Abstract
Stress induced by early life social isolation leads to long-lasting alterations in stress responses and serotonergic activity. Corticotropin-releasing factor (CRF) is a neurotransmitter that mediates stress responses and alters serotonergic activity. We tested the hypothesis that the stress of early life isolation enhances responses to CRF in adulthood by determining the effect of CRF infusions into the dorsal raphe nucleus (dRN) on serotonin (5-HT) release in the nucleus accumbens (NAc) of adult rats using in vivo microdialysis. Juvenile male rats were either isolated or housed in groups of 3 for a 3 week period beginning on postnatal day 21 after which, all rats were group-reared for an additional 2 weeks. Following the isolation/re-socialization procedure, infusion of 100 ng CRF into the dRN decreased 5-HT release in the NAc of group-reared rats. This treatment did not significantly affect 5-HT release in the NAc of isolation-reared animals. In contrast, infusion of 500 ng CRF into the dRN transiently increased 5-HT release in the NAc of both group-reared and isolated animals with isolated animals showing a more prolonged serotonergic response. Western blot and immunofluorescent staining for CRF receptors in the dRN showed that CRF2 receptor levels were increased in the dRN of isolation-reared animals when compared to group-reared rats. Taken together, the results suggest that isolation during the early part of development causes alterations in both CRF receptor levels and CRF-mediated serotonergic activity. These effects may underlie the increased sensitivity to stress observed in isolates.
Keywords: microdialysis, stress, serotonin, nucleus accumbens, dorsal raphe nucleus
Introduction
Isolation from social counterparts during development is an animal model of early-life stress that leads to long-lasting alterations in behavior and monoaminergic activity (Hall, 1998, Varlinskaya et al., 1999, Heidbreder et al., 2000). Social activity in adolescent rodents is essential for developing an ability to express and understand intraspecific communicative signals (Meaney and Stewart, 1981, Varlinskaya et al., 1999). Social isolation of rats has the most potent effects during a critical phase from weaning to early adulthood (Einon and Morgan, 1977, Leng et al., 2004, Weiss et al., 2004, Arakawa, 2005, Ferdman et al., 2007). These changes last into adulthood, even when the isolated rats are returned to group-housing for re-socialization (Einon and Morgan, 1977, Wright et al., 1991, Nunes Mamede Rosa et al., 2005, Pascual et al., 2006). Adult rats exposed to early-life social isolation exhibit locomotor hyperactivity in a novel open field, increased aggression and anxiety, and altered responses to reward-related stimuli (Valzelli, 1973, Sahakian et al., 1975, Einon and Morgan, 1977, Sahakian and Robbins, 1977, Stanford et al., 1988, Wright et al., 1991). Furthermore, early-life social isolation affects cognitive function as evidenced by spatial memory deficits and impaired maze learning (Einon and Morgan, 1977, Einon, 1980).
The hypothalamic-pituitary-adrenal (HPA) axis is more responsive to stressors in early life compared to adulthood (Gomez et al., 2002), and neural monoaminergic levels do not reach adult levels until postnatal days 30-50 (Coyle and Henry, 1973). Therefore, it is not surprising that early-life social isolation induces long-lasting changes in the monoaminergic activity, which is known to play an important role in the response to stress (Miura et al., 2002b, Weiss et al., 2004). For example, altered serotonin (5-HT) and dopamine concentrations in the medial prefrontal cortex and nucleus accumbens (NAc) have been observed following social isolation in rats (Blanc et al., 1980, Jones et al., 1992, Miura et al., 2002b). Interestingly, the direction of monoaminergic change is related to the length of the isolation period and the time during development that isolation occurred.
Altered serotonergic activity in the NAc has been implicated as a substrate for many of the behavioral disturbances associated with post-weaning social isolation (Jones et al., 1990, Jones et al., 1992, Hall, 1998, Howes et al., 2000). For example, isolation-reared rats show elevated 5-HT turnover in the NAc in response to novelty (Miura et al., 2002a). Isolation-reared rats also show elevated NAc shell 5-HT release during exposure to a foot-shock or to the contextual cue associated with foot-shock (Fulford and Marsden, 1998). It has been suggested that alterations to serotonergic activity in the NAc, as a consequence of stressful events early in life, may contribute to the development of symptoms characterizing psychiatric disorders such as schizophrenia, anxiety disorders and depression (Weiss et al., 2004).
Corticotropin-releasing factor (CRF) is a neurotransmitter involved in integrating multiple components of the stress response. The central nucleus of the amygdala provides CRF innervation to the dorsal raphe nucleus (dRN; (Gray, 1993), which in turn provides 5-HT innervation to the NAc (Van Bockstaele et al., 1993). Behavioral effects induced by CRF, such as increased anxiety and stress responses, are thought to be mediated, in part, by CRF effects on 5-HT release (Kirby et al., 2000, Hammack et al., 2002, Forster et al., 2006, Lowry and Moore, 2006). Both CRF1 and CRF2 receptors are present in the dRN (Day et al., 2004) and have opposing effects on 5-HT release (Kirby et al., 2000, Pernar et al., 2004, Lukkes et al., 2008). While CRF activates both CRF1 and CRF2 receptors, CRF2 receptors have a lower affinity for CRF (Bale, 2005). Lower concentrations of CRF activate CRF1 receptors in the dRN and result in decreased 5-HT release in the NAc (Lukkes et al., 2008). In contrast, higher concentrations of CRF infused into the dRN activate CRF2 receptors which, in turn, increase 5-HT release in the NAc (Lukkes et al., 2008).
Previous work has implicated alterations of adult 5-HT levels in the NAc in the neural disruptions induced by early life social isolation of rats (Jones et al., 1992, Fulford and Marsden, 1998, Hall, 1998, Heidbreder et al., 2000). However, potential effects of early-life social isolation on the modulation of 5-HT systems by CRF have not been investigated. Given the role of CRF in modulating 5-HT activity, fear, anxiety and stress responses, it appears likely that alterations to CRF modulation of 5-HT systems could underlie some of the neural and behavioral disruptions resulting from early-life social isolation. We hypothesized that isolation-rearing of male rats during the period encompassing pre to early adolescence would alter CRF-induced serotonergic activity in the NAc when tested in early adulthood. Concentrations of 100 ng and 500 ng CRF were infused into the dRN of adult rats exposed to early life social isolation, since these doses activate CRF1 verses CRF2 receptors, respectively (Lukkes et al., 2008). We also predicted that any change in CRF-modulation of NAc 5-HT activity would be related to alterations of CRF receptor levels in the dRN of isolation-reared rats, as measured by western immunoblots and immunocytochemistry.
Experimental Procedures
Animals and social isolation protocol
Male rats were used in the following experiments for consistency with the majority of previous studies investigating the behavioral effects of early-life social isolation. Fifty-seven male Sprague-Dawley rats (University of South Dakota Laboratory Animal Services, Vermillion, SD, USA), were maintained at a constant room temperature (RT; 22°C, 60% relative humidity) and a reverse 12h light: 12h dark cycle. Food and water were available ad libitum. Experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of South Dakota, and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
On postnatal day 21 (P21; weaning age corresponding to pre-adolescence), male rats were housed either individually or in groups of 3 for a period of 3 weeks during pre-adolescent to mid-adolescent development (P21-P42)(Bowling and Bardo, 1994, Hall, 1998, Spear, 2000, Andersen, 2003). After 3 weeks of isolation, rats were weighed and housed in groups of 3 with other isolates for a re-socialization period of 2 weeks (McCormick et al., 2004, Nunes Mamede Rosa et al., 2005, Pascual et al., 2006). At this time, group-reared rats were also randomly re-housed with two new group-reared rats to ensure that any observed differences between isolates and group-reared rats were attributable to isolation rearing and not the re-socialization procedure. The re-socialization following the critical isolation period (pre-adolescence) allowed the rats to complete their development from mid-adolescence to early adulthood. Furthermore, re-socialization ensured that any observed behavioral or neurochemical changes were due to social isolation during the pre-adolescent critical period instead of at any other point during early life. At the end of the 5-week isolation/re-socialization procedure, rats reached early adulthood (P56) and were used in the following experiments.
