Abstract
Objective
To clarify aspects of the association between physical activity and breast cancer, such as activity intensity required, and possible effect modification by factors such as menopausal hormone therapy (MHT) use.
Methods
We prospectively examined physical activity in relation to breast cancer risk among 45,631 women participating in the U.S. Radiologic Technologists cohort. Participants provided information at baseline regarding hours spent per week engaging in strenuous activity, walking/hiking for exercise, and walking at home or work. We estimated multivariable relative risks (RR) and 95% confidence intervals (CI) of breast cancer using Cox regression.
Results
We identified 864 incident invasive breast cancers. Greatest risk reduction was observed among women who reported walking/hiking for exercise 10 or more hours per week (RR, 0.57;95%CI, 0.34-0.95) compared with those reporting no walking/hiking. The association between walking/hiking for exercise and breast cancer was modified by MHT use (p for interaction=0.039). Postmenopausal women who never used MHT had reduced risks of breast cancer associated with physical activity whereas no relation was observed among ever users of MHT.
Conclusion
Our study suggests moderate intensity physical activity, such as walking, may protect against breast cancer. Further, the relation between physical activity and breast cancer may be modified by MHT use.
Keywords: breast cancer, physical activity, cohort studies, hormones
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer among women in the United States [1]. Physical activity represents one of the few established risk factors for breast cancer that can be modified through behavior changes. Numerous observational studies have reported a reduced risk of breast cancer in relation to increasing levels of physical activity [2-6], with evidence of this association classified as “convincing” in 2002 by IARC [7]. Several biological mechanisms have been proposed to explain the relationship between physical activity and breast cancer risk, including a decrease in endogenous hormone levels, reduction of insulin and insulin-like growth factors, favorable modification of menstrual characteristics, and enhanced immune function [8-11].
Several aspects of the association between physical activity and breast cancer risk remain uncertain including the type of activity, timing in life of activity, dose of activity required (including duration, frequency, and intensity), and whether risks differ among certain population subgroups. The current investigation will primarily focus on the intensity level of physical activity and the association between physical activity and breast cancer according to menopausal status and menopausal hormone therapy use. Inconsistencies exist regarding the intensity of physical activity required for risk reduction, and it is important to investigate whether moderate intensity physical activity can also reduce risk. Some studies observe risk reduction with strenuous or moderate forms of physical activity [5, 12, 13] and others suggest that strenuous activity is required for risk reduction [14, 15]. A recent review reported that although moderate intensity activity has been associated with reduced risk of breast cancer, the reductions are even stronger with vigorous intensity activity [16]. In addition, results of epidemiologic studies also suggest that the association between physical activity and breast cancer may differ by menopausal status. The majority of studies report a reduced risk of breast cancer related to increased physical activity among postmenopausal women, but the evidence for this association among premenopausal women is much less consistent [6, 16-19]. Further, few studies have evaluated the modifying effects of menopausal hormone therapy among postmenopausal women [4, 5, 13, 19-23].
In the present study, we examine the association between physical activity of varying intensities and breast cancer risk in the U.S. Radiologic Technologists (USRT) cohort, with special emphasis on potential effect modification of the physical activity and breast cancer relation by menopausal hormone therapy among postmenopausal women.
MATERIALS AND METHODS
The U.S. Radiologic Technologists Cohort
The USRT cohort is a collaborative effort between the U.S. National Cancer Institute, the University of Minnesota, and the American Registry of Radiologic Technologists (ARRT). The study is comprised of a cohort of radiologic technologists residing in the United States and who were certified by the ARRT for at least 2 years between 1926 and 1982. Detailed information on the study population and methods has been published previously [24,25].
Briefly, an initial questionnaire was mailed in 1983-1989 that collected detailed information on employment history, demographic and lifestyle factors, and reproductive and medical history. The current study, however, uses as its baseline the second self-administered questionnaire (1994-1998), which ascertained incident cancers and collected information on demographic, reproductive, and other potential risk factors, including physical activity. Of the 94,495 known living female technologists that were mailed the second questionnaire, 69,998 responded (74%). A third questionnaire distributed in 2004-2005 collected additional information on cancer risk factors and updated health outcomes. Of the 69,998 female responders to the second questionnaire, 52,563 women responded to the third questionnaire (75%).
Study Population
We included 51,473 women who responded to the second questionnaire, were cancer-free (except for non-melanoma skin cancer) at completion of the second questionnaire and who responded to the third questionnaire or died during the intervening period. We excluded 5,842 women with missing data on any of the three physical activity questions (N=5,759) or unrealistic data on physical activity(defined as women who reported spending 80 or more hours a week participating in a combination of strenuous activity and walking/hiking for exercise) (N=83), resulting in an analytic cohort consisting of 45,631 women. The 5,842 excluded women did not differ appreciably from women in the analytic cohort on most covariates; however, excluded women tended to be older at baseline and were more likely to be smokers.
Cohort maintenance
Annual follow-up is conducted through re-certification with the ARRT. For individuals who fail to renew certification, vital status is determined through linkage to mortality and national address change databases, including the Social Security Death Index and National Death Index Plus. The Institutional Review Boards of the National Cancer Institute and the University of Minnesota approved this study.
