not known. We show here that a single amino acid exchange in position 79 from serine (nonself) to threonine (self) in myelin basic protein peptide MBP68–85, which is a major encephalitogenic determinant for Lewis rats, dramatically alters the protection. Moreover, vaccines encoding the encephalitogenic sequence MBP68–85 do not protect against the second encephalitogenic sequence MBP89–101 in Lewis rats and vice versa. Thus, protective immunity conferred by DNA vaccination exquisitely discriminates between peptide target autoantigens. No bystander suppression was observed. The exact underlying mechanisms remain elusive because no simple correlation between impact on ex vivo responses and protection against disease were noted.
DNA vaccination can protect from autoimmune diseases. The mechanisms involved are poorly understood and may well differ between different DNA constructs and ways of application. A mechanism involving immune deviation was suggested in experiments in which DNA vaccination with a construct encoding a T cell receptor (TCR) β chain resulted in a T helper 2 (Th2) shift of encephalitogenic T cells, thus protecting Pl/J mice from experimental autoimmune encephalomyelitis (EAE) (1). With another approach, a form of anergy was implied, because myelin basic protein (MBP) peptides in tandem as DNA vaccine in contrast resulted in reduction of a Th1 immune response of encephalitogenic T cells without a measurable increase of Th2 type responses (2).
Immune responses after DNA vaccination have been analyzed more thoroughly when applied for infectious diseases (3). Sequences encoding foreign antigenic stretches induce strong, antigen-specific T and B cell responses that are able to protect from subsequent disease. MHC class I-restricted CD8+ T cell responses mainly have been described, whereas less is known about MHC class II-restricted CD4+ T cell responses. Even in these more well studied situations, there is little information on the specificity requirements of the vaccines. Does protective vaccination also act on closely related peptides both in context of infectious disease and autoimmunity? Crossreactivity of the resulting immune response would be plausible in view of the recently emphasized high degeneracy of T cell recognition of antigens (4, 5). These questions are of importance for several reasons. First, DNA vaccination with foreign antigens might lead to unwanted recognition of self-antigens with autoimmunity as a consequence. Second, DNA vaccination against autoimmunity might affect natural immunity against infections. Third, in human autoimmune disease, the disease-promoting triggers are not defined with regard to exact amino acid sequence (exogenous host-mimicking vs. purely self) (6). Furthermore, epitope spreading might result in a disease-promoting immune response (7). Thus, if DNA vaccines are to be used therapeutically or prophylactically, one would need to know the potential degeneracy of any protective immune response and whether any bystander suppression, perhaps through immune deviation, might affect both closely related and strikingly dissimilar peptide responses. With this background, we study here specificity requirements for DNA vaccines in the well defined MBP peptide-induced Lewis rat EAE model.
The autologous MBP peptide MBPRAT68–85 and the heterologous MBP peptide MBPGP68–85 differ in a single amino acid exchange from threonine (T) to serine (S) in position 79. We had shown recently that this minor difference has a strong impact on the expansion on TCRBV8S2+ T cells (8). Furthermore, we had shown that MBPGP68–85- induced EAE can be protected from by immunization with a DNA vaccine consisting of tandem repeats of MBPGP68–85 and targeting of the gene product to IgG (2). Now, we wanted to investigate whether DNA encoding the autologous vs. the heterologous MBP peptides could protect against subsequent challenge with both peptides and how different encephalitogenic stretches of MBP can protect against each other. Furthermore, we investigated whether DNA encoding the whole rat MBP 21.5-kDa sequence can protect against peptide-induced EAE.
Materials and Methods
Rats.
Lewis (LEW) rats were obtained from Harlan Netherlands (Zeist, The Netherlands). All rats were housed under specific pathogen-free conditions to keep the influence of additional environmental factors as well as immunization as low as possible. They were checked routinely for specific pathogens. In all experiments, female rats, 8–9 weeks of age, were used.
Induction and Evaluation of EAE.