Experiment 1: Effects of social isolation on CRF-mediated 5-HT release in NAc
Microdialysis and pharmacological procedures
To remain consistent with our previous methodology that identified the CRF receptor subtypes in the dRN meditating accumbal serotonin release (Lukkes et al., 2008), the following experiments were conducted under urethane anesthesia. Urethane is a long-lasting anesthetic that has minimal effects neuronal firing rates and neurotransmitter release, and urethane-anesthetized rats show similar patterns of serotonergic release in response to CRF when compared to awake rats (Maggi and Meli, 1986, Forster et al., 2006, Forster et al., 2008). After induction of anesthesia, the overall timeline of these experiments followed that of Lukkes et al. (2008). A probe was implanted in the NAc and a guide cannula implanted above the dRN, and a CRF or vehicle-filled cannula was inserted into the dRN 3 hours later, with a further hour allowed before microdialysis sampling of 5-HT levels in the NAc.
Rats were anesthetized with urethane (1.8 g/kg i.p; Sigma, St. Louis, MO) and placed in a stereotaxic frame (David Kopf Institute, CA, USA) with the incisor bar set at -3.3 mm. Body temperature was maintained at 37 ± 0.5°C with a temperature regulated heating pad (CMA, North Chelmsford, MA). A concentric microdialysis probe (membrane length 2 mm, with MW cut-off 5000; (Hoffman et al., 2002, Lukkes et al., 2008) was inserted into the NAc (AP: +1.2 from bregma; ML: -1.4 from midline; DV: -8.1 from dura; Paxinos and Watson, 1997). A single stainless-steel infusion guide cannula (26 gauge) was implanted 2 mm above the dRN (AP: -7.4 from bregma; ML: +2.8 from midline; DV: -4.6 from dura; Paxinos and Watson, 1997) at a 26° lateral to medial angle to avoid the cerebral aqueduct (Forster et al., 2006, Lukkes et al., 2008).
Artificial CSF (aCSF; (Moghaddam et al., 1989) was perfused continuously through the probe at a rate of 0.4 μl/min with a microinfusion pump (CMA) via PE-20 tubing connected to a 1 ml syringe. Three hours after probe insertion, a silica cannula (194 μm od, 2 mm longer than guides; Polymicro Technologies, Phoenix, AZ) was lowered through a cannula. The silica cannula was fixed to PE-20 tubing connected to a 10 μl Hamilton syringe, and rat-human CRF (100 or 500 ng/0.5 μl; Sigma Aldrich) or vehicle (aCSF) was back-loaded into the cannula before implantation as described previously (Forster and Blaha, 2000). Microdialysis sampling began 4 hours after the implantation of the probe (Lukkes et al., 2008), with perfusates collected at 20 min intervals and analyzed for 5-HT. Following at least 3 stable baseline 5-HT samples, either aCSF (0.5 μl; vehicle for CRF) or CRF (100 or 500 ng; 0.5 μl;(Lukkes et al., 2008) was infused over a one minute period through the silica cannula directed at the dRN. Sampling of 5-HT in perfusates was continued until 5-HT levels returned to pretreatment levels.
Serotonin analysis
Analysis of 5-HT in dialysates was accomplished by using high-performance liquid chromatography (HPLC) with electrochemical detection (Bradberry et al., 1991, Hoffman et al., 2002). Samples were injected into a chromatographic system with a 5 μl loop. The mobile phase used for 5-HT separation (0.08 g EDTA, 0.54 g of 1-decanesulfonic acid, 2.8 g sodium phosphate monobasic, 180 ml acetonitrile, 300 μl triethylamine in 1 L of deionized water, pH 5.83; typical elution time for 5-HT=11.4 min) was pumped through a UniJet 5 μm C18 silica column (Bioanalytical Systems, West Lafayette, IN) under nitrogen gas pressure (2000 psi). The collection rate of 0.4 μl/ min resulted in approximately 8 μl of dialysate to insure that the loop was overfilled during each sample period. Following separation by the column, 5-HT was detected by a glassy carbon electrode (Bioanalytical Systems) maintained at +0.65 V with respect to the Ag/AgCl2 reference electrode with a LC-4C potentiostat (Bioanalytical Systems). The voltage output was recorded by CSW32 v1.4 Chromatography Station for Windows (DataApex, Prague, Czech Republic). Serotonin peaks were identified by comparison to a 5-HT standard (7.9 pg/5 μl 5-HT). The 2:1 signal to noise detection limit for 5-HT using this system was 0.57 ±0.07 pg for group-reared and 0.64 ±0.11 pg for isolation-reared animals. Pre-infusion (baseline) levels of 5-HT (uncorrected for probe recovery) were 2.65±0.72 pg/5 μl for group-reared rats and 2.26±0.31 pg/5 μl for isolates. Baseline 5-HT levels were not significantly different between the two groups (P=0.636).
Histology
At the conclusion of each microdialysis experiment, rats were administered a lethal dose of Fatal-plus (Vortech, Dearborn, MI, 0.5 ml, ip.). Brains were removed and fixed in 10% formalin. Sections (60 μm) were cut at −12 °C, and analyzed under a light microscope to confirm appropriate placements of microdialysis probes and drug infusion cannulae. Only data from rats with correct probe and cannulae placements were included in the data analyses (n=7-11 per group).
Experiment 2: Effects of social isolation on CRF receptor levels within the dRN
Western blot analysis of primary antibody specificity
Western blots were performed with rats not subjected to the isolation/re-socialization procedure to determine the specificity of the primary antibodies used. Rats (n = 2) were decapitated and tissue from the hindbrain was dissected free, frozen on dry ice, and stored at -80 °C until processed. Tissue was then homogenized in extraction buffer (pH 7.4, 4 °C) containing 50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% Triton-X, 20 μl/ml protease inhibitor stock “complete” (Roche Diagnostics, IN), and 2.5 μl/ml PMSF. The homogenates were centrifuged at 1000 × g for 10 min and aliquoted, frozen, and stored at -20 °C. Protein concentration was determined by the Bradford method (BioRad Laboratories, Hercules, CA, Bradford, 1976) and 36.6 μg of protein was mixed in 1 × SDS/β-mercaptoethanol, vortexed and boiled for 3 min prior to separation by 8% SDS-PAGE. Following electrophoresis (BioRad Laboratories), proteins were transferred to an Immuno-Blot PVDF membrane (0.2 μm, BioRad Laboratories). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for 20 minutes at RT and incubated with primary polyclonal antibodies to CRF1 receptors (1:100; Santa Cruz Biotech, Santa Cruz, CA, #SC-12381) and CRF2 receptors (1:100; Santa Cruz Biotech, #SC-1826, no reported reactivity with CRF1 receptors; (Tian et al., 2006) in 5% nonfat dry milk in TBS-T overnight 4 °C. The membranes were rinsed 3 times for 10 minutes at RT in TBS-T. After the rinsing procedure, the membranes were incubated for 2 hours at room temperature in IRDye 800-conjugated affinity purified anti-goat IgG (H & L) (Rockland Inc, Gilbertsville, PA, #605-732-125) 1:1000 for CRF1 receptors and 1:2000 for CRF2 receptors, in 5% nonfat dry milk in TBS-T. For controls, primary antibodies were incubated for 48 hours at 4 °C with purified CRF1 blocking peptide (1:12 ratio of primary antibody to blocking peptide; Santa Cruz Biotech, #SC-12381P) or CRF2 blocking peptide (1:12 ratio of primary antibody to blocking peptide; Santa Cruz Biotech, #SC-1826P). Control for protein loading was achieved by using primary antibodies to actin (1:2000; #MAB1501R; Chemicon International, USA) and secondary antibodies to actin at 1:5000 for IRDye 80-conjugated affinity purified anti-mouse IgG (H&L; #610-132-121; Rockland Inc) in 5% nonfat dry milk in TBS-T. Proteins were detected using the Odyssey infrared imaging system (excitation/emission filters at 780 nm/820 nm range, LI-COR Biosciences, Lincoln, NE).
