Abstract
Since manifestations of prion diseases include disturbances of hypothalamic and pituitary functions, we tested the hypothesis that the cellular prion protein (PrPC) has a role as modulator of the hypothalamic-pituitary-adrenal axis. Level of corticosterone and adrenocorticotropic hormone were compared in PrPC null (PrP0/0) and wild type (PrP+/+) mice. PrP0/0 showed hypercorticism during the dark part of day. Following acute stress, corticosterone and adrenocorticotropic hormone increased similarly in PrP+/+ and PrP0/0 mice. However, adrenocorticotropic hormone remained elevated in PrP0/0 mice at corticosterone levels that are inhibitory in PrP+/+ mice. Pretreatment with corticosterone or dexamethasone inhibited stress-induced elevation of adrenocorticotropic hormone in PrP+/+ but not in PrP0/0 mice. Thus, PrPC may play a role in the negative feedback regulation of axis.
Keywords: Prion, Corticosteroid, Adrenal, Hypothalamus, Hypothalamic-Pituitary-Adrenal, Stress, Adrenocorticotropic Hormone
Introduction
Transmissible spongiform encephalopathies are lethal neurodegenerative disorders characterized by astrocytosis, spongiform degeneration of neurons and neuronal loss (for review see [1]). Neurodegeneration associated with TSE requires post-translational modification of the cellular prion protein (PrPC) leading to the accumulation of an abnormal protease-resistant isoform (PrPSc). While PrPC is required for PrPSc amplification and subsequent deposition associated with neuropathology, the normal physiological role of PrPC remains largely unknown.
The hypothalamic-pituitary-adrenal axis is a vital homeostatic system regulating neuroendocrine functions [2]. Its activation occurs when a stimulus, primarily stress, stimulates hypothalamic secretion of corticotropic releasing hormone that, in turn, induces the pituitary gland to release adrenocorticotropic hormone into the circulation. Finally, adrenocorticotropic hormone activates synthesis and secretion of corticosteroids from the adrenal glands. The activation is counter-regulated by corticosteroids that inhibit the secretion of corticotropic releasing and adrenocorticotropic hormones.
Disturbances of hypothalamic and pituitary functions have long been recognized as clinical signs of natural scrapie [3–6]. Sheep with naturally occurring scrapie have hypertrophy of the median eminence of hypothalamic pathway indicating alterations of adrenocorticotropic hormone regulation possibly through vasopressin and corticotropic releasing factor neurons [6]. Other indications include adrenal glands enlargement of ewes naturally affected with scrapie, and of experimentally infected hamsters [5, 7, 8]. Hypercorticism was observed in scrapie-affected ewes, mice inoculated with scrapie and humans with fatal familial insomnia [8–13]. Infected ewes showed hyper-responsive adrenal cortex, lowered corticosteroid binding globulin (CBG) and sustained adrenocorticotropic hormone secretion despite the high level of glucocorticoids indicating a disruption of the pathways involved in the negative feedback inhibition of the axis [9, 12, 13].
Scrapie-associated hypercorticism could be a secondary consequence of the neurodegenerative nature of the disease or the result of loss of endogenous PrPC. To begin addressing these two possibilities we compared circadian and stress induced adrenocorticotropic hormone and corticosterone levels in disease-free mice devoid of PrPC (mPrP0/0) and their wt littermate (mPrP+/+).
Methods
All procedures were approved by The Scripps Research Institute Animal Care and Use Committee. mPrP0/0 and mPrP+/+ mice both in the 129/Ola background and were obtained by Jean Manson (Institute for Animal Health, Edinburgh, UK) [1, 14–17]. Aanimals (4 per cage) were kept on a 12:12-h light/dark cycle with lights on at 6:00 AM, ad libitum food and water. Stress was delivered between 6:00 and 8:00 when corticosterone levels were lowest (Figure 1) by placing animals in ventilated plexiglas restrainers. Trunk blood was collected from animals anesthetized by 1 minute exposure to 5% isoflurane. Corticosterone and adrenocorticotropic hormone levels were determined on EDTA plasma using commercially available radioimmunoassay kits (MP Biomedicals, Orangeburg, NY). For statistical analysis ANOVA and a pos-hoc Sheffé test were performed, results are expressed as means ± standard error. A significance of p <0.05 was considered in all cases.