Assessment of physical activity
Participants provided information at baseline (1994-1998) regarding the number of hours spent per week during the previous year engaging in each of the following activities as written on the questionnaire: exercising strenuously (e.g. aerobics, jogging, swimming), walking or hiking for exercise and walking at home or at work. Response options for each question included: ‘none’, ‘<1hour’, ‘1-3 hours’, ‘4-9 hours’, ‘10-19 hours’, ‘20-39 hours’, or ‘over 40 hours’. A midpoint value was assigned for the number of hours per week spent engaging in physical activity (the ‘over 40 hours’ category was assigned a value of 41) and multiplied by the estimated metabolic equivalent task (MET) value specific to that intensity level. MET values for strenuous exercise, walking for exercise, and walking at home or work were assigned MET values of 7, 4, and 3, respectively [26]. We created a total physical activity score by summing the MET-hours per week for all three physical activity variables. The MET-hours per week were divided into quintiles for the analyses, based on their distribution in the total population. Studies assessing the reliability and validity of self-reported physical activity have concluded that questionnaires, in general, are a reasonably useful method of estimating physical activity in large epidemiologic studies [27, 28].
Breast cancer validation
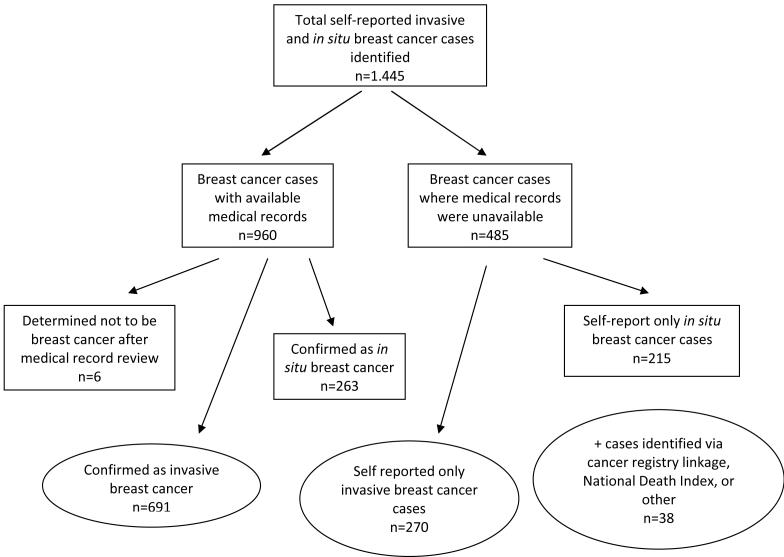
A total of 1,445 self-reported breast cancers were identified between the second and third surveys (Figure 1). Participants were asked to say whether they had been diagnosed with invasive or in situ breast cancer. The case definition was limited to invasive breast cancer and in situ breast cancers were excluded. Pathology reports or medical records were obtained for 960 (66.4%) patients reporting breast cancer (invasive or in situ); of which 954 breast cancer cases were confirmed, resulting in a 99% confirmation rate. We excluded the 6 incorrectly reported “breast cancers”. The 954 validated breast cancers included 263 in situ breast cancer cases, which we excluded, leaving 691 confirmed invasive breast cancers. Since such a large percentage of self-reported breast cancer cases were confirmed among women for whom medical records could be obtained, we included 270 self-reported invasive breast cancer cases for whom medical records were unavailable and excluded 215 self-reported in situ breast cancers. We also included 38 breast cancer cases identified via cancer registry linkage (N=26), linkage with the National Death Index with breast cancer as the underlying cause of death (N=8), and incidental reports (originally reported as non-breast cancers but later found to be invasive breast cancer through validation) (N=4). Of the 999 total invasive breast cancer cases available (N=691+270+38), 864 breast cancer cases remained from 45,631 women for analysis after exclusions due to missing physical activity data. Cases identified using death certificates had a diagnosis date imputed by subtracting the average breast cancer survival time (based on data from the Surveillance, Epidemiology, and End Results Program) from the date of death.
Figure 1.
Diagram showing case validation and inclusion steps
Statistical analysis
To assess the association between physical activity and breast cancer, we used multivariable Cox proportional hazards regression to estimate relative risks (RRs) and corresponding confidence intervals (CIs), with age as the underlying time scale. Analyses were stratified at baseline for 5-year birth cohorts to control for secular trends. Person-time began at the completion of the second questionnaire and ended at the date of first reported cancer diagnosis (except non-melanoma skin cancers), death, response to the third questionnaire, whichever occurred first.
In the analysis, we assessed risk using three models, one adjusting for age only, one adjusting for age and potential confounders (described below), and an additional model that was mutually adjusted for all three categories of physical activity. For strenuous activity and walking for exercise, we selected as the reference group women who reported never engaging in physical activity. To evaluate the relations with time spent walking at home or work, <1 hour/week served as the referent group. Women who reported never walking at home or work were not used as the referent group because their inactivity may have been due to underlying disease potentially related to breast cancer. A similar approach has been used previously to account for this effect [22, 29].