The rats were anaesthetized by inhalation anesthesia with methoxyflurane (Metofane; Pitman–Moore, Mundelein, IL) and injected intradermally at the base of the tail with a total volume of 200 μl of inoculum, consisting of 100 μg of peptide emulsified (1:1) with complete Freund's adjuvant (Sigma) containing 500 μg of heat-inactivated mycobacterium tuberculosis (strain H 37 RA from Difco).
Animals were scored for clinical signs of EAE and weighed daily up to 25 days postimmunization. The signs were scored as follows: grade 1, tail weakness or tail paralysis; grade 2, hind leg paraparesis or hemiparesis; grade 3, hind leg paralysis or hemiparalysis; grade 4, complete paralysis (tetraplegy), moribund state, or death.
Synthetic Peptides and Antigens.
The synthetic peptides were synthesized by Fmoc/HBTU strategy (Å. Engström, Department of Medical and Physiological Chemistry, University of Uppsala, Sweden). Peptides were purified by reversed-phase chromatography and, subsequently, analyzed by plasma desorption MS. The degree of purity of the used peptides was >99%. The peptide sequences are shown in Table 1. GPMBP, purified MBP from central nervous system tissue of guinea pigs, was bought from Sigma.
Table 1.
Peptide sequences
| MBPGP68–85 | HYGSLPQKSQRSQDENPV |
| MBPGP63–88 | AARTTHYGSLPQKSQRSQDENPVVHF |
| MBPRAT68–85 | HYGSLPQKSQRTQDENPV |
| MBPRAT63–88 | HTRTTHYGSLPQKSQRTQDENPVVHF |
| MBP89–101 | VHFFKNIVTPRTP |
| MOG37–54 | VGWYRSPFSRVVHLYRNG |
Fractionation and Cultivation of Mononuclear Cells from Spleen.
Spleens were dissected out under deep anesthesia and disrupted, and mononuclear cells (MNC) were washed twice in DMEM (Life Technologies, Paisley, Scotland), resuspended in complete medium (CM) containing DMEM supplemented with 1% rat serum, 1% penicillin/streptomycin (Life Technologies), 1% glutamine (Life Technologies), and 50 μM 2-mercaptoethanol (Life Technologies), and flushed through a 70-μm plastic strainer (Falcon; Becton Dickinson). Red blood cells were lysed with lysing buffer, consisting of 0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM Na2EDTA adjusted to pH 7.4.
MNC were cultured at a concentration of 2 × 106 cells/ml in either 96-well, round-bottomed microtiter plates (Nunc) with 100 μl of cell suspension per well or in flat-bottomed nitrocellulose plates for enzyme-linked immunospot assay (ELISPOT) (MAHA; Millipore) with 200 μl of cell suspension per well at 37°C in a humidified atmosphere containing 5% CO2.
Assays of Antigen-Induced Proliferation.
All proliferative experiments were performed in triplicate in 96-well, round-bottomed microtiter plates. MNC (2 × 105/well) in 100 μl of CM were cultured with or without the relevant Ag for 60 h and, subsequently, pulsed with 0.5 mCi of [3H]thymidine (Amersham Pharmacia) per well for 12 h. DNA was collected on glass-fiber filters (Skatron, Sterling, VA), and [3H]thymidine-incorporation was measured in a β-counter (Beckman Coulter).
Enumeration of Cells Secreting Antigen-Specific IFN-γ.
To enumerate T cells secreting IFN-γ after Ag exposure, an ELISPOT method was used (8). Nitrocellulose-bottomed, 96-well plates were coated with the mouse mAb DB1 (a generous gift of Peter van der Meide, TNO Primate Centre, Rijswijk, The Netherlands), which reacts with rat IFN-γ. After washing with PBS, the plates were blocked with DMEM containing 5% FCS (Life Technologies). Cells (4 × 105/well) in 200 μl of CM were added to the plates and incubated for 48 h at 37°C in a humidified atmosphere containing 5% CO2. For each Ag, triplicate determinations were performed. Cells then were discarded and plates were washed four times with PBS. Secreted and bound IFN-γ was visualized with biotinylated DB12 (also a generous gift of Peter van der Meide), which has a binding site on IFN-γ other than DB1—avidin-biotin peroxidase (Vector Laboratories)—and, subsequently, by staining with carbazole (Sigma).