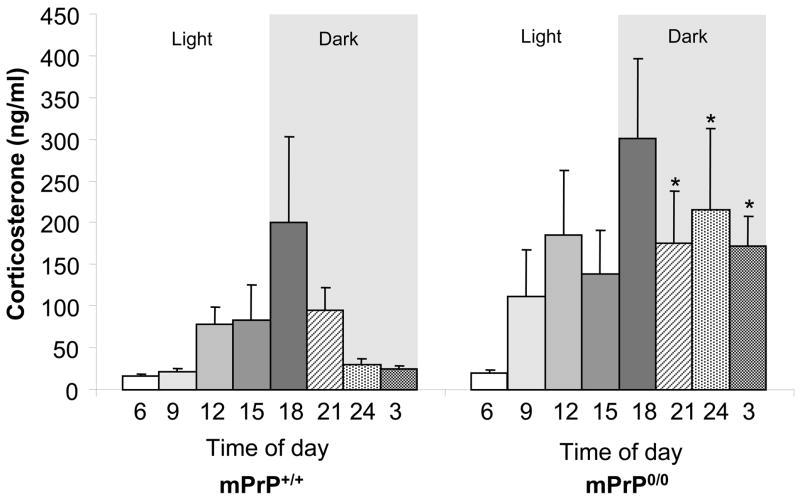
Figure 1.
Circadian profile of corticosterone level in mPrP+/+ and mPrP0/0 mice. Plasma corticosterone levels were determined at three hrs intervals during 24 hrs starting at 6:00 AM (n= 5 per point; *p< 0.05)
Results
To determine whether mice devoid of endogenous cellular PrP have hypercorticism similar to what is observed in animals infected with PrPSc, levels of circulating corticosterone were assessed at 3 hrs intervals over a 24 hrs period. The values (ng/ml) in mPrP+/+ vs mPrP0/0 were (6:00)17.4 ± 1 vs 19.8 ± 3.4; (9:00) 22.1 ± 3.4 vs 111.6 ± 55.8; (12:00) 78.8 ± 20.1 vs 186.5 ± 75.4; (15:00) 83.0 ± 41.8 vs 139.1 ± 51.6; (18:00) 200.6 ± 101.7 vs 300.9 ± 95.9; (21:00) 95.2 ± 26.3 vs 176.0* ± 61.9; (24:00) 29.4 ± 7.6 vs 215.6* ± 97.5; (3 AM) 25.5 ± 3.2 vs 171.7* ± 35.7 (n= 5 per point; *p< 0.05) (Figure 1).
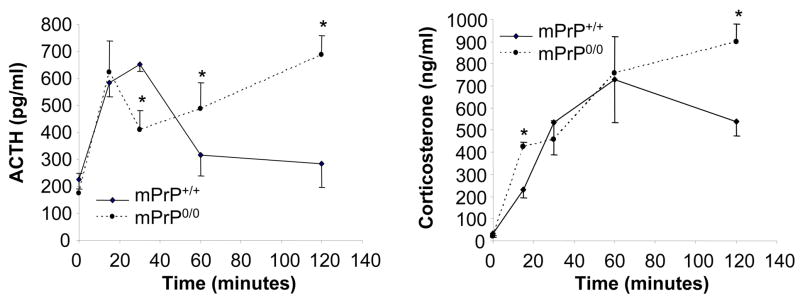
Hypothalamic-pituitary-adrenal axis activity was investigated by determining the levels of adrenocorticotropic hormone and corticosterone in animals not stressed or following 15, 30, 60 or 120 minutes of restraint stress. Experiments were carried out between 6AM and 8AM when circadian corticosterone levels were found to be lowest in a room adjacent to that hosting the colony. Control mice were sacrificed 1–2 minutes after transfer to the procedure room. The levels of adrenocorticotropic hormone (pg/ml) in mPrP+/+ vs mPrP0/0 were (non-stressed) 226.6 ± 34 vs 172.8 ± 75; (15 min restraint stress) 582.7 ± 50 vs 621.8 ± 118; (30 min restraint stress) 650.4 ± 23 vs 410.9* ± 69; (60 min restraint stress) 317.1 ± 77 vs 488.7* ± 95; (120 min restraint stress) 283.2 ± 84 vs 685.7* ± 72. The levels of corticosterone (ng/ml) in mPrP+/+ vs mPrP0/0 were (non-stressed) 30.7 ± 13 vs 22.2* ± 3; (15 min restraint stress) 232.4 ± 37 vs 425.3 ± 21; (30 min restraint stress) 534.4 ± 145 vs 455.7 ± 85; (60 min restraint stress) 728.2 ± 194 vs 757.6 ± 166; (120 min restraint stress) 540.4 ± 68 vs 898.2* ± 80 (n= 5 per point; *p< 0.05) (Figure 2).