Analyses that adjusted for confounding included the following factors, which are commonly included in breast cancer analyses: age at menarche (<11, 11-14, 15+, unknown), number of live births (none, 1-3, 4+, unknown), age at first live birth (<20, 20-<25, 25-<30, 30-<35, 35+ and nulliparous, unknown), age at menopause (pre-menopausal, <35, 35-<40, 40-<45, 45-<50, 50-<55, 55+), family history of breast cancer (no, yes, unknown), personal history of breast disease (no, yes, unknown), use of oral contraceptives (never, ever, unknown), race (white, black, Asian, other/unknown), menopausal hormone therapy use (MHT) (never/premenopausal, ever, unknown), smoking (never, quit and ≤20 cigarettes/day, quit and >20 cigarettes/day, current smoker and ≤20 cigarettes/day, current smoker and >20 cigarettes/day, unknown), alcohol (<1 drink/week, 1-6 drinks/week, 7-12 drinks/week, 13+ drinks/week, unknown), and body mass index (BMI) (<25 kg/m2, 25-<30 kg/m2, 30+kg/m2, unknown). Missing values for menopausal status and/or age at menopause were imputed for 7.3% of participants using mean values for women of similar age. Other covariates (height, years spent working, marital status, and vitamin use) were evaluated for potential confounding but were not included in the models because they did not appreciably affect the risk estimates and have generally not been shown to confound an association with physical activity in previous studies.
Trend tests for each physical activity variable were calculated by assigning the median value to each exposure category and treating each as a single continuous variable in the model. Effect modification was tested using the resulting P-value of a cross-product interaction term between the physical activity variable and the covariate of interest together with the main effects in the appropriate model. The coefficient of the interaction term was evaluated using a Wald test. All tests of statistical significance were two-sided and P<0.05 was considered statistically significant. The statistical analyses were conducted using the PHREG procedure of the Statistical Analysis System (SAS) software package (version 8.2, SAS Institute, Inc., Cary, NC).
RESULTS
During a mean length of follow-up of 8.9 years (total person-years: 404,457), we identified 864 incident cases of breast cancer. Age-adjusted study population characteristics stratified by total physical activity score (in quintiles) are listed in Table 1. The mean age of study participants at baseline was 47.2 years. More active women were slightly younger at first birth, had lower parity, consumed more alcohol, and had a lower BMI than their inactive counterparts. Unexpectedly, more active women were more likely to be current smokers. Modest differences were observed across physical activity quintiles for other variables.
Table 1.
Age-adjusted baseline characteristics according to total physical activity: U.S. Radiologic Technologists Studya
| Quintiles of total physical activity score (MET hr/wk) | |||||
|---|---|---|---|---|---|
| Characteristic | Q1 (0.0-9.5) | Q2 (11.5-23.0) | Q3 (23.5-45.5) | Q4 (46.0-96.5) | Q5 (≥97.0) |
| Number of participants | 9,154 | 9,058 | 9,509 | 9,000 | 8,910 |
| Mean age (years) | 48.9 | 47.0 | 47.0 | 46.6 | 46.1 |
| Mean body mass index (kg/m2) | 26.6 | 25.5 | 25.1 | 24.8 | 24.8 |
| Race (%) | |||||
| White | 96.4 | 97.1 | 97.5 | 97.6 | 96.8 |
| Black | 2.8 | 2.2 | 1.8 | 1.9 | 2.6 |
| Asian | 0.8 | 0.7 | 0.7 | 0.5 | 0.6 |
| Family history of breast cancer (%)b | 9.6 | 9.9 | 10.1 | 9.8 | 9.4 |
| Personal history of breast disease (%) | 33.0 | 34.9 | 34.8 | 34.5 | 34.7 |
| Currently married (%) | 76.8 | 76.8 | 77.6 | 77.0 | 73.9 |
| Age at menarche (%), years | |||||
| <11 | 7.0 | 7.2 | 6.4 | 6.7 | 7.6 |
| 11-14 | 85.3 | 85.2 | 85.4 | 85.6 | 83.6 |
| 15+ | 7.7 | 7.6 | 8.2 | 7.7 | 8.8 |
| Age at first birth (%), years | |||||
| <20 | 2.9 | 3.3 | 2.9 | 3.5 | 3.6 |
| 20-24 | 36.8 | 37.9 | 38.1 | 38.9 | 39.5 |
| 25-29 | 39.0 | 38.4 | 40.1 | 39.4 | 39.3 |
| 30+ | 21.4 | 20.5 | 18.8 | 18.2 | 17.6 |
| Parity (%) | |||||
| 0 | 19.4 | 19.8 | 19.1 | 19.3 | 20.3 |
| 1-3 | 72.4 | 72.4 | 72.8 | 72.4 | 72.1 |
| 4+ | 8.2 | 7.8 | 8.1 | 8.3 | 7.6 |
| Age at menopause (%), years | |||||
| Premenopausal | 58.4 | 59.6 | 58.9 | 59.2 | 58.0 |
| <40 | 13.1 | 12.3 | 12.4 | 12.6 | 13.8 |
| 40-44 | 9.1 | 8.7 | 9.0 | 8.8 | 9.4 |
| 45-49 | 9.3 | 9.3 | 9.2 | 9.4 | 9.5 |
| 50-54 | 8.5 | 8.7 | 8.7 | 8.6 | 8.1 |
| 55+ | 1.6 | 1.4 | 1.7 | 1.4 | 1.2 |
| Current smoker (%) | 13.2 | 12.3 | 10.3 | 11.5 | 15.4 |
| Alcohol consumption, drinks/week (%) | |||||
| <1 | 67.7 | 61.3 | 58.7 | 57.0 | 58.6 |
| 1-6 | 24.0 | 29.4 | 31.4 | 32.4 | 30.8 |
| 7-12 | 4.2 | 5.2 | 5.7 | 5.9 | 6.0 |
| 13+ | 4.1 | 4.1 | 4.2 | 4.8 | 4.6 |
| Menopausal hormone therapy, current use (%) | 22.0 | 22.8 | 24.0 | 23.5 | 24.4 |
| Oral contraceptive use, ever (%) | 73.2 | 76.3 | 76.3 | 75.8 | 77.0 |
Cohort restricted to respondents of the baseline questionnaire who were cancer free (except nonmelanoma skin cancer) at time of response. Values calculated from participants with nonmissing data for each variable and standardized to the age distribution of the study population.