Cytokine ELISA.
ELISA kits for detection of secreted IL-4 and IL-10 were purchased from BioSource International (Camarillo, CA). Supernatants from MNC from spleens, which had been incubated at a concentration of 2 × 106 cells/ml with or without relevant antigens or Con A, were analyzed (2). The procedure was performed as recommended by the manufacturer.
Plasmid Construction.
For pZZ/MBPGP68–85, pZZ/MBPRAT68–85, and pZZ/MBP89–101, a 94-bp fragment containing a murine heavy chain IgG signal sequence (ss) was ligated upstream and in-frame of a 385-bp fragment encoding ZZ (2). Directly downstream of the coding sequence ZZ and upstream of the stop codon, seven AvaI-AvaI fragments encoding MBPGP68–85 or MBP89–101 or six AvaI-AvaI fragments encoding MBPRAT68–85 were ligated in-frame. cDNA encoding a 21.5-kDa isoform of rat MBP (GenBank accession no. AJ132898), which is formed by alternative splicing of MBP mRNA, was cloned by standard reverse transcription–PCR and PCR procedure. After sequencing, obtaining two identical sequences from different PCRs, a single copy of the MBPRAT21.5-coding cDNA was cloned into expression vector pCI, downstream of ZZ. For pZZ, a fragment containing ss and ZZ in frame was cloned into pCI (Promega). Expression is driven by an immediate/early human CMV enhancer/promoter. The Escherichia coli host was XL1-Blue (Stratagene).
Plasmid Preparation.
Plasmid DNA was prepared by Qiagen plasmid preparation protocol. Endotoxins were removed in an additional step (Endofree buffer set; Qiagen) (2).
Plasmid DNA Injections and Cardiotoxin Pretreatment.
Five- to 6-week-old LEW (RT1l) male rats were injected with 100 μl of 10 μM cardiotoxin (Latoxan, Rosans, France) into the musculi (Mm.) tibialii and Mm. gastrocnemii. Seven days later, the rats were injected with 800 μg of DNA vaccine at 2.0 μg/ml in PBS, divided into four 200-μg injections administered in the Mm. tibialii and Mm. gastrocnemii (2).
Statistics.
Student's t test was used for normally distributed variables. When the data did not fulfill the criteria of being normally distributed, nonparametric statistics (Mann–Whitney U test) were used.
Results
Exquisite Epitope Specificity of Protection by DNA Vaccination.
As shown in Table 2 in experiment 1, only pZZ/MBPGP68–85 protected from MBPGP68–85-induced EAE (P < 0.01), whereas DNA constructs encoding pZZ/MBPRAT68–85 and pZZ/MBPRAT21.5 failed to result in significant protection. Vice versa, pZZ/MBPRAT68–85 resulted in significant protection against challenge with MBPRAT68–85 (P < 0.01), whereas constructs pZZ/MBPGP68–85 and pZZ/MBPRAT21.5 did not. These results demonstrate a high level of antigen specificity in protection by DNA vaccination.
Table 2.