Figure 2.
Adrenocorticotropic hormone (ACTH) and corticosterone (Cort) levels during acute restraint stress. mPrP+/+ and mPrP0/0 mice subject to restraint stress for 15, 30, 60 or 120 minutes (n=5, *p< 0.05)
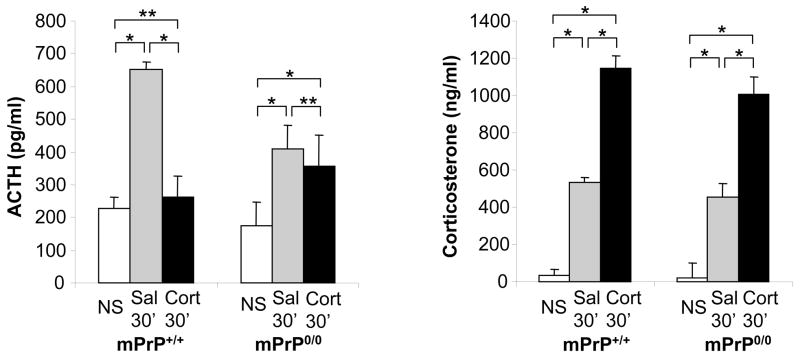
To test the hypothesis that prolonged adrenocorticotropic hormone elevation in mPrP0/0 mice might be due to impaired corticosterone-mediated feed-back inhibition, the response to acute stress in mPrP0/0 and mPrP+/+ mice was measured following pretreatment with corticosterone or the synthetic corticosteroid dexamethasone. Intraperitoneal injection of 5 mg/kg corticosterone (Sigma Aldrich, Saint Luis, MO) or saline1 hr prior to 30 minute restraint stress resulted in a large elevation of corticosterone levels that inhibited stress-induced adrenocorticotropic hormone elevation. In contrast, similarly high levels of corticosterone did not inhibit adrenocorticotropic hormone secretion during stress in mPrP0/0 mice: adrenocorticotropic hormone (pg/ml) (non-stressed vs saline + 30 min restraint stress vs corticosterone+ 30 min restraint stress) mPrP+/+ 226.6 ± 34 vs 650.4 ± 23 vs 261.0± 64; mPrP0/0 172.8 ± 75 vs 410.9 ± 69 vs ± 356.7 ± 93; Corticosterone (ng/ml) (non-stressed vs saline + 30 min restraint stress vs corticosterone+ 30 min restraint stress) 30.7 ± 14 vs 534.4 ± 145 vs 1148.9 ± 46; mPrP0/022.2 ± 3; 455.7 ± 85 vs 1003.8 ± 52 (Figure 3).
Figure 3.