Any first-degree relative with breast cancer
Table 2 shows age- and multivariable-adjusted risk estimates for associations of four indices of physical activity with breast cancer incidence. Risk estimates were similar after multivariable adjustment for several risk factors, including BMI. Risk reduction was greatest among women who reported walking/hiking for exercise 10 or more hours per week (RR, 0.57; 95% CI, 0.34-0.95) compared to women who never walk/hike for exercise, although the trend was not significant (P=0.321). Breast cancer risk was slightly reduced among women reporting 40 or more hours per week of walking at home or at work (RR, 0.88; 95% CI, 0.67-1.16), compared to women walking less than 1 hour per week. After mutually adjusting for the three categories of physical activity evaluated in the questionnaire, observed risk estimates for all three exercise variables were slightly attenuated. Further, the association between walking/hiking for exercise and breast cancer was similarly attenuated among women who reported that they never engaged in strenuous physical activity (data not shown). Total physical activity (based on MET-score) was not statistically significantly associated with breast cancer risk (P=0.174), however risk was suggestively decreased among women in the highest two quintiles (RR, 0.87; 95% CI, 0.70-1.08 and RR, 0.91; 95% CI, 0.74-1.13, respectively).
Table 2.
Risk of breast cancer according to various measures of physical activity
| Exercise variable | Cases | Person-years | Age-adjusted HR | Multivariatea HR | Multivariatea HR + other activity variables |
|---|---|---|---|---|---|
| Strenuous exercise (hours/week) | |||||
| Never | 469 | 200,372 | 1.0 | 1.0 | 1.0 |
| <1 | 169 | 90,368 | 0.94 (0.78-1.12) | 0.93 (0.78-1.11) | 0.93 (0.77-1.11) |
| 1-3 | 161 | 75,989 | 1.06 (0.88-1.27) | 1.03 (0.86-1.24) | 1.02 (0.85-1.23) |
| 4-9 | 55 | 31,415 | 0.86 (0.65-1.14) | 0.83 (0.62-1.10) | 0.82 (0.61-1.09) |
| 10+ | 10 | 6,312 | 0.76 (0.41-1.43) | 0.76 (0.40-1.42) | 0.90 (0.47-1.71) |
| P trend | 0.298 | 0.196 | 0.320 | ||
| Walking/hiking for exercise (hours/week) | |||||
| Never | 187 | 82,030 | 1.0 | 1.0 | 1.0 |
| <1 | 223 | 110,068 | 0.96 (0.79-1.17) | 0.97 (0.80-1.18) | 1.01 (0.82-1.24) |
| 1-3 | 295 | 139,260 | 1.01 (0.84-1.21) | 1.02 (0.84-1.22) | 1.06 (0.87-1.29) |
| 4-9 | 143 | 60,083 | 1.08 (0.87-1.34) | 1.10 (0.88-1.37) | 1.16 (0.92-1.46) |
| 10+ | 16 | 13,016 | 0.56 (0.33-0.92) | 0.57 (0.34-0.95) | 0.63 (0.37-1.07) |
| P trend | 0.264 | 0.321 | 0.662 | ||
| Walking at home or work (hours/week) | |||||
| Never | 57 | 20,851 | 1.20 (0.88-1.63) | 1.21 (0.88-1.65) | 1.21 (0.88-1.67) |
| <1 | 131 | 58,598 | 1.0 | 1.0 | 1.0 |
| 1-3 | 233 | 105,771 | 1.00 (0.81-1.24) | 1.01 (0.81-1.25) | 1.00 (0.80-1.24) |
| 4-9 | 177 | 83,370 | 0.98 (0.78-1.23) | 0.99 (0.79-1.24) | 0.97 (0.77-1.23) |
| 10-19 | 82 | 41,678 | 0.92 (0.70-1.21) | 0.92 (0.70-1.21) | 0.92 (0.69-1.22) |
| 20-39 | 101 | 48,537 | 0.98 (0.76-1.28) | 1.00 (0.77-1.29) | 0.99 (0.76-1.29) |
| 40+ | 83 | 45,652 | 0.87 (0.66-1.14) | 0.88 (0.67-1.16) | 0.90 (0.68-1.20) |
| P trend | 0.330 | 0.401 | 0.623 | ||
| Total MET-score | |||||
| Q1 (0.0-9.5) | 188 | 79,694 | 1.0 | 1.0 | |
| Q2 (11.5-23.0) | 181 | 80,264 | 1.04 (0.85-1.27) | 1.02 (0.83-1.26) | |
| Q3 (23.5-45.5) | 190 | 84,697 | 1.03 (0.84-1.26) | 1.02 (0.83-1.25) | |
| Q4 (46.0-96.5) | 152 | 80,381 | 0.88 (0.71-1.09) | 0.87 (0.70-1.08) | |
| Q5 (≥97.0) | 153 | 79,420 | 0.92 (0.74-1.14) | 0.91 (0.74-1.13) | |
| P trend | 0.170 | 0.174 |
Adjusted for entry age, body mass index, age at menarche, parity, age at first birth, age at menopause, family history of breast cancer, personal history of breast disease, OC use, menopausal hormone therapy, race, smoking, and alcohol consumption.