Western blot analysis of CRF1 and CRF2 receptor levels in the dRN of isolation- and group-reared rats
At P56, following the 5 week isolation/re-socialization procedure, group-reared and isolation-reared rats (n = 12 per treatment) were decapitated and brains rapidly removed. Brains were frozen and stored at -80°C, and sectioned frozen (300 μm) within a cryostat (Lecia Jung CM 1800; North Central Instruments, Plymouth, MN) at -12 °C. The dRN was dissected from frozen sections on a freezing stage (Physiotemp; North Central Instruments) using a 23 gauge cannula, and homogenized in 40 μl of HEPES buffer. Protein concentrations were determined within 5 μl sample duplicates using a Bradford Kit (BioRad Laboratories, Hercules, CA) and a microplate reader (Bio-Tek Instruments, Winooski, TV, USA. Samples (25 μg) were processed for western blotting, with CRF1 receptor, CRF2 receptor, and actin levels detected using the methods described above. Optical density of each protein band was obtained using Odyssey software (LI-COR Biosciences), and normalized against background. Optical density for each of the CRF1 and CRF2 receptors from each individual sample were then corrected against actin levels.
Immunocytochemistry for CRF1 and CRF2 receptor levels in the dRN of isolation- and group-reared rats
Immunocytochemisty was used to measure receptor levels in the treatment groups because this method provides the high spatial resolution required to analyze the lateral and medial portions of the dRN separately. This was considered important because the lateral portions of the dRN provide serotonergic innervation to the NAc (Van Bockstaele et al., 1993). Group-reared and isolation-reared rats (n = 6-8 per treatment) were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) immediately following the 5 week isolation/re-socialization period (P56). Rats were transcardially perfused with 150 ml of 0.1 M phosphate-buffered saline (PBS, pH 7.4) at RT, followed by 200 ml of cold (4°C) 4% paraformaldehyde (pH 7.4). Brains were removed from the cranium, post-fixed for 1 hour in 4% paraformaldehyde at 4°C, then immersed in 0.1 M PBS with 25% sucrose and stored for 2 days at 4°C.
Following cryopreservation with sucrose, brains were frozen with dry ice and 30 μm coronal sections were taken using a sliding microtome. Sections of the dRN from group- and isolation-reared rats were processed together, and consecutive serial sections from each treatment group were processed separately for CRF1 and CRF2 receptors. Sections containing the dRN were placed in 0.01 M PBS and pre-incubated in blocking buffer (0.01 M PBS pH 7.4 containing 10% normal rabbit serum, Jackson ImmunoResearch, West Grove, PA) at RT for 2 hours to reduce non-specific labeling. Sections were then incubated in rabbit serum dilutent (2 % normal rabbit serum, Jackson ImmunoResearch, in 0.01 M PBS) containing goat CRF1 receptor-selective polyclonal antibody (1:200; Santa Cruz Biotech, #SC-12381) or goat CRF2 receptor-selective polyclonal antibody (1:300; Santa Cruz Biotech, #SC-1826) and 0.2 % Triton-X for 20 hours at 4°C with gentle agitation (Radulovic et al., 1998, Radulovic et al., 1999). Sections were washed in rabbit serum dilutent 3 times for 10 minutes, and incubated for 2 hours at RT in Cy2-conjugated rabbit anti-goat IgG secondary antibody (1:200; peak emission 510 nm, green fluorescence, obtained from Jackson ImmunoResearch #305-225-003) to visualize either CRF1 or CRF2 receptors. After incubation in secondary antibody, sections were washed in 0.01 M PBS 3 times for 10 minutes and mounted on gelatin-coated slides. Sections were dehydrated using xylene, permanently cover-slipped, and visualized using a fluorescence microscope equipped with video camera (Zeiss Axioskop 2-Mot, Carl Zeiss Microimagin, Thornwood, NY).
Additional sections were processed in parallel with the omission of the primary antibodies to determine the extent of non-specific secondary antibody binding. To determine the specificity of the primary antibodies, primary antibodies were incubated for 48 hours at 4 °C with purified CRF1 blocking peptide (1:3 ratio of primary antibody to blocking peptide; Santa Cruz Biotech, #SC-12381P) or CRF2 blocking peptide (1:3 ratio of primary antibody to blocking peptide; Santa Cruz Biotech, #SC-1826P). Primary antibody pre-incubated with 0.01 M PBS served as a control. After the 48 hour pre-incubation period, sections were processed as described above.
Quantitative image analysis
Quantitative image analysis of stained sections was performed using Adobe Photoshop software, which calculated the average luminosity level of the pixels in the selected field as a measure of receptor density (Ouyang et al., 1999, Keifer, 2001, Keifer et al., 2003, Lindahl and Keifer, 2004, Meyer et al., 2004). Luminosity levels were subtracted from background values obtained from regions within the section where no cellular staining was observed (Lindahl and Keifer, 2004). For each region (lateral wings and medial dRN; according to Paxinos and Watson, 1997; Figure 1), luminosity levels obtained from CRF1 and CRF2 receptor labeled sections were determined for isolated and group reared rats. These analyses were performed blind to experimental treatment. This approach has been used and validated in many previous studies (Ouyang et al., 1999, Keifer, 2001, Keifer et al., 2003, Lindahl and Keifer, 2004).
Fig. 1. Regions of dRN Analyzed for CRF Receptor Immunofluorescence.
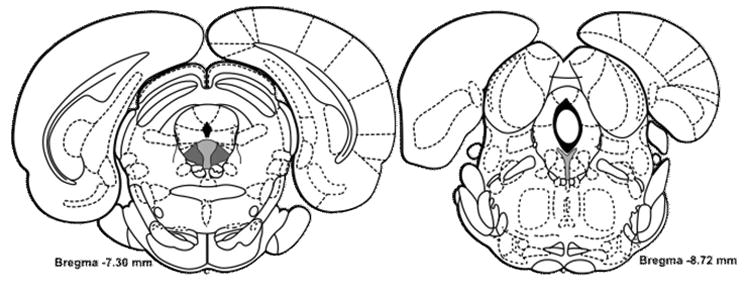
Coronal sections of the dRN illustrating the anterioposterior range of the dRN that were analyzed for CRF receptor immunofluorescence. The dRN was divided into lateral (dark gray fill) and medial (light gray fill) regions for -7.3 mm to -8.0 mm from bregma. At -8.3 mm to -8.72 mm from bregma, only the medial region was analyzed, since the lateral portions of the dRN at this level are not present. Figures adapted from Paxinos and Watson (1997).
Data analysis
Significance levels for all statistical tests were set at P<0.05 (SigmaStat v2.03 and SPSS 13.0 SPSS Inc., Point Richmond, CA). For microdialysis experiments, 5-HT levels were expressed as a percentage change (±SEM) from baseline levels where the baseline level was determined by averaging the 3 baseline measurements (Lukkes et al., 2008). The effects of housing and CRF dose on NAc 5-HT release were analyzed using a three-way ANOVA (rearing × CRF treatment × time) with one repeated measure (time). A significant interaction between the three factors was analyzed further within each CRF dose using separate two-way ANOVA (rearing × time) with one repeated measure (time). When a significant main effect of time, or rearing × time interaction was present within a CRF dose, the effects of CRF infusion across time was analyzed separately for each rearing group using a one-way repeated measures ANOVA, and significant effects over time were identified using the Dunnett's post-hoc test where the sample immediately preceding CRF infusion was used as the control 5-HT value. Significant main effects of rearing within each CRF dose were further analyzed by one-way ANOVAs for each time point, followed by Student Newman-Keuls post hoc tests for multiple comparisons.
Separate one way ANOVAs were used to measure the effect of rearing condition on CRF1 and CRF2 receptor optical density (western blots) or luminosity levels (immunocytochemistry) within the dRN.