Mean accumulated EAE score
| Treatment | Immunogen
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| MBPGP68–85 | P | MBPRAT68–85 | P | MBP89–101 | P | GPMBP | P | ||
| Experiment 1 | pZZ/MBPGP68–85 | 3.5 | <0.01 | 8.0 | NS | ||||
| pZZ/MBPRAT68–85 | 8.1 | NS | 3.6 | <0.01 | |||||
| pZZ/MBPRAT21.5 | 8.8 | NS | 8.3 | NS | |||||
| pZZ | 12.9 | 11.8 | |||||||
| Experiment 2 | pZZ/MBPGP68–85 | 7.0 | <0.001 | 8.5 | NS | 7.6 | <0.01 | ||
| pZZ/MBP89–101 | 11.8 | NS | 5.9 | <0.05 | 14.8 | NS | |||
| pZZ/MBPGP68–85 and pZZ/MBP89–101 | 8.5 | <0.05 | 7.2 | NS | 9.1 | <0.05 | |||
| pZZ | 13.9 | 10.1 | 13.9 | ||||||
The table shows mean accumulated EAE score in relation to DNA vaccine and subsequent immunization with either MBP peptide MBPGP68–85, MBPRAT68–85, MBP89–101, or full length GPMBP. There was a high specificity of the protective effect of the DNA vaccine. Each group represents eight rats. Vaccinations, immunizations, and scoring were performed as described in Materials and Methods. P values relate to statistical comparisons with the indicated DNA vaccine in relation to pZZ.
In experiment 2 we investigated how far pZZ/MBPGP68–85 can protect against induction of disease with the second dominant epitope in the LEW rat, MBP89–101. As shown, there was no protective effect, but there was significant protection against EAE induction with GPMBP (P < 0.05). On the other hand, the construct pZZ/MBP89–101 protected against disease induced with the peptide MBP89–101 (P < 0.05), but not against disease induced with MBPGP68–85. A mixture of both pZZ/MBPGP68–85 and pZZ/MBP89–101 ameliorated EAE induced with MBPGP68–85 (P < 0.05) and GPMBP (P < 0.05). In contrast, pZZ/MBP89–101 did not protect against GPMBP.
Cellular Responses After Vaccination with Different MBP Constructs and Subsequent Challenge with MBP Peptides.
Immune responses to MBP and myelin-oligodendrocyte glycoprotein (MOG) peptides after DNA vaccination with different MBP constructs and subsequent immunization with MBP peptides by proliferation and ELISPOT assay for detection of IFN-γ-secreting cells and cytokine ELISAs for IL-4 and IL-10 were monitored.
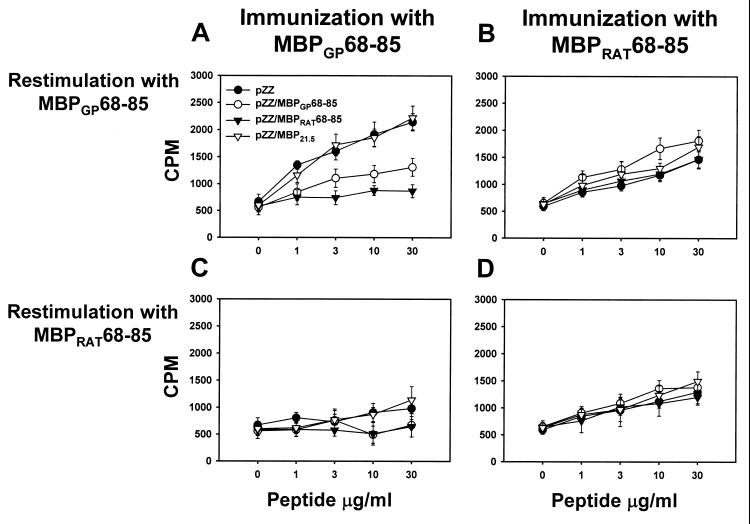
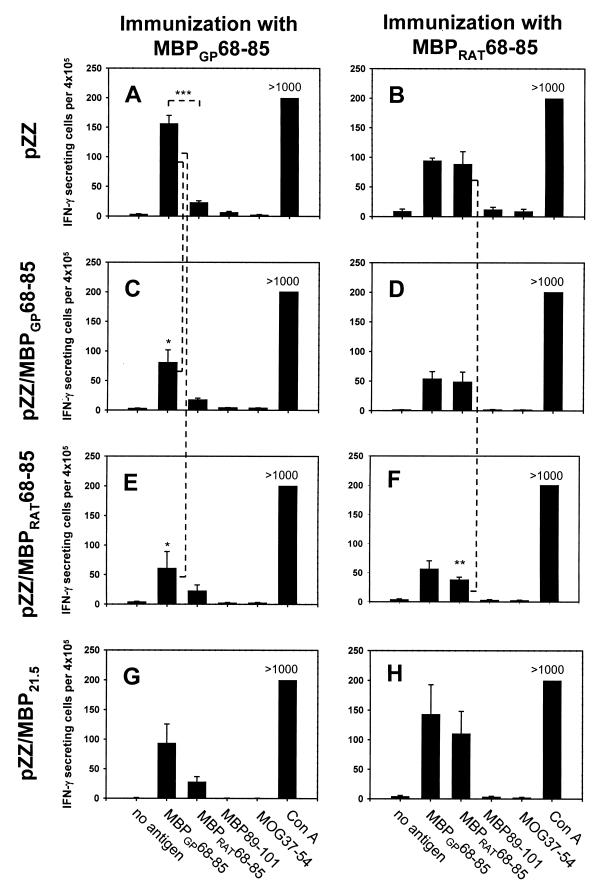