Adrenocorticotropic hormone (ACTH) and corticosterone (Cort) levels following corticosterone pretreatment. Mice received no stress (NS) or 30 minutes of restraint stress (30′) 1 hr after pretreatment with saline (Sal) or corticosterone (Cort) (n=5; *p<0.05; **= not significant)
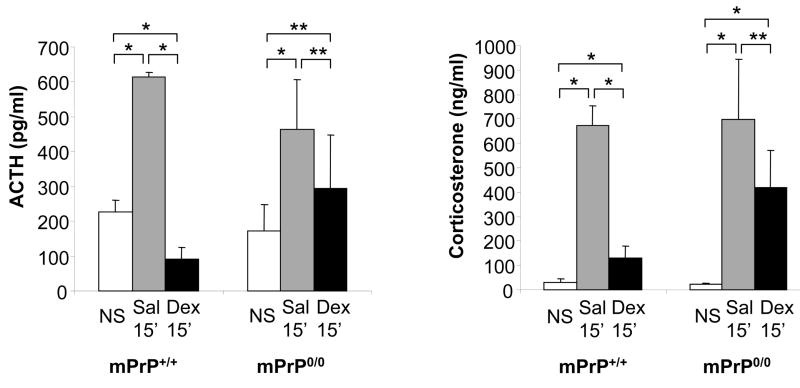
The same results were obtained in similar experiments carried out pre-treating mice with 5 mg/kg of the synthetic corticosteroid dexamethasone (Sigma Aldrich) or with saline 1 hr prior to 15 minute restraint stress. The use of dexamethasone allowed the measurement of endogenous corticosterone that also demonstrated impaired inhibition of hypothalamic-pituitary-adrenal axis in mPrP0/0 mice. adrenocorticotropic hormone (pg/ml) (non-stressed vs saline +15 min restraint stress vs dexamethasone + 15 min restraint stress) mPrP+/+ 226.6 ± 35 vs 614.1 ± 13 vs 91.6 ± 34; mPrP0/0 172.8 ± 75 vs 463.6 ± 142 vs 292.9 ± 155; Corticosterone (ng/ml) (non-stressed vs saline +15 min restraint stress vs dexamethasone + 15 min restraint stress) mPrP+/+ 30.7 ± 14 vs 672.7 ± 81 vs 129.1 ± 49; mPrP0/0 22.2 ± 3 vs 696.1 ± 249 vs 417.7 ± 152 (Figure 4).
Figure 4.
Adrenocorticotropic hormone (ACTH) and corticosterone (Cort) levels following dexamethasone (Dex) pretreatment. Mice received no stress (NS) or 15 minutes of restraint stress (15′) 1 hr after pretreatment with saline (Sal) or Dex (n=5; *p<0.05; **= not significant)
Discussion
We demonstrated for the first time that healthy mice devoid of PrPC have hypercorticism similar to that observed in animals naturally affected by scrapie. This suggests that PrPC may have a direct role in the modulation of the hypothalamic-pituitary-adrenal axis and that some of the symptoms caused by TSE might be, at least partly, due to lack of PrPC rather than to the presence of PrPSc or its deposition.
Mice with experimental prion disease showed hypercorticism as well as late disruption of the circadian pattern of corticosterone profile [15]. Compared to mPrP+/+ mice, the circulating level of corticosterone in mPrP0/0 animals was elevated at all points throughout the 24 hrs except at the beginning of the light/inactive phase, and reached statistically significant differences only during the dark/active phase. However, mPrP0/0 mice did not present a disruption of the circadian profile of corticosterone release nor of the core body temperature profile (not shown). Circadian rythmicity is controlled by the hypothalamic suprachiasmatic and paraventricular nuclei. PrPC is expressed in the suprachiasmatic nucleus where, similarly to what is observed in other brain regions including the supraoptic nucleus, caudate putamen and cortex, its mRNA is regulated in a circadian fashion with levels peaking to nearly double 2 hrs into the dark phase of the day [18]. The present observation that mPrP0/0 mice did not lose circadian rythmicity suggested that PrPC is subject to circadian fluctuation rather than contributing to its regulation.
Both mPrP0/0 and mPrP+/+ mice showed an activation of the hypothalamic-pituitary-adrenal axis during acute stress, but different profiles were observed in the maintenance of the response (Figure 2). Adrenocorticotropic hormone levels increased readily in mPrP+/+ mice, peaked at 30 minutes and decreased thereafter as a consequence of the paralleled increased in corticosterone levels that eventually inhibited adrenocorticotropic hormone secretion. In mPrP0/0 mice, adrenocorticotropic hormone levels increased similarly to mPrP+/+ in the first 15 minutes but temporarily decreased before steadily increasing at 30, 60 and 120 minutes even when circulating levels of corticosterone reached a concentration that was inhibitory in mPrP+/+ mice. Overall, these results demonstrate that during acute restraint stress mPrP0/0 mice have normal activation, but delayed feedback inhibition of hypothalamic-pituitary-adrenal axis. This hypothesis was further supported by demonstrating that both corticosterone and dexamethasone pretreatments display a reduced ability to prevent acute stress-induced hypothalamic-pituitary-adrenal axis activation in mPrP0/0 mice.