We examined the relation of physical activity to risk of breast cancer according to menopausal status and menopausal hormone therapy use (Table 3). In pre-menopausal women, decreased risk of breast cancer was greatest among women walking/hiking for exercise for 10 or more hour per week (RR, 0.37; 95% CI, 0.16-0.84), although the trend was not statistically significant. The relationship at ≥ 10 hours/week remained even after adjustment for participation in other types of physical activity. Slightly reduced breast cancer risks were also observed for pre-menopausal women engaging in strenuous exercise and time spent walking at home/work, but risks for strenuous activity were attenuated after adjusting for other types of physical activity. No statistically significant effect modification was observed for any of the physical activity variables and menopausal status (data not shown).
Table 3.
Risk of breast cancer according to physical activity, menopausal status, and menopausal hormone therapy use
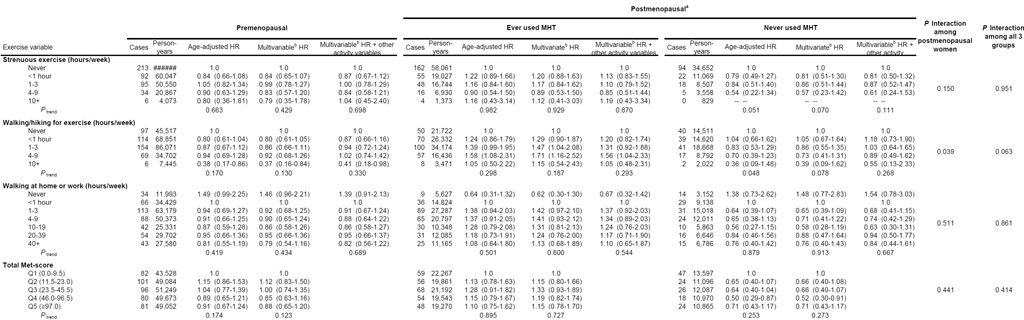 |
Postmenopausal women with “unknown MHT status (n=143) were excluded from the ever used/never used stratified analysis.
Adjusted for entry age, body mass index, age at menarche, parity, age at first birth, family history of breast cancer, personal history of breast disease, OC use, race, smoking, and alcohol consumption. Model conducted among postmenopausal women were additionally adjusted for age at menopause.
Postmenopausal women who never used MHT experienced reduced risks of breast cancer with all three types of physical activity. Associations approaching statistical significance were observed for increasing levels of strenuous activity (RR comparing extreme categories, 0.57; 95% CI, 0.23-1.42, P=0.070) and walking/hiking for exercise (RR comparing extreme categories, 0.39; 95% CI, 0.09-1.62, P=0.078). There was, however, no clear association between physical activity and breast cancer risk among postmenopausal MHT users. We observed a slight decrease in breast cancer risk among women who reported engaging in no walking at home/work compared to the reference group. A significant interaction was observed between MHT use and walking/hiking for exercise among postmenopausal women (P=0.039), although the interaction was not significant for strenuous activity (P=0.150).
The association between physical activity and breast cancer risk was not modified by age, body mass index, race, age at menarche, parity, family history of breast cancer, personal history of breast disease, oral contraceptive use, smoking, or alcohol consumption. Additionally, to investigate the effect of undiagnosed cancer on physical activity levels, we excluded the first year of follow-up. We found reported associations between physical activity and breast cancer remained largely unchanged.
DISCUSSION
Results from this large prospective cohort of radiologic technologists support a modest inverse relationship between physical activity and breast cancer risk. The strongest associations were observed for walking for exercise among premenopausal women and postmenopausal women who never used MHT, although dose-response relationships were not observed. This is one of few studies to evaluate physical activity and breast cancer risk among postmenopausal women according to MHT use. Further, these data provide additional support that even forms of physical activity of moderate intensity may confer protection against breast cancer.