As shown, DNA vaccination with pZZ and subsequent challenge with MBPGP68–85 resulted in a strong, proliferative response against MBPGP68–85 (Fig. 1A) and a lower response to MBPRAT68–85 (Fig. 1C), whereas disease induction with MBPRAT68–85 resulted in comparable responses to both peptides (Fig. 1 B and D). This was also the case for the number of IFN-γ-secreting cells as assessed by ELISPOT with higher numbers of such cells after in vitro stimulation of MBPGP68–85-immunized rat spleen cells with MBPGP68–85 compared with MBPRAT68–85 (Fig. 2A; P < 0.001). Equal numbers of such cells were noted after immunization with MBPRAT68–85 and subsequent in vitro stimulation with either MBPGP68–85 or MBPRAT68–85 in pZZ-vaccinated rats (Fig. 2B).
Figure 1.
Ag-specific proliferation of T cells from MBPGP68–85 (A and C) and MBPRAT68–85 (B and D) primed LEW rats that had received different DNA constructs 4 weeks before immunization i.m. as indicated in Materials and Methods. Each value represents mean values (±SEM) from four LEW rats. Splenocytes were obtained on day 12 postimmunization, and MNC were cultured for 72 h in triplicates in the presence of either MBPGP68–85 (A and B) or MBPRAT68–85 (C and D) at various antigen concentrations or medium alone. Stimulation with MBP89–101 or MOG37–54 did not result in cpm values over background (data not shown). T cell proliferation was assessed by [3H]thymidine incorporation during the last 12 h of culture.
Figure 2.
Enumeration of cells secreting Ag-specific IFN-γ of T cells from MBPGP68–85- and MBPRAT68–85-primed LEW rats that had received different DNA constructs 4 weeks before immunization i.m. as indicated in Materials and Methods. Responses are shown after DNA vaccination with pZZ (A and B), after vaccination with pZZ/MBPGP68–85 (C and D), after vaccination with pZZ/MBPRAT68–85 (E and F), and after vaccination with pZZ/MBPRAT21.5 (G and H). Each value represents data from four LEW rats. Spleens were obtained on day 12 postimmunization, and MNC were cultured for 48 h in triplicate in the presence of either MBPGP68–85, MBPGP63–88, MBPRAT68–85, MBPRAT63–88, MBP89–101, or MOG37–54 at a concentration 10 μg/ml peptide, medium alone, or Con A at a concentration of 3 μg/ml. Asterisks indicate significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Compared with DNA vaccination with the control construct pZZ, vaccination with pZZ/MBPGP68–85 followed by immunization with MBPGP68–85 led to a reduced proliferative capacity of MBPGP68–85-reactive cells (Fig. 1A). In contrast, the proliferative capacity after immunization with MBPRAT68–85 was not affected (Fig. 1B). Both constructs pZZ/MBPGP68–85 and pZZ/MBPRAT68–85 led, after immunization with MBPGP68–85, to a reduction of IFN-γ-secreting cells induced by MBPGP68–85 (both P < 0.05). pZZ/MBPRAT21.5 encoding the full-length rat MBP 21.5-kDa molecule did not result in a decrease of MBPGP68–85-reactive cells.