These finding suggests that PrPC plays an important role in the modulation of the corticosteroid-dependent negative feedback of hypothalamic-pituitary-adrenal axis and may contribute to the elusive understanding of the physiological role of PrPC. The mechanisms of such action of PrPC were not addressed in the present study and will require extended investigation, given the complexity of the feedback regulation of hypothalamic-pituitary-adrenal axis. Corticosteroid-mediated negative feedback occurs primarily by inhibition of CRH and adrenocorticotropic hormone secretion [19–21]. However, vasopressin, oxytocin and epinephrine also act as secretagogues of adrenocorticotropic hormone and may thus be involved [22]. In addition, the feedback occurs in at least three time domains (fast, intermediate and slow) [19], that may or may not require mineralcorticoids and glucocorticoid receptors, and can be modulated by different brain regions including hypothalamus, hippocampus and frontal cortex [2, 22–24].
Conclusion
In summary, these data indicate that healthy mice devoid of PrPC display hypercorticism resembling that observed in animals naturally affected by scrapie. This phenotype may be, at least partly, attributed to impaired corticosteroid-mediated negative inhibition of the hypothalamic-pituitary-adrenal axis. This suggests that some of the symptoms caused by transmissible spongiform encephalopathy might be due to lack of PrPC-mediated biological activity rather than to the presence and deposition of PrPSc per se, an hypothesis that was never clearly demonstrated nor refuted [25].
Acknowledgments
We thank Michael BA Oldstone and Tamas Bartfai for comments and Jean Manson of for providing the mice. Supported partly by USHP AG04342 and by NIH N504350103, AG028040, HL088083.
References
- 1.Weissmann C, Enari M, Klohn PC, Rossi D, Flechsig E. Molecular biology of prions. Acta Neurobiol Exp. 2002;62:153–166. doi: 10.55782/ane-2002-1434. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Reviews. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 3.Parry HB. Studies in scrapie. Veterinary Record. 1957;69:1318–1324. [Google Scholar]
- 4.Parry HB. Scrapie: a transmissible and hereditary disease of sheep. Heredity. 1962;17:75–105. doi: 10.1038/hdy.1962.4. [DOI] [PubMed] [Google Scholar]
- 5.Beck E, Daniel PM, Parry HB. Degeneration of the cerebellar and hypothalamoneurohypophysial systems in sheep with scrapie; and its relationship to human system degenerations. Brain Pathol. 1964;87:153–176. doi: 10.1093/brain/87.1.153. [DOI] [PubMed] [Google Scholar]
- 6.Parry HB, Livett BG. A new hypothalamic pathway to the median eminence containing neurophysin and its hypertrophy in sheep with natural scrapie. Nature. 1973;242(5392):63–65. doi: 10.1038/242063a0. [DOI] [PubMed] [Google Scholar]
- 7.Carp RI, Kim YS, Callahan SM. Pancreatic lesions and hypoglycemia-hyperinsulinemia in scrapie-injected hamsters. Journal of Infectious Diseases. 1990;161(3):462–466. doi: 10.1093/infdis/161.3.462. [DOI] [PubMed] [Google Scholar]
- 8.Portaluppi F, Cortelli P, Avoni P, Vergnani L, Contin M, Maltoni P, et al. Diurnal blood pressure variation and hormonal correlates in fatal familial insomnia. Hypertension. 1994;23(5):569–576. doi: 10.1161/01.hyp.23.5.569. [DOI] [PubMed] [Google Scholar]
- 9.Picard-Hagen N, Gayrard V, Laroute V, Grandjean C, Andreoletti O, Elsen JM, et al. Discriminant value of blood and urinary corticoids for the diagnosis of scrapie in live sheep. Veterinary Record. 2002;150(22):680–684. doi: 10.1136/vr.150.22.680. [DOI] [PubMed] [Google Scholar]