Our finding of a decrease in breast cancer risk with physical activity is consistent with most previous research [6, 7]. However, the intensity of physical activity required to decrease breast cancer risk is an important public health issue, on which results from previous studies are largely inconclusive. Among studies that differentiate between exercise intensity, most report stronger breast cancer risk reduction associated with strenuous physical activity [14, 15, 30, 31]. However, several studies have also observed risk reductions with moderate forms of physical activity [5, 12, 13, 19, 21, 32-34]. With regard to strenuous activity, results of the present study are similar to those reported by Dorn et al. [14] where breast cancer risk reduction was modest in the highest level of activity and no evidence of a dose-response relationship was observed.
Few studies [5, 14, 35] have specifically evaluated the effects of walking on breast cancer risk. In the current study, women reporting the highest levels of walking/hiking for exercise (10 or more hours per week) experienced the greatest reduction in breast cancer risk, even after accounting for strenuous activity. Modest risk reduction was also observed for women reporting the highest levels of walking at home or work. These findings are promising and suggest that women may not necessarily have to engage in the most strenuous activities to reduce their breast cancer risk. Discrepancies across study results may be attributed to difficulty in accurately recalling moderate levels of intensity as opposed to more strenuous forms of exercise as has been described previously [36, 37].
Findings from our study of pre-menopausal women add to the data suggesting that increased physical activity may decrease risk of breast cancer in this population. Results from previous studies of premenopausal women have been largely inconsistent [6]. Some studies have observed a decreased risk of breast cancer among premenopausal women [3, 32, 38, 39], while others have not [18, 30, 35, 40-43]. Results from the Nurses’ Health Study I observed an approximately 20% risk reduction associated with the highest category of physical activity in both pre- and post-menopausal women [12]. However the Nurses’ Health Study II, which was conducted in a cohort of pre-menopausal women only, found no overall association between physical activity and risk of breast cancer [35].
Studies among postmenopausal women have consistently observed an inverse relationship between physical activity and breast cancer risk [3, 5, 6, 22, 29, 41]. A recent review [6] classified the evidence as strong for this association, with risk reductions in postmenopausal women ranging from 20% to 80%. In our study, MHT use among postmenopausal women modified the relationship between physical activity and risk of breast cancer. We found that among postmenopausal women who never used MHT, both moderate and strenuous physical activity reduced the risk of breast cancer. No reduction in risk with physical activity was observed for women who reported ever using MHT at baseline.
Evidence is accumulating that MHT use may modify the association between physical activity and breast cancer risk. A study by Patel and colleagues [22] observed a stronger association between recreational physical activity and breast cancer among women who were not currently using MHT, similar to the results of our investigation. In a more recent study, both Hispanic and non-Hispanic white women who were postmenopausal and not recently exposed to hormones experienced the most consistent reduction in breast cancer risk [44]. Based on this pattern, it has been suggested that physical activity may affect breast cancer risk in postmenopausal women through hormonal pathways.
Several biologic mechanisms have been proposed to explain the association between physical activity and breast cancer risk. One likely explanation is that physical activity may reduce risk by diminishing adipose tissue. In postmenopausal women, adiposity increases the production of estrogen, which is the main source of circulating estrogen in women not taking MHT [18, 45]. Moderate levels of physical activity on hormone levels in women already at lower levels of baseline circulating estrogen may be sufficient to reduce breast cancer risk whereas more intense activity may be required in women with higher baseline levels of estrogen [22]. Other biological mechanisms proposed to explain to protective effect of physical include changes in insulin-related factors, regulation of the immune system, and hormonal and cellular metabolism pathways [16].
We found a slight decrease in breast cancer risk among postmenopausal ever-users of MHT who engaged in no walking at home/work compared to the reference group. A similar observation among all postmenopausal women was noted in a previous study [22]. As suggested in that report, this pattern may be due to the presence of conditions such as osteoporosis in “never walkers”. Osteoporosis is associated with lower levels of circulating estrogens and consequently lower breast cancer risk; women with osteoporosis are also less likely to engage in physical activity. In the subgroup of postmenopausal women who reported never walking at home/work, women who ever took MHT were more likely to report osteoporosis than those reporting some walking. However, results did not change appreciably after excluding women who reported osteoporosis at baseline.
Strengths of this study include its prospective design, large cohort size and number of incident breast cancers, and extensive information on potential confounders and effect modifiers. Potential limitations of this study result from the use of self-reported physical activity measures. Since physical activity was not a primary study aim in the USRT study, only limited indicators of physical activity were included in the questionnaire for analysis, incomplete dietary information was available, and physical activity measures in our questionnaire were not specifically validated. Our basic measures of physical activity may also introduce confounding of type of physical activity with the intensity of physical activity. Information on occupational physical activity other than walking was not collected, which may be an additional source of exercise [21]. However, the USRT cohort comprises current and former radiologic technologists and current technologists may have similar occupational physical activity levels, suggesting that confounding by occupational activity may be minimal. We also did not collect information about physical activity performed at other ages. Physical activity reported at baseline in this study reflects activity by women who have consistently exercised over their lifetime as well as activity by women who recently began exercising. Thus, our results may reflect the benefit of long term exercise or current exercise with no ability to differentiate between these two effects. Another limitation of this study is the moderate amount of missing physical activity data. Some misclassification of menopausal status may be present since we did not have updated information on menopausal status, thus some women who were premenopausal at baseline may have become postmenopausal during the follow-up period. Lack of significant associations may also be due to small numbers of breast cancer cases in some categories of physical activity.