DNA vaccination with pZZ/MBPGP68–85 and pZZ/MBPRAT68–85 compared with pZZ, all subsequently immunized with MBPRAT68–85, did not result in significant reduction of IFN-γ-secreting cells reactive for MBPGP68–85 (Fig. 2 B, D, and F). pZZ/MBPGP68–85 also did not lead to reduction of MBPRAT68–85-reactive cells compared with pZZ (Fig. 2 B and D). In contrast, vaccination with pZZ/MBPRAT68–85 resulted in significant reduction of MBPRAT68–85-reactive cells secreting IFN-γ compared with vaccination with pZZ (P < 0.01) (Fig. 2 B and F). There were no differences in proliferative responses (Fig. 1D). We did not measure an effect of pZZ/MBPRAT21.5 compared with pZZ (Figs. 1 and 2).
We never found any measurable in vitro response over background toward MBP89–101, the second minor dominant epitope in MBP for the LEW rat, or to MOG37–54, the dominant MOG epitope in the LEW rat (Fig. 2; not shown for proliferative data). There was no measurable antigen-induced IL-4 or IL-10 in ELISA after stimulation with peptides except for that for the mitogen Con A (data not shown).
Discussion
We demonstrate that (i) there is an exquisite epitope specificity of DNA vaccination, (ii) protection from disease is associated with reduction of T cell reactivity from spleen to the disease-inducing peptide, (iii) DNA vaccination with sequences for a certain myelin peptide does not cross-tolerize against other encephalitogenic MBP or MOG peptides, and (iv) DNA encoding the dominant encephalitogenic stretch in a high copy number has a higher protective potential compared with DNA encoding the full-length autoantigen in a single copy number.
We consider two principle mechanisms by which DNA vaccination with autoantigens could act protectively through deletion or active suppression of antigen-specific T cell clones. First, previous exposure of the immune system to the autoantigenic peptide by the DNA vaccine might have deviated the autoreactive MBP peptide-directed immune response to a T2 or T3 response. Potentially, no disease would evolve with such an immune response as evidenced in MHC congenic LEW rats (9). Second, DNA vaccination might have triggered a subencephalitogenic immune response, insufficient to cause disease. This response, however, might be sufficient to trigger a series of down-regulatory, antiidiotypic circuits specific for a particular MBP peptide. This scenario would be in line with that described for vaccination with attenuated encephalitogenic T cell lines and clones (10). Several lines of evidence presented here and elsewhere argue against the first and support the second possibility.
In this and our preceding studies, using the same protocols, we have analyzed the ex vivo MBP peptide-induced cytokine production and found no increase in production of T2 cytokines, e.g., IL-4 and IL-10. Instead, we found a global decrease in the antigen reactivity measured by IFN-γ production from draining lymph node cells (2). Moreover, we have shown that CpG DNA and a T1-type response are necessary for induction of protection in autoimmune conditions by DNA vaccination (11). Deliberate use of altered peptide ligands, thus implicating some form of degeneracy in the immune recognition, can lead to protection through T2-biased immunity (12). This was not the case here. Moreover, such an active immune deviation should have been able to protect against other encephalitogenic peptide stretches by bystander suppression (cross-tolerance) as in certain forms of oral tolerance (13, 14). Also, this was not the case in this study. Instead, the antigen specificity of suppression is consistent with either clonal anergy/deletion or an antiidiotypic protective immune response, as long as the encephalitogenic T cell repertoires differ after immunization with MBPGP68–85 and MBPRAT68–85. This, indeed, is the case, because we had shown that after immunization with MBPGP63–88 compared with MBPRAT63–88, differences in the T cell repertoire evolve with a predominant activation of TCRBV8S2+ T cells and accumulation of these cells in the central nervous system (8). The difference between these peptides lies in a serine-to-threonine exchange, which represents a minor difference. Nevertheless, this conservative amino acid exchange resulted in dramatic differences in the repertoire. For the immune system this exchange cannot be considered as small, because one peptide represents a non-self-determinant, whereas the other is a self-determinant. MBPGP68–85 is considered more immunogenic in rats compared with MBPRAT68–85, but in equimolar high concentrations both peptides result in disease, which indicates that there is no lack of an encephalitogenic repertoire. Moreover, we have shown that the MBPGP68–85-specific T cells induce EAE by cross-reactivity with self-MBP (8).