- 10.Schelcher F, Picard-Hagen N, Laroute V, Gayrard V, Popot MA, Andreoletti O, et al. Corticoid concentrations are increased in the plasma and urine of ewes with naturally occurring scrapie. Endocrinology. 1999;140(5):2422–2425. doi: 10.1210/endo.140.5.6896. [DOI] [PubMed] [Google Scholar]
- 11.Voigtlander T, Unterberger U, Touma C, Palme R, Polster B, Strohschneider M, et al. Prominent corticosteroid disturbance in experimental prion disease. Eur J Neurosci. 2006 May;23(10):2723–2730. doi: 10.1111/j.1460-9568.2006.04801.x. [DOI] [PubMed] [Google Scholar]
- 12.Picard-Hagen N, Gayrard V, Alvinerie M, Laroute V, Touron C, Andreoletti O, et al. Naturally occurring scrapie is associated with a lower CBG binding capacity in ewes. Journal of Endocrinology. 2000;165(2):527–532. doi: 10.1677/joe.0.1650527. [DOI] [PubMed] [Google Scholar]
- 13.Gayrard V, Picard-Hagen N, Grino M, Sauze N, Grandjean C, Galea J, et al. Major hypercorticism is an endocrine feature of ewes with naturally occurring scrapie. Endocrinology. 2000;141(3):988–994. doi: 10.1210/endo.141.3.7345. [DOI] [PubMed] [Google Scholar]
- 14.Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol. 1994;8(2–3):121–127. doi: 10.1007/BF02780662. [DOI] [PubMed] [Google Scholar]
- 15.Manson JC, Hope J, Clarke AR, Johnston A, Black C, MacLeod N. PrP gene dosage and long term potentiation. Neurodegeneration. 1994;4:113–115. doi: 10.1006/neur.1995.0014. [DOI] [PubMed] [Google Scholar]
- 16.Moore RC, Lee IY, Silverman GL, Harrison PM, Strome R, Heinrich C, et al. Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J Mol Biol. 1999;292(4):797–817. doi: 10.1006/jmbi.1999.3108. [DOI] [PubMed] [Google Scholar]
- 17.Silverman GL, Qin K, Moore RC, Yang Y, Mastrangelo P, Tremblay P, et al. Doppel is an N-glycosylated, glycosylphosphatidylinositol-anchored protein. Expression in testis and ectopic production in the brains of Prnp(0/0) mice predisposed to Purkinje cell loss. J Biol Chem. 2000;275(35):26834–26841. doi: 10.1074/jbc.M003888200. [DOI] [PubMed] [Google Scholar]
- 18.Cagampang FR, Whatley SA, Mitchell AL, Powell JF, Campbell IC, Coen CW. Circadian regulation of prion protein messenger RNA in the rat forebrain: a widespread and synchronous rhythm. Neuroscience. 1999;91(4):1201–1204. doi: 10.1016/s0306-4522(99)00092-5. [DOI] [PubMed] [Google Scholar]
- 19.Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocrine Reviews. 1984;5(1):1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 20.Plotsky PM, Vale W. Hemorrhage-induced secretion of corticotropin-releasing factor-like immunoreactivity into the rat hypophysial portal circulation and its inhibition by glucocorticoids. Endocrinology. 1984 Jan;114(1):164–169. doi: 10.1210/endo-114-1-164. [DOI] [PubMed] [Google Scholar]
- 21.Widmaier EP, Dallman MF. The effects of corticotropin-releasing factor on adrenocorticotropin secretion from perifused pituitaries in vitro: rapid inhibition by glucocorticoids. Endocrinology. 1984 Dec;115(6):2368–2374. doi: 10.1210/endo-115-6-2368. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005 Jun;34(2):271–292. vii. doi: 10.1016/j.ecl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993 Sep;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003 Jul;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996 Jan 25;379(6563):339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]