Results
Probe and injection placement
The placement of microdialysis probes ensured that the 2 mm length of dialysis membrane sampled from both the NAc shell and core of group-reared (Fig. 2A) and isolation-reared rats (Fig. 2C). Drug infusion cannulae were located in the mid to posterior aspect of the dRN of group- (Fig.2B) and isolation-reared rats (Fig. 2D). The area of the dRN targeted by the cannulae has been shown to provide 5-HT innervation to the NAc (Van Bockstaele et al., 1993). Although infusions into the dRN were close to the cerebral aqueduct, cannulae were placed on a lateral to medial angle, which has been shown to minimize diffusion into the cerebral aqueduct (Forster et al., 2006, Lukkes et al., 2008). Furthermore, previous work from our laboratory has established that infusion of CRF (0.5 μl) adjacent to, but outside the dRN (including into the adjacent central gray), does not affect NAc or cortical 5-HT levels (Forster et al., 2006, Lukkes et al., 2008), suggesting minimal spread of this volume from the dRN. Probe and cannula placements were similarly distributed in isolation and group-housed rats.
Fig. 2. Probe and Cannula Placements in the NAc and dRN.
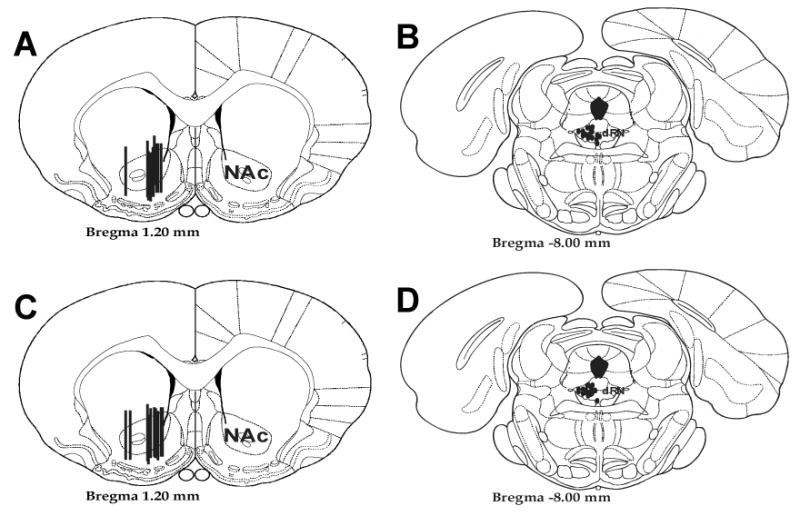
Schematic representations of microdialysis probe (A,C) and dRN drug infusion cannulae (B, D) placements for CRF dose-response experiments in group-reared (A, B) and isolation-reared (C, D) animals. Microdialysis probes were placed in the NAc with a 2 mm membrane to sample from both the shell and the core. Drug cannulae were placed in regions encompassing areas of the dRN that project to the NAc (Van Bockstaele et al, 1993). Figures adapted from Paxinos and Watson (1997).
Effects social isolation on CRF-mediated 5-HT levels
Isolation-rearing from early adolescence to mid-adolescence (P21-P42) caused alterations in CRF-mediated NAc 5-HT release when compared to group-reared rats (Fig. 3). There was a significant interaction between rearing, CRF dose, and time (F16, 240 = 3.55, P < 0.001). Infusions of vehicle (aCSF) into the dRN had no significant effect on NAc 5-HT levels over time (F8, 63= 1.606, P = 0.141; Fig. 3A), and there was no effect of rearing (F1, 63 = 0320, P = 0.863), nor a significant interaction between rearing and time (F8, 63 = 0.194, P = 0.991) (Fig 3A). For 100 ng CRF infusions, there was a significant effect of time (F8, 110 = 4.051, P < 0.001) and a significant interaction between rearing and time (F8, 110 = 7.05, P < 0.001). One-way ANOVA revealed that infusion of 100 ng CRF into the dRN of group-reared rats produced a significant effect on NAc 5-HT release over time (F8, 55 = 11.12, P < 0.001; Fig. 3B); however, an effect across time was not observed in isolation-reared rats (F8, 56 = 0.73, P = 0.670; Fig. 3B). Dialysate 5-HT concentrations were significantly lower in the NAc in the first twenty minutes following 100 ng CRF infusions into the dRN of group-reared rats when compared to pre-treatment 5-HT levels (P < 0.05; Fig. 3B). The decrease in NAc 5-HT levels detected in group-reared rats after the infusion of 100 ng in the dRN was significant when compared to isolation-reared rats (P< 0.001).
Fig. 3. Effects of CRF Infusion into the dRN on NAc 5-HT Release in Group- and Isolation-reared Rats.
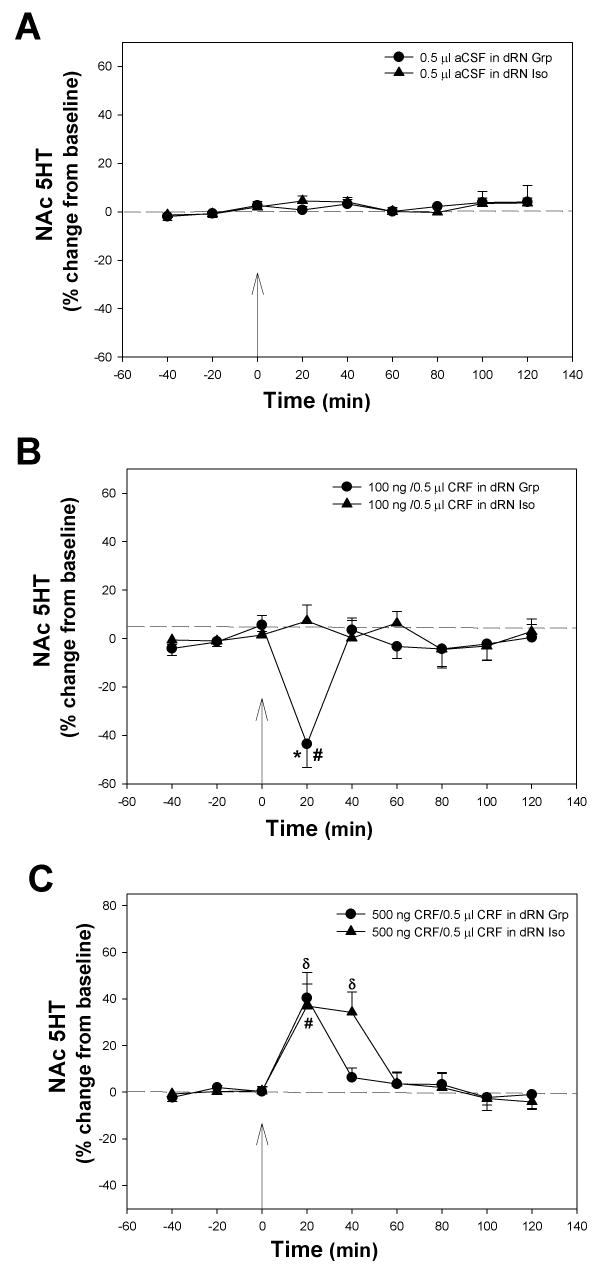
Isolation rearing resulted in differences in CRF-mediated NAc 5-HT release in adulthood when compared to group-reared rats. (A) Infusion of vehicle (aCSF) into the dRN did not significantly affect 5-HT levels in the NAc of either group-reared (circles) or isolated (triangles) animals. (B) Infusion of 100 ng CRF into the dRN resulted in a significant decrease in NAc 5-HT of group-reared animals (circles), but this effect was completely absent in isolates (triangles). (C) Infusion of 500 ng CRF into the dRN resulted in an acute significant increase in NAc 5-HT in group-reared animals (circles), which was prolonged in isolates (triangles). *significant differences between group- and isolation-reared animals. #significant differences from baseline levels for group-reared rats. δsignificant differences from baseline levels for isolation-reared rats. P < 0.05. Arrow indicates time of intra-dRN infusion.