In summary, our results provide modest support that moderate physical activity may protect against breast cancer. Effect modification by MHT use among postmenopausal women suggests that physical activity may reduce risk through hormonal mechanisms. Further epidemiologic and mechanistic research among postmenopausalwomen is required to clarify the potential hormonal association between physical activity and breast cancer.
ACKNOWLEDGEMENTS
We are indebted to Jeremy Miller, Information Management Services, Rockville, MD, for expert computer support and data management. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
REFERENCES
- 1.American Cancer Society . Cancer facts and figures 2008. American Cancer Society; Atlanta, GA: 2008. [Google Scholar]
- 2.Mittendorf R, Longnecker MP, Newcomb PA, et al. Strenuous physical activity in young adulthood and risk of breast cancer. Cancer Causes Control. 1995;6:347–353. doi: 10.1007/BF00051410. [DOI] [PubMed] [Google Scholar]
- 3.Thune I, Brenn T, Lund E, et al. Physical activity and the risk of breast cancer. N Engl J Med. 1997;336:1269–1275. doi: 10.1056/NEJM199705013361801. [DOI] [PubMed] [Google Scholar]
- 4.Gammon MD, Schoenberg JB, Britton JA, et al. Recreational physical activity and breast cancer risk among women under age 45 years. Am J Epidemiol. 1998;147:273–280. doi: 10.1093/oxfordjournals.aje.a009447. [DOI] [PubMed] [Google Scholar]
- 5.McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 6.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 7.IARC . Weight control and physical activity. Vol. 6. IARC Press; Lyon (France): 2002. IARC handbooks on cancer prevention. [Google Scholar]
- 8.Hoffman-Goetz L, Apter D, Demark-Wahnefried W, Goran MI, McTiernan A, Reichman ME. Possible mechanisms mediating an association between physical activity and breast cancer. Cancer. 1998;83:621–628. doi: 10.1002/(sici)1097-0142(19980801)83:3+<621::aid-cncr4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control. 1998;9:487–509. doi: 10.1023/a:1008853601471. [DOI] [PubMed] [Google Scholar]
- 10.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002;32:180–184. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 11.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8:205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 12.Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Colditz GA. A prospective study of recreational physical activity and breast cancer risk. Arch Intern Med. 1999;159:2290–2296. doi: 10.1001/archinte.159.19.2290. [DOI] [PubMed] [Google Scholar]
- 13.Tehard B, Friedenreich CM, Oppert JM, et al. Effect of physical activity on women at increased risk of breast cancer: results from the E3N Cohort Study. Cancer Epidemiol Biomarkers Prev. 2006;15:57–64. doi: 10.1158/1055-9965.EPI-05-0603. [DOI] [PubMed] [Google Scholar]
- 14.Dorn J, Vena J, Brasure J, Freudenheim J, Graham S. Lifetime physical activity and breast cancer risk in pre- and postmenopausal women. Med Sci Sports Exerc. 2003;35:278–285. doi: 10.1249/01.MSS.0000048835.59454.8D. [DOI] [PubMed] [Google Scholar]
- 15.Dallal CM, Sullivan-Halley J, Ross RK, et al. Long-term recreational physical activity and risk of invasive and in situ breast cancer. Arch Intern Med. 2007;167:408–415. doi: 10.1001/archinte.167.4.408. [DOI] [PubMed] [Google Scholar]
- 16.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42:636–647. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 17.Friedenreich CM. Physical activity and breast cancer risk: the effect of menopausal status. Exerc Sport Sci Rev. 2004;32:180–184. doi: 10.1097/00003677-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Margolis KL, Mucci L, Braaten T, et al. Physical activity in different periods of life and the risk of breast cancer: the Norwegian-Swedish Women’s Lifestyle and Health cohort study. Cancer Epidemiol Biomarkers Prev. 2005;14:27–32. [PubMed] [Google Scholar]
- 19.Lahmann PH, Friedenreich C, Schuit AJ, et al. Physical activity and breast cancer risk: The European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2007;16(1):OF1–7. doi: 10.1158/1055-9965.EPI-06-0582. [DOI] [PubMed] [Google Scholar]
- 20.Moore DB, Folsom AR, Mink PJ, et al. Physical activity and incidence of postmenopausal breast cancer. Epidemiology. 2000;11:292–296. doi: 10.1097/00001648-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Friedenreich CM, Bryant HE, Courneya KS. Case-control study of lifetime physical activity and breast cancer risk. Am J Epidemiol. 2001;154:336–347. doi: 10.1093/aje/154.4.336. [DOI] [PubMed] [Google Scholar]
- 22.Patel AV, Callel EE, Bernstein L, et al. Recreational physical activity and risk of postmenopausal breast cancer in a large cohort of US women. Cancer Causes Control. 2003;14:519–529. doi: 10.1023/a:1024895613663. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein L, Patel AV, Ursin G, et al. Lifetime physical recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst. 2005;97:1671–1679. doi: 10.1093/jnci/dji374. [DOI] [PubMed] [Google Scholar]