Together with our preceding work, our data reinforce that after immunization with self-MBPRAT68–85 vs. non-self-MBPGP68–85, differences in T cell repertoires evolve. Proliferative responses and numbers of IFN-γ-secreting cells thus differ greatly depending on the DNA vaccine used and the subsequent immunization with self- vs. non-self-MBP. Importantly, protection evolved only to the specific vaccination stretch. Furthermore, there were no T cell responses measurable against MBP89–101 or MOG37–54 after DNA vaccination with pZZ/MBPGP68–85, pZZ/MBPRAT68–85, or pZZ/MBPRAT21.5 and subsequent immunization with MBP peptides MBPGP68–85 or MBPRAT68–85. This imposes that there is no cross-priming or antigen spreading (7) of myelin-reactive T cells by DNA vaccines and supports the high degree of specificity of the DNA vaccination approach. We thus failed to observe some general immunomodulatory capacities of DNA vaccines resulting in bystander suppression, as has been shown for altered peptide ligands (15). Also, in oral tolerance the situation differs: MBPGP68–88 orally fed to Lewis rats resulted in protection from MBPGP68–88- or MBPRAT68–88-induced disease (14). This was not the case when MBPRAT68–88 was fed, resulting in no protection against either peptide (14).
DNA vaccination with full-length MBPRAT21.5 did not result in protection from EAE induced by either MBPGP68–85 or MBPRAT68–85. The reason could be that (i) the single-copy construct does not result in a high enough copy number of the potential encephalitogenic stretch to induce either T cell tolerance or a subencephalitogenic T cell response, (ii) potentially, a MHC class I-restricted T cell response is induced, which might not result in disease or protection, or (iii) there is no creation and presentation on MHC class II molecules of the encephalitogenic peptide stretch.
Our studies altogether suggest that a subencephalitogenic Th1 response can result in protection from EAE. This was supported by the finding that immune responses of spleen cells analyzed after DNA vaccination but before induction of EAE with peptides have shown a slight increase of peptide-reactive cells secreting IFN-γ rather than unresponsiveness (data not shown). Antigen-specific CD4+ T cell responses are difficult to investigate on the clonal level, because they might persist only for a short time and the clonal expansions might be small (16). Use of tetrameric MHC–peptide complexes (17) and immunoscope analysis (18) will allow easier study of such responses in the future.
What are the actual molecular mechanisms dampening the encephalitogenic response? For example, the functional differentiation of the ensuing immune response with regard to expression of costimulatory molecules can be different after DNA vaccination (19). We have documented that DNA vaccination indeed dampened the ex vivo peptide specific reactivity, but the mechanism is unclear. Furthermore, this dampening occurred irrespective of the success or failure of the vaccination, i.e., DNA vaccination with MBPGP68–85 reduced the immune response against MBPRAT68–85 despite no significant effect on the accumulated disease score. Thus, the cellular and/or molecular mediators responsible for protection remain unclear.
Our results indicate that before using DNA vaccines as potential therapeutic agents in humans, the exact T cell epitopes that drive disease may have to be known. The data also demonstrate that more knowledge about the actual mode of disease induction has to be gathered. Finally, our data imply that DNA vaccines for autoimmune conditions can be made without inducing unwanted immune alterations.