In rats treated with 500 ng CRF infusions into the dRN, there was a significant effect of time (F8, 95 = 13.74, P< 0.001; Fig. 3C), but no significant effect of rearing (F1, 95 = 0.000, P = 0.990), and no significant interaction between rearing and time (F8, 95 = 0.684, P = 0.705). Infusion of 500 ng CRF into the dRN of grouped-reared rats resulted in a significant increase in 5-HT levels over time (F8, 48 = 10.180, P< 0.001) with values approximately 40% greater than baseline values at 20 min post-treatment (P < 0.05). Infusion of 500 ng CRF into the dRN of isolation-reared animals also resulted in a significant effect on 5-HT over time (F8, 48 = 12.3, P< 0.001) and, in contrast to group-reared rats, increases in 5-HT were detected at both 20 min and 40 min post treatment (P < 0.05).
Effect of social isolation on CRF1 and CRF2 receptor levels
Figure 4 illustrates western blots from hindbrain tissue for primary antibodies raised against the internal region of the CRF1 receptor and the N-terminus of the CRF2 receptor. A single protein band was detected at 43 kDa using the CRF1 receptor antibody and at 40 kDa using the CRF2 receptor antibody, consistent with the predicted molecular weight (40-45 kDa) of these receptors based on cDNA data (Grigoriadis and De Souza, 1989, Chen et al., 1993). Binding of each CRF receptor antibody was blocked by peptides raised against the antibody recognition site (Fig. 4).
Fig. 4. Western blots Illustrating CRF1 and CRF2 Receptor Antibody Specificity.
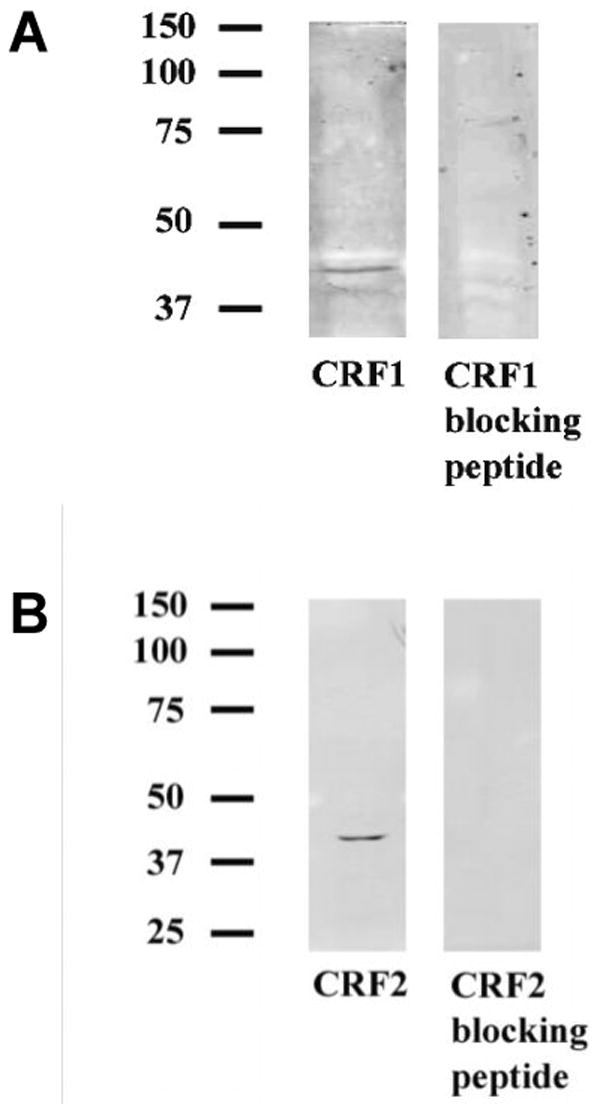
(A) The CRF1 receptor antibody recognized the full length CRF1 receptor at approximately 43 kDa. Incubation of CRF1 blocking peptide with CRF1 receptor antibody blocked CRF1 receptor binding in hindbrain tissue. (B) The CRF2 receptor antibody recognized the full-length CRF2 receptor at approximately 40 kDa. Incubation of CRF2 blocking peptide with the CRF2 receptor antibody blocked CRF2 receptor antibody binding in hindbrain tissue.
Analysis of CRF1 and CRF2 receptor levels within dRN tissue using western blot optical density revealed that there was no difference between group- and isolation-reared rats in CRF1 receptor levels (F1, 22 = 0.159, P = 0.695; Fig. 5A and 5B). However, isolation-reared rats exhibited higher levels of CRF2 receptors in dRN tissue as compared to group-reared rats (F1, 22 = 4.471, P = 0.046; Figs. 5A and 5C).
Fig. 5. CRF1 and CRF2 Receptor Levels in dRN Tissue of Isolation- and Group-Reared Rats.
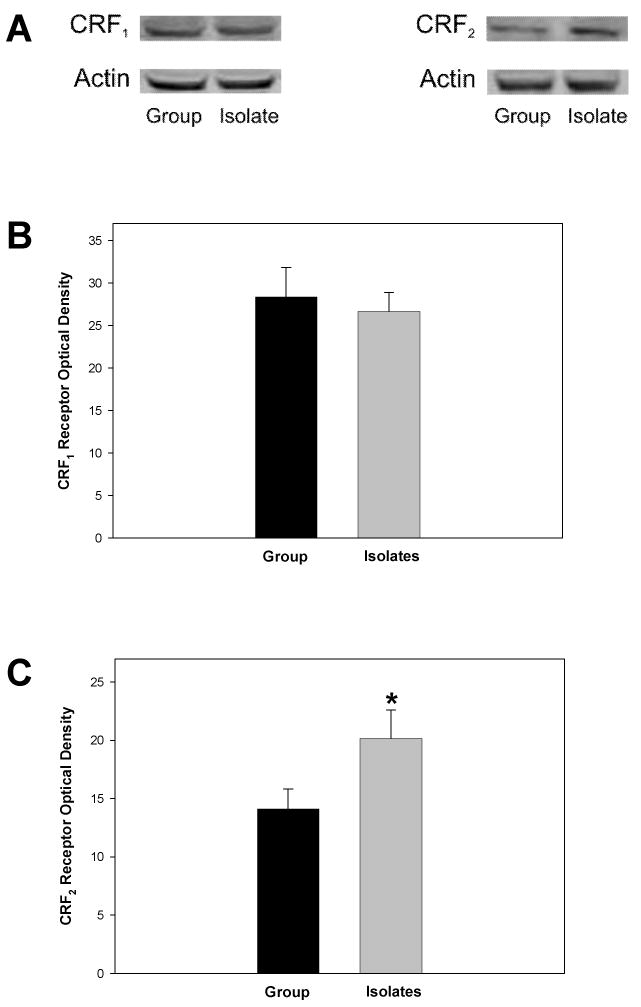
(A) Representative examples of CRF1 (approximately 43 kDa) and CRF2 (approximately 40 kDa) receptor bands in the dRN obtained from an isolation-reared and a group-reared rat, with actin loading controls. (B) No effects of rearing condition were observed on CRF1 receptor levels within dRN tissue obtained from adult rats. (C) Isolation-reared rats exhibited significantly greater levels of CRF2 receptors within dRN tissue as compared to group-reared rats (P < 0.05).
Immunocytochemical staining showed that CRF1 and CRF2 receptors were distributed within the lateral and medial portions of the dRN. The receptors were predominantly localized on soma, but some labeling of processes was noted (Fig. 6). Levels of CRF2 receptors in the lateral wings of the dRN were significantly higher in isolates when compared to group-reared rats (F1, 10 = 5.492, P = 0.041; Figs. 7 and 8B), but CRF1 levels in this region were not affected by rearing (F1, 14 = 0.008, P = 0.932; Figs. 8A). Rearing did not affect CRF1 (F1, 13 = 1.102, P = 0.333) or CRF2 (F1,10 = 1.501, P =0.241) receptor levels in the medial portion of the dRN (Figure 8).