- 24.Boice JD, Jr, Mandel JS, Doody MM, Yoder RC, McGowan R. A health survey of radiologic technologists. Cancer. 1992;69:586–98. doi: 10.1002/1097-0142(19920115)69:2<586::aid-cncr2820690251>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Sigurdson AJ, Doody MM, Rao RS, et al. Cancer incidence in the US radiologic technologists health study, 1983-1998. Cancer. 2003;97:3080–9. doi: 10.1002/cncr.11444. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Bowles HR, FitzGerald SJ, Morrow JR, Jr, Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;160:279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- 28.Jackson AW, Morrow JR, Jr, Bowles HR, FitzGerald SJ, Blair SN. Construct validity evidence for single-response items to estimate physical activity levels in large sample studies. Res Q Exerc Sport. 2007;78:24–31. doi: 10.1080/02701367.2007.10599400. [DOI] [PubMed] [Google Scholar]
- 29.Cerhan J, Chiu B, Wallace R, et al. Physical activity, physical function, and the risk of breast cancer in a prospective study among elderly women. J Gerontol. 1998;53A:M251–M256. doi: 10.1093/gerona/53a.4.m251. [DOI] [PubMed] [Google Scholar]
- 30.Steindorf K, Schmidt M, Kropp S, et al. Case-control study of physical activity and breast cancer risk among premenopausal women in Germany. Am J Epidemiol. 2003;157:121–130. doi: 10.1093/aje/kwf181. [DOI] [PubMed] [Google Scholar]
- 31.Shoff SM, Newcomb PA, Trentham-Dietz A, et al. Early-life physical activity and postmenopausal breast cancer: effect of body size and weight change. Cancer Epidemiol Biomarkers Prev. 2000;9:591–5. [PubMed] [Google Scholar]
- 32.Yang D, Bernstein L, Wu AH. Physical activity and breast cancer risk among Asian-American women in Los Angeles: a case-control study. Cancer. 2003;97:2565–2575. doi: 10.1002/cncr.11364. [DOI] [PubMed] [Google Scholar]
- 33.Verloop J, Rookus MA, van der Kooy K, van Leeuwen FE. Physical activity and breast cancer risk in women aged 20-54 years. J Natl Cancer Inst. 2000;92:128–35. doi: 10.1093/jnci/92.2.128. [DOI] [PubMed] [Google Scholar]
- 34.John EM, Horn-Ross PL, Koo J. Lifetime physical activity and breast cancer risk in a multiethnic population: the San Francisco Bay area breast cancer study. Cancer Epidemiol Biomarkers Prev. 2003;12:1143–52. [PubMed] [Google Scholar]
- 35.Colditz GA, Feskanich D, Chen WY, et al. Physical activity and risk of breast cancer in premenopausal women. Br J Cancer. 2003;89:847–851. doi: 10.1038/sj.bjc.6601175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slattery ML, Jacobs DR., Jr. Assessment of ability to recall physical activity of several years ago. Ann Epidemiol. 1995;5:292–6. doi: 10.1016/1047-2797(94)00095-b. [DOI] [PubMed] [Google Scholar]
- 37.Hagstromer M, Oja P, Sjostrom M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755–762. doi: 10.1079/phn2005898. [DOI] [PubMed] [Google Scholar]
- 38.Wyshak G, Frisch RE. Breast cancer among former college athletes compared to non-athletes: a 15-year follow-up. Br J Cancer. 2000;82:726–730. doi: 10.1054/bjoc.1999.0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews CE, Shu XO, Jin F, et al. Lifetime physical activity and breast cancer risk in the Shanghai Breast Cancer Study. Br J Cancer. 2001;84:994–1001. doi: 10.1054/bjoc.2000.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sesso HD, Paffenbarger RS, Jr, Lee IM. Physical activity and breast cancer risk in the College Alumni Health Study. Cancer Causes Control. 1998;9:433–439. doi: 10.1023/a:1008827903302. [DOI] [PubMed] [Google Scholar]
- 41.Breslow RA, Ballard-Barbash R, Munoz K, et al. Long-term recreational physical activity and breast cancer in the National Health and Nutrition Examination Survey I epidemiologic follow-up study. Cancer Epidemiol Biomarkers Prev. 2001;10:805–808. [PubMed] [Google Scholar]
- 42.Luoto R, Latikka P, Pukkala E, et al. Physical activity on breast cancer risk: a cohort study of 30,548 women. Eur J Epidemiol. 2000;16:973–980. doi: 10.1023/a:1010847311422. [DOI] [PubMed] [Google Scholar]
- 43.Magnusson CMK, Roddam AW, Pike MC, et al. Body fatness and physical activity at young ages and the risk of breast cancer in premenopausal women. Br J Cancer. 2005;93:817–824. doi: 10.1038/sj.bjc.6602758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slattery ML, Edwards S, Murtaugh MA, et al. Physical activity and breast cancer risk among women in the Southwestern United States. Ann Epidemiol. 2007;17:342–353. doi: 10.1016/j.annepidem.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siiteri P. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45:277–82. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]