Summary
Rationale
Pulmonary hypertension (PH) commonly complicates the course of patients with idiopathic pulmonary fibrosis (IPF). It has a significant impact on outcomes and is, therefore, important to detect.
Objectives
We sought to characterize the accuracy and performance characteristics of the right ventricular systolic pressure (RVSP) as estimated by echocardiography (ECHO) alone and in conjunction with physiologic indices in predicting the presence of PH in IPF patients.
Methods
Cross-sectional study of IPF patients from two large tertiary centers in whom both ECHO and right-heart catheterization (RHC) were available.
Measurements and main results
There were 110 patients with available ECHOs and RHCs. Estimates of RVSP were reported in 60 of these patients (54.5%) of whom 22 (36.6%) had PH, while 16 of the 50 patients without RVSP estimate (32%) had PH. Twenty-four of 60 (40%) ECHOs accurately reflected the pulmonary arterial systolic pressure as measured by RHC. An optimal RVSP threshold for the screening of PH could not be detected. When assessed in combination with various thresholds of PFT and 6-minute walk test (6MWT) parameters, the performance characteristics of the RVSP were slightly improved.
Conclusion
The RVSP is not an accurate test for the assessment of PH in IPF patients. Awareness of the various combinations of threshold values for RVSP with and without PFT and 6MWT might nonetheless assist clinicians in risk stratifying IPF patients for the presence of PH.
Keywords: Hypertension, Pulmonary, Oximetry, Pulmonary fibrosis, Pulmonary function tests, Pressure, Pulmonary artery
Introduction
IPF is a disease that carries with it a poor prognosis with an estimated survival of 2.5-5 years.1-3 There are multiple factors that impact on the prognosis and clinical course of patients with this disease. One such factor is the development of pulmonary hypertension (PH).4,5 This can occur at any stage during the course of the disease and has been shown to impact patients' functional status as well as outcomes. For this reason, it appears to be an important clinical measure to assess for.
The gold standard for the measurement of PH is right heart catheterization (RHC), but this is invasive with the inherent risk of complications. As yet, no non-invasive measurement has been shown to suffice as an adequate screening or diagnostic tool for the presence of PH in IPF.6 Echocardiography (ECHO) has been touted as providing an accurate measurement of the right ventricular systolic pressure (RVSPecho) based on the estimated flow of the tricuspid regurgitant jet.7-9 We sought to evaluate the utility of the RVSPecho as a surrogate for the Pulmonary Artery Systolic Pressure (PASPcath) obtained by RHC in a cohort of patients with IPF.
Methods
We performed a retrospective cross-sectional study of IPF patients, diagnosed as per the ATS/ERS criteria,10 from two large tertiary centers. All patients evaluated from 1996 to 2006 qualified for the analysis if they had an ECHO and RHC performed as part of their evaluation. Some but not all of the patients were seen and evaluated as part of a transplant work-up. Contemporaneous pulmonary function tests were also recorded. The ECHO reports were scrutinized and patients were then stratified as to whether or not there was a RVSPecho reported by the echocardiographers. For the group in whom there was a RVSPecho, this number was recorded and then correlated to the PASPcath. RVSPecho was estimated using standard techniques.7-9 Specifically, the peak pressure gradient between the right ventricle and atrium during systole was calculated using the modified Bernoulli's equation, while the right atrial pressure was estimated from the degree of inspiratory collapse of the inferior vena cava. These were then summed to provide an estimate of the RVSPecho.
The accuracy of the RVSPecho in relation to the PASPcath was assessed. Accuracy was arbitrarily defined as a RVSPecho within 10 mmHg of the PASPcath. A further analysis was undertaken to assess whether the severity of disease based on the FVC% predicted and DLCO% predicted, affected the accuracy of this measure. A similar analysis was performed to assess if the severity of PH influenced the accuracy.
The performance characteristics of various incremental threshold values of the RVSP as a predictor of PH were assessed. We defined PH as resting mean pulmonary artery pressure (mPAP) from RHC of >25 mmHg. We further sought to determine whether a combination of PFT or 6-minute walk test (6MWT) data could improve the predictive value of the RVSPecho. In this regard, we assessed the performance characteristics of different values of the RVSPecho in conjunction with different thresholds of the following: the FVC% predicted, the DLCO% predicted, the ratio of the FVC% to DLCO% predicted, the resting room air oxygen saturation obtained via pulse oximetry (RAsatrest), the room air oxygen saturation nadir with exercise (RAsatexercise) and the 6MWT distance (6MWD). All 6MWTs were performed as per the ATS standard and only those that were performed on room air were included in the analysis.11
Statistical methods
Continuous data are presented as mean ± standard deviation (SD). Categorical data are presented as frequency and percent. Student's t-test, Pearson correlation coefficients and Chi-square tests were used to determine statistical significance where appropriate. P-values ≤ 0.05 were considered statistically significant. Estimates of positive and negative predictive values were calculated using Bayes' Theorem with an estimated PH background prevalence set at 34.5%. All analyses were conducted in SAS (Version 9, Cary, NC).
Results
There were 110 patients who qualified for the analysis over a 10-year period (1996-2006). All of the patients fulfilled the ATS/ERS guidelines for the diagnosis of IPF; of these 60.9% had the diagnosis confirmed by surgical lung biopsy. Although all these patients had ECHOs performed, RVSPecho was reported in only 60 of the patients (54.5%). Demographic, PFT and 6MWT data of the patient cohort are shown in Table 1. Most of these patients had advanced disease as evidenced by their PFTs, but there were some who had more “mild-moderate” disease, with 15/60 (25%) having FVCs > 60% predicted. There was no discernable demographic or disease severity difference between this final cohort and the group in whom there was no RVSPecho reported. As per the International Society for Heart and Lung Transplantation guidelines, the majority of these patients were also potential transplant candidates, specifically 33/60 were <65 years of age.12 Of the 60 patients, 22 (36.6%) had PH, while 16 of the 50 patients without ECHO estimates (32%) had PH. The mean time between the RHC and ECHO was 32 ± 78 days while the mean difference between the RVSPecho and PASPcath from RHC was 8 ± 14.2 mmHg.