Fig. 6. CRF1 and CRF2 Receptor Immunoreactivity and Antibody Specificity in the dRN.
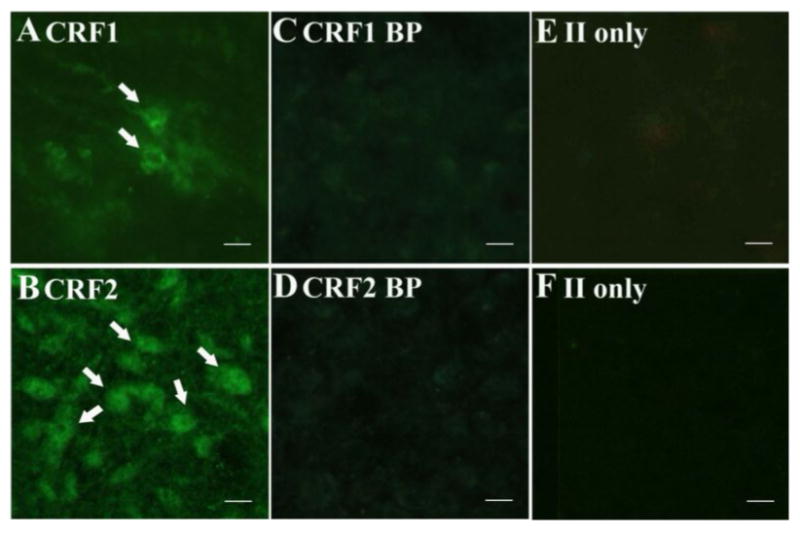
Photomicrographs of CRF1 and CRF2 receptor immunoreactivity in the rat dRN (A and B), antibody specificity of primary (C and D), and antibody specificity of secondary (E and F). Arrow heads indicate labeled somas of neurons immunoreactive for CRF1 (Panel A) and CRF2 (Panel B) receptor types. Cellular staining was not observed after incubation with a blocking peptide for the CRF1 antibody (Panel C) and the CRF2 antibody (panel D). Cellular staining was also not observed when sections were incubated with secondary antibody only (E and F). Scale bar = 10 μm for all panels.
Fig. 7. Example of CRF2 Receptor Immunoreactivity in the Lateral Wings of the dRN.
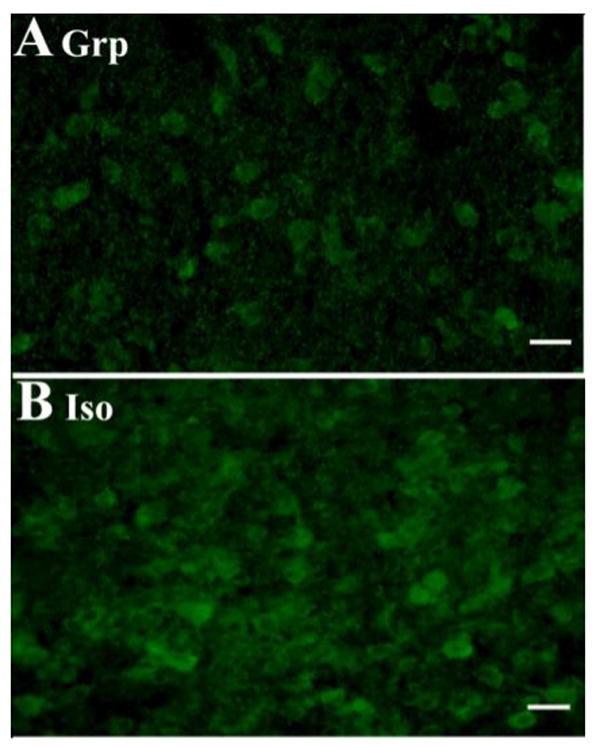
Representative photomicrograph of CRF2 immunoreactivity in the lateral wings of the dRN (-8.00 mm from bregma; scale bar=10 μm) showing increased luminosity levels in isolation-reared (B) animals when compared to group-reared (A) rats.
Fig. 8. Effects of Isolation Rearing on CRF1 and CRF2 Receptor Luminosity Levels in the dRN of Adult Rats.
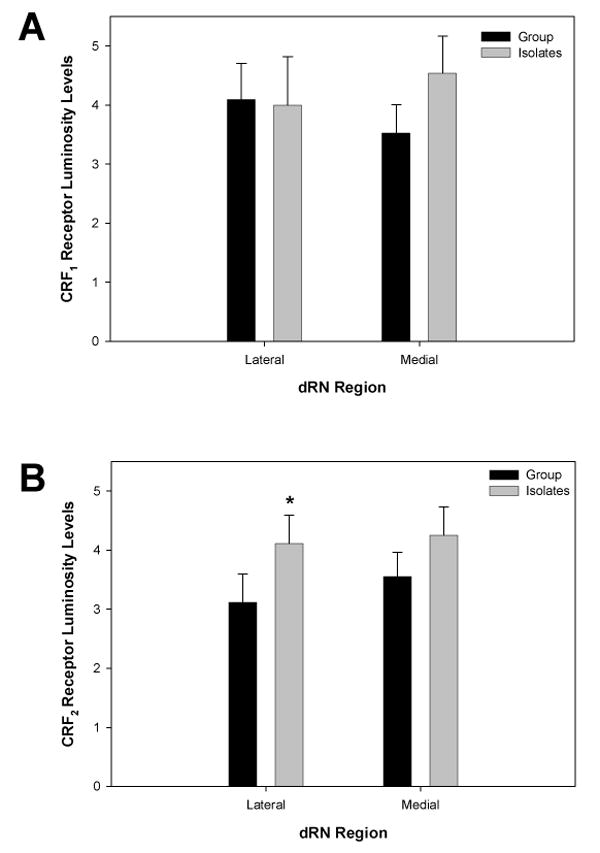
(A) No changes in CRF1 receptor luminosity levels (corrected for background) were observed in either the lateral wings or the medial portion of the dRN following isolation rearing. (B) In contrast, a significant increase in CRF2 receptor levels (corrected for background) was observed in the lateral wings of the dRN of isolation-reared animals when compared to group-reared rats. However, there were no significant differences in CRF2 receptor levels in the medial portion of the dRN between rearing groups. * significant difference in CRF2 receptor levels as compared to group-housed animals (P < 0.05).
Discussion
Our results demonstrate that exposure to early-life stress alters CRF-mediated 5-HT release in the NAc of adult male rats. Infusion of 100 ng CRF into the dRN decreased 5-HT release in the NAc of group-reared rats. This effect is consistent with recent evidence which suggests that CRF1 receptor activation in the dRN decreases 5-HT release in the NAc (Lukkes et al., 2008). In contrast, the effect of 100 ng CRF infused into the dRN on 5-HT release in the NAc was completely absent in isolation-reared rats. Previously, we found that infusion of 500 ng of CRF into the dRN increases 5-HT release in the NAc through the activation of CRF2 receptors (Lukkes et al., 2008). Consistent with this finding, infusion of 500 ng CRF into the dRN increased, NAc 5-HT release in group-reared rats. Rats reared in isolation also had increased 5-HT release in the NAc in response to infusion of 500 ng CRF into the dRN. However, the serotonergic response to CRF in these rats was prolonged when compared to the response detected in group-reared rats.
In addition to differences in NAc 5-HT release in response to CRF, isolates showed increased CRF2 receptor levels in the dRN. Further experiments using immunocytochemistry revealed increased CRF2 receptor density in the lateral wings of the dRN of isolates when compared to group-reared rats in adulthood. This result suggests that early-life social isolation leads to an up-regulation of CRF2 receptors in this region of the dRN. Importantly, the lateral wings of the dRN provide 5-HT innervation to the NAc (Van Bockstaele et al., 1993). CRF2 receptor densities in the medial portion of the dRN were similar in both treatment groups. Furthermore, no changes were observed in CRF1 receptor levels in either region of the dRN in adult rats exposed to early life isolation when compared to group-reared animals.