Acknowledgments
This study was supported by the Swedish Medical Research Council, the Swedish Association of Neurologically Disabled, the Swedish Cancer Society, Pharmacia and Upjohn, State Biological Laboratory Vaccine Company, and the Deutsche Forschungsgemeinschaft (We 1947/1-1 and 2-1).
Abbreviations
- EAE
experimental autoimmune encephalomyelitis
- MBP
myelin basic protein
- MOG
myelin-oligodendrocyte glycoprotein
- TCR
T cell receptor
- Th
T helper
- MNC
mononuclear cell(s)
- LEW
Lewis
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database [accession no. AJ132898 (cDNA encoding 21.5-kDa isoform of rat MBP)].
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030390097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030390097
References
- 1.Waisman A, Ruiz P J, Hirschberg D L, Gelman A, Oksenberg J R, Brocke S, Mor F, Cohen I R, Steinman L. Nat Med. 1996;2:899–905. doi: 10.1038/nm0896-899. [DOI] [PubMed] [Google Scholar]
- 2.Lobell A, Weissert R, Storch M K, Svanholm C, de Graaf K L, Lassmann H, Andersson R, Olsson T, Wigzell H. J Exp Med. 1998;187:1543–1548. doi: 10.1084/jem.187.9.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 4.Hemmer B, Fleckenstein B T, Vergelli M, Jung G, McFarland H, Martin R, Wiesmuller K H. J Exp Med. 1997;185:1651–1659. doi: 10.1084/jem.185.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason D. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 6.Wucherpfenning K W, Strominger J L. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehmann P V, Forsthuber T, Miller A, Sercarz E E. Nature (London) 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 8.Weissert R, Svenningsson A, Lobell A, de Graaf K L, Andersson R, Olsson T. J Immunol. 1998;160:681–690. [PubMed] [Google Scholar]
- 9.Mustafa M, Vingsbo C, Olsson T, Issazadeh S, Ljungdahl A, Holmdahl R. J Immunol. 1994;153:3337–3344. [PubMed] [Google Scholar]
- 10.Zhang J, Medaer R, Stinissen P, Hafler D, Raus J. Science. 1993;261:1451–1454. doi: 10.1126/science.7690157. [DOI] [PubMed] [Google Scholar]
- 11.Lobell A, Weissert R, Eltayeb S, Svanholm C, Olsson T, Wigzell H. J Immunol. 1999;163:4754–4762. [PubMed] [Google Scholar]
- 12.Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D, et al. Nature (London) 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo V K, Weiner H L. Nature (London) 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 14.Javed N H, Gienapp I E, Cox K L, Whitacre C C. J Immunol. 1995;155:1599–1605. [PubMed] [Google Scholar]
- 15.Nicholson L B, Murtaza A, Hafler B P, Sette A, Kuchroo V K. Proc Natl Acad Sci USA. 1997;94:9279–9284. doi: 10.1073/pnas.94.17.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maini M K, Casorati G, Dellabona P, Wack A, Beverley P C L. Immunol Today. 1999;20:262–266. doi: 10.1016/s0167-5699(99)01472-3. [DOI] [PubMed] [Google Scholar]
- 17.Gallimore A, Glithero A, Godkin A, Tissot A C, Pluckthun A, Elliot T, Hengartner H, Zinkernagel R. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douillard P, Pannetier C, Josien R, Menoret S, Kourilsky P, Soulillou J P, Cuturi M C. J Immunol. 1996;157:1250–1260. [PubMed] [Google Scholar]
- 19.Ruiz P J, Garren H, Ruiz I U, Hirschberg D L, Nguyen L V, Karpuj M V, Cooper M T, Mitchell D J, Fathman C G, Steinman L. J Immunol. 1999;162:3336–3341. [PubMed] [Google Scholar]