The up-regulation of dRN CRF2 receptors in isolates may account for the extended increase in NAc 5-HT release after infusion of 500 ng CRF into the dRN. Infusion of a CRF2 receptor antagonist into the dRN completely abolishes the stimulatory effect of 500 ng CRF on NAc 5-HT release, whereas CRF1 receptor antagonism has no effect (Lukkes et al., 2008). Since the inhibitory effects of 100 ng CRF on 5-HT release in the NAc are blocked by CRF1 receptor antagonism (Lukkes et al., 2008), we hypothesized that a down-regulation of CRF1 receptors might explain the lack of response to 100 ng CRF infusion into the dRN of isolates. However, no changes in CRF1 receptor densities were detected in the dRN of isolates when compared to group-reared rats. It is possible that CRF1 receptors in the dRN of isolates may have reduced affinity for CRF or altered efficacy. A more plausible explanation for the absence of an increase in NAc 5-HT following 100 ng CRF infusion into the dRN of isolates may be related to the increase in the density of CRF2 receptors. The presence of higher densities of CRF2 receptors in the dRN of isolates could mask the inhibitory effects of CRF1 receptor actions in response to CRF, since CRF2 receptor activation generally increases 5-HT neuronal activity in the dRN (Pernar et al., 2004). Therefore, it is likely that infusions of CRF into the dRN altered 5-HT responses in the NAc in adult rats exposed to early-life social isolation as a result of increased levels of CRF2 receptors. The current study did not localize CRF2 receptors to specific neuronal types within the dRN. Generally, the lateral dRN is high in GABAergic neurons (Day et al., 2004) and CRF2 receptor activation inhibits GABAergic neurons to presumably disinhibit 5-HT neurons in the dRN (Pernar et al., 2004). Thus, it is possible that the increase in CRF2 receptor levels in the lateral dRN of isolates represents increased density of CRF2 receptors on GABAergic neurons, but this remains to be tested. This would prove to be an interesting direction to follow for future work.
The current results are consistent with the finding that CRF2 receptor antagonism in the dRN reduces the behavioral effects of repeated uncontrollable stress in an inescapable foot-shock paradigm (Hammack et al., 2003), suggesting a role for dRN CRF2 receptors in mediating the consequences of repeated stress. However, the mechanisms by which early-life stress up-regulates CRF2 receptors in the dRN are unknown. Some attention has been paid to alterations in neural CRF receptors following CRF infusion. Intracranial CRF infusion presumably mimics stress, since stress causes the release of CRF from cell body regions in the amygdala (Merlo Pich et al., 1995, Merali et al., 1998). Reyes et al. (2006) showed that infusion of CRF into the locus coeruleus of rats causes rapid internalization of CRF receptors. In contrast, Imaki and colleagues (1996) found that 120 min of restraint stress or an intracerebroventicular infusion of CRF caused an increase in CRF1 receptor mRNA in the hypothalamus of rats. This latter study suggests that acute inescapable stress or increased neural levels of CRF are sufficient to increase CRF1 mRNA, although the effects of these treatments on CRF2 receptors or the effects of long-term stress were not examined. Like the CRF1 receptor, the CRF2 receptor is predominantly a Gs-coupled receptor (Dautzenberg and Hauger, 2002). Ligand activation of these receptors stimulates adenylate cylase (AC) which increases intracellular cAMP levels to result in activation of regulatory subunits of protein kinase A (PKA), protein phosphorylation, and induction of a variety of genes (Dautzenberg and Hauger, 2002). Activation of AC by forskolin results in up-regulation of other types of Gs-coupled receptors, which is dependent upon increased cAMP levels, PKA activation, and protein synthesis (Wanderoy and Westlind-Danielsson, 1997). Wanderoy and Westlind-Danielsson (1997) suggest that up-regulation of such Gs-coupled receptors is mediated by the nuclear transcription factor CREB, since this is a target for PKA-mediated phosphorylation. A similar mechanism may underlie up-regulation of CRF2 receptors. Future studies should examine whether isolates have increased CRF levels or increased levels of high affinity CRF2 receptor ligands such as urocortin II (Dautzenberg and Hauger, 2002) in the dRN, which could stimulate cAMP and CREB pathways to result in up-regulation of CRF2 receptors.
Early-life social isolation of rats has many effects on both behavior and monoaminergic activity. Rats isolated from 3 to 15 weeks of age (pre-adolescence to adulthood) exhibit decreased basal 5-HT turnover in the NAc (Jones et al., 1992). Isolation reared rats exhibit increased locomotion in novel environments (Jones et al., 1992) and show increased 5-HT turnover in the NAc in response to novelty (Miura et al., 2002b). Early-life social isolation also heightens 5-HT release in the NAc during acute foot-shock stress (Fulford and Marsden, 1998). Furthermore, preliminary results suggest that isolation-reared rats show increased freezing behavior in response to foot-shock when compared to group-reared rats (Lukkes et al., 2007). Acute stress is known to increase CRF levels (Merlo Pich et al., 1995, Merali et al., 1998), and 500 ng CRF infused into the dRN elicits freezing behavior in rats (Forster et al., 2006). Therefore, the prolonged serotonergic response to 500 ng CRF infused into the dRN of rats exposed to early-life social isolation suggests greater neural sensitivity to CRF concentrations that produce freezing behavior. The behavioral consequences of 100 ng CRF infusion into the dRN are not known, although many studies suggest that the resultant decrease in 5-HT levels could be related to increased impulsivity and aggression (Linnoila et al., 1983, Coccaro, 1992, Winstanley et al., 2005). Therefore, the absence of 100 ng CRF-induced reductions in NAc 5-HT release observed in isolates may also predict greater behavioral inhibition in response to stressors. Overall, the behavioral and increased 5-HT response in the NAc to stressors observed in isolation-reared rats may result from altered CRF-5-HT interactions at the level of the dRN.
Conclusions and Future Considerations
This study shows that early-life stress alters CRF-mediated 5-HT release in the NAc. Specifically, the results suggest that isolation during the early part of development causes up-regulation of CRF2 receptor levels in the dRN, which may underlie altered CRF-induced serotonergic activity in the NAc observed in male adult rats exposed to early-life social isolation. Furthermore, our findings suggest that the observed behavioral alterations after isolation-rearing found in many previous studies using male rats, could be due to modifications in the mechanisms regulating stress-related CRF activity in the dRN. Importantly, whether similar disruptions to CRF mediation of 5-HT activity following early-life stress are observed in females should be determined, given that human females have greater prevalence of affective disorders compared to males (Hirschfeld and Weissmann, 2002).
Acknowledgments
This work was supported by NIH grants R01 DA019921, R03 MH068303 and COBRE P20 RR15567 which is designated a Center for Biomedical Research Excellence, but is solely the responsibility of the authors and does not necessarily represent the official views of NIH. We would like to thank Dr. Michael Watt, Dr. Joyce Keifer, and Dr. Maxim Mokin, for their valuable contributions to this study.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- CRF
corticotropin-releasing factor
- dRN
dorsal raphe nucleus
- NAc
nucleus accumbens
- 5-HT
serotonin
- TBS-T
Tris-buffered saline containing 0.1% Tween-20
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Arakawa H. Interaction between isolation rearing and social development on exploratory behavior in male rats. Behav Processes. 2005;70:223–234. doi: 10.1016/j.beproc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Hormones and behavior. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Blanc G, Herve D, Simon H, Lisoprawski A, Glowinski J, Tassin JP. Response to stress of mesocortico-frontal dopaminergic neurones in rats after long-term isolation. Nature. 1980;284:265–267. doi: 10.1038/284265a0. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacology, biochemistry, and behavior. 1994;48:459–464. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Sprouse JS, Aghajanian GK, Roth RH. Sub-picogram determination of serotonin using HPLC with electrochemical detection for microdialysis studies of serotonin release. Advances in experimental medicine and biology. 1991;294:81–89. doi: 10.1007/978-1-4684-5952-4_7. [DOI] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF. Impulsive aggression and central serotonergic system function in humans: an example of a dimensional brain-behavior relationship. International clinical psychopharmacology. 1992;7:3–12. doi: 10.1097/00004850-199200710-00001. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Henry D. Catecholamines in fetal and newborn rat brain. Journal of neurochemistry. 1973;21:61–67. doi: 10.1111/j.1471-4159.1973.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends in pharmacological sciences. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. The Journal of comparative neurology. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einon D. Spatial memory and response strategies in rats: age, sex and rearing differences in performance. Q J Exp Psychol. 1980;32:473–489. doi: 10.1080/14640748008401840. [DOI] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ. A critical period for social isolation in the rat. Developmental psychobiology. 1977;10:123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Ferdman N, Murmu RP, Bock J, Braun K, Leshem M. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav Brain Res. 2007;180:174–182. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SW, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus. Eur J Neurosci. 2008 doi: 10.1111/j.1460-9568.2008.06333.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford AJ, Marsden CA. Conditioned release of 5-hydroxytryptamine in vivo in the nucleus accumbens following isolation-rearing in the rat. Neuroscience. 1998;83:481–487. doi: 10.1016/s0306-4522(97)00423-5. [DOI] [PubMed] [Google Scholar]
- Gomez F, Houshyar H, Dallman MF. Marked regulatory shifts in gonadal, adrenal, and metabolic system responses to repeated restraint stress occur within a 3-week period in pubertal male rats. Endocrinology. 2002;143:2852–2862. doi: 10.1210/endo.143.8.8929. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Annals of the New York Academy of Sciences. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, De Souza EB. Heterogeneity between brain and pituitary corticotropin-releasing factor receptors is due to differential glycosylation. Endocrinology. 1989;125:1877–1888. doi: 10.1210/endo-125-4-1877. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Critical reviews in neurobiology. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Hirschfeld R, Weissmann M. Secondary risk factors for major depression and bipolar disorder. In: Davis KL, et al., editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia: Lippincott Williams & Wilkin; 2002. pp. 1018–1025. [Google Scholar]
- Hoffman CS, Westin TM, Miner HM, Johnson PL, Summers CH, Renner KJ. GABAergic drugs alter hypothalamic serotonin release and lordosis in estrogen-primed rats. Brain research. 2002;946:96–103. doi: 10.1016/s0006-8993(02)02867-6. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacology, biochemistry, and behavior. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, Robbins TW. Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms of the nucleus accumbens. Psychopharmacology. 1990;102:364–372. doi: 10.1007/BF02244105. [DOI] [PubMed] [Google Scholar]
- Keifer J. In vitro eye-blink classical conditioning is NMDA receptor dependent and involves redistribution of AMPA receptor subunit GluR4. J Neurosci. 2001;21:2434–2441. doi: 10.1523/JNEUROSCI.21-07-02434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer J, Brewer BT, Meehan PE, Brue RJ, Clark TG. Role for calbindin-D28K in in vitro classical conditioning of abducens nerve responses in turtles. Synapse. 2003;49:106–115. doi: 10.1002/syn.10219. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Leng A, Feldon J, Ferger B. Long-term social isolation and medial prefrontal cortex: dopaminergic and cholinergic neurotransmission. Pharmacology, biochemistry, and behavior. 2004;77:371–379. doi: 10.1016/j.pbb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Lindahl JS, Keifer J. Glutamate receptor subunits are altered in forebrain and cerebellum in rats chronically exposed to the NMDA receptor antagonist phencyclidine. Neuropsychopharmacology. 2004;29:2065–2073. doi: 10.1038/sj.npp.1300485. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life sciences. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Moore FL. Regulation of behavioral responses by corticotropin-releasing factor. General and comparative endocrinology. 2006;146:19–27. doi: 10.1016/j.ygcen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. European journal of pharmacology. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia. 1986;42:109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Gleason E, Kelsey JE. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Hormones and behavior. 2004;46:458–466. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Hormones and behavior. 1981;15:197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich Merlo E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer WN, Keifer J, Korzan WJ, Summers CH. Social stress and corticosterone regionally upregulate limbic N-methyl-D-aspartatereceptor (NR) subunit type NR(2A) and NR(2B) in the lizard Anolis carolinensis. Neuroscience. 2004;128:675–684. doi: 10.1016/j.neuroscience.2004.06.084. [DOI] [PubMed] [Google Scholar]
- Miura H, Qiao H, Ohta T. Attenuating effects of the isolated rearing condition on increased brain serotonin and dopamine turnover elicited by novelty stress. Brain research. 2002a;926:10–17. doi: 10.1016/s0006-8993(01)03201-2. [DOI] [PubMed] [Google Scholar]
- Miura H, Qiao H, Ohta T. Synapse. Vol. 46. New York, NY: 2002b. Influence of aging and social isolation on changes in brain monoamine turnover and biosynthesis of rats elicited by novelty stress; pp. 116–124. [DOI] [PubMed] [Google Scholar]
- Moghaddam MF, Gerwick WH, Ballantine DL. Discovery of 12-(S)-hydroxy-5,8,10,14-icosatetraenoic acid [12-(S)-HETE] in the tropical red alga Platysiphonia miniata. Prostaglandins. 1989;37:303–308. doi: 10.1016/0090-6980(89)90065-8. [DOI] [PubMed] [Google Scholar]
- Nunes Mamede Rosa ML, Nobre MJ, Ribeiro Oliveira A, Brandao ML. Isolation-induced changes in ultrasonic vocalization, fear-potentiated startle and prepulse inhibition in rats. Neuropsychobiology. 2005;51:248–255. doi: 10.1159/000085820. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Rosenstein A, Kreiman G, Schuman EM, Kennedy MB. Tetanic stimulation leads to increased accumulation of Ca(2+)/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J Neurosci. 1999;19:7823–7833. doi: 10.1523/JNEUROSCI.19-18-07823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual R, Zamora-Leon SP, Valero-Cabre A. Effects of postweaning social isolation and re-socialization on the expression of vasoactive intestinal peptide (VIP) and dendritic development in the medial prefrontal cortex of the rat. Acta Neurobiol Exp (Wars) 2006;66:7–14. doi: 10.55782/ane-2006-1582. [DOI] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Sydow S, Spiess J. Characterization of native corticotropin-releasing factor receptor type 1 (CRFR1) in the rat and mouse central nervous system. Journal of neuroscience research. 1998;54:507–521. doi: 10.1002/(SICI)1097-4547(19981115)54:4<507::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Robbins TW. Isolation-rearing enhances tail pinch-induced oral behavior in rats. Physiology & behavior. 1977;18:53–58. doi: 10.1016/0031-9384(77)90093-2. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Robbins TW, Morgan MJ, Iversen SD. The effects of psychomotor stimulants on stereotypy and locomotor activity in socially-deprived and control rats. Brain research. 1975;84:195–205. doi: 10.1016/0006-8993(75)90975-0. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanford SC, Parker V, Morinan A. Deficits in exploratory behaviour in socially isolated rats are not accompanied by changes in cerebral cortical adrenoceptor binding. Journal of affective disorders. 1988;15:175–180. doi: 10.1016/0165-0327(88)90087-0. [DOI] [PubMed] [Google Scholar]
- Tian JB, Shan X, Bishop GA, King JS. Presynaptic localization of a truncated isoform of the type 2 corticotropin releasing factor receptor in the cerebellum. Neuroscience. 2006;138:691–702. doi: 10.1016/j.neuroscience.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Valzelli L. The “isolation syndrome” in mice. Psychopharmacologia. 1973;31:305–320. doi: 10.1007/BF00421275. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain research. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Wanderoy MH, Westlind-Danielsson A. Molecular mechanisms underlying forskolin-mediated up-regulation of human dopamine D2L receptors. Cellular and molecular neurobiology. 1997;17:547–555. doi: 10.1023/A:1026367023458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behavioural brain research. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiology & behavior. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]