Table 1.
Demographics of the patient cohort (n = 60)
| Male, n (%) | 33 (55.0%) |
| Age, mean ± SD | 62.9 ± 8.6 |
| aFVC% | 50.6 ± 14.8 |
| aFEV1% | 58.5 ± 16.5 |
| bDLCO% | 29.6 ± 34.4 |
| mPAP | 24.9 ± 9.2 |
| c6MWD | 242.4 ± 171.7 |
FVC% = forced vital capacity percent predicted; FEV1% = forces expired volume in the first one second percent predicted; DLCO% = diffusing capacity for carbon monoxide percent predicted; mPAP = mean pulmonary artery pressure; and 6MWD = 6-minute walk test distance.
FVC% and FEV1% data available in 58 patients.
DLCO% data available in 36 patients.
Twenty-eight patients completed 6MWT on room air.
Twenty-four of 60 (40%) ECHOs accurately reflected the PASPcath using the definition of accuracy as a RVSP to PASPcath difference of ±10 mmHg. RVSPecho overestimated the PASPcath in 29/60 (48.3%) cases, while it underestimated the PASPcath in 7/60 (11.6%). In 15 of the cases, the time interval between the two studies was 1 and 3 months, while in the remaining 13 cases it was beyond 3 months. The longest time interval between the two studies in any patient was 7.5 months. In 51/60 cases, the ECHO was obtained concurrently or before the RHC. In 32 of the cases, the ECHOs were within 1 month of the RHC. In this group, the mean difference between the RVSPecho and PASPcath was also 8±12.3 mmHg. Only 37.5% (12/32) of these cases fell within the range of accuracy.
There was no correlation between the severity of IPF based on the FVC% and the DLCO% predicted and the degree of accuracy in the RVSPecho estimate. The FVC% predicted to RVSP-PAS PASPcath difference is depicted in Fig. 1. Similarly, there did not appear to be a relationship between the RHC-measured mPAP and the accuracy of the RVSPecho (Fig. 2).
Figure 1.

Accuracy of the RVSPecho compared to the PAScath pressure as measured by right-heart catheterization in relation to the FVC% predicted.
Figure 2.
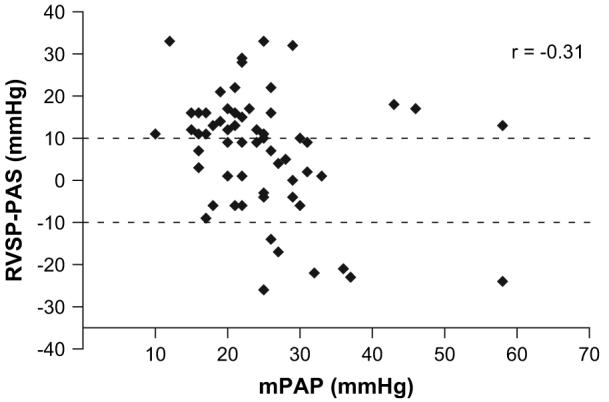
Accuracy of the RVSPecho compared to the PASPcath pressure as measured by right-heart catheterization in relation to the mean PAP.
The performance characteristics of RVSPecho as a diagnostic tool for PH are depicted in Fig. 3. As expected, the lower the threshold RVSPecho value, the greater the sensitivity. The specificity increases with the threshold value, but does so at the expense of the sensitivity.
Figure 3.

Diagnostic accuracy of incremental thresholds of the RVSPecho for the detection of PH in IPF depicted as a receiver operator characteristic curve.
The PFT parameters that had the best performance characteristics for predicting PH when used in conjunction with the RVSPecho were first the DLCO% predicted, then the FVC/DLCO% ratio and lastly, the FVC% predicted. In general, the higher the RVSPecho and the lower the DLCO are, the greater is the likelihood that the patient has PH (Table 2). Similarly, the higher the FVC/DLCO% ratio and the higher the RVSPecho, the greater the likelihood of PH.
Table 2.
Performance characteristics of PFTs and six-minute walk data alone and in combination with the RVSPecho for the detection of pulmonary hypertension
| RVSPecho excludeda | RVSPecho (mm Hg) |
|||||
|---|---|---|---|---|---|---|
| >30 | >40 | >50 | >60 | |||
| DLCO% | <30 | 62.5/66.7 | 36.8/78.9 | 30.0/86.0 | 25.0/91.5 | 17.4/98.0 |
| <40 | 87.5/23.1 | 66.7/46.7 | 52.6/68.6 | 31.6/82.1 | 18.2/97.6 | |
| <50 | 95.8/10.3 | 77.8/32.1 | 63.2/63.6 | 36.8/81.1 | 22.7/97.4 | |
| RAsatrest | <95 | 90.9/50.0 | 63.6/57.1 | 54.5/60.7 | 36.4/78.6 | 18.2/100 |
| <90 | 9.1/88.9 | 5.9/93.0 | 5.9/93.0 | 5.9/100 | 5.9/100 | |
| RAsatexercise | <85 | 100/61.9 | 45.5/83.3 | 41.7/89.5 | 23.1/94.7 | 14.3/97.6 |
| <80 | 56.1/72.2 | 8.3/97.4 | 7.7/100 | 7.1/100 | 6.7/100 | |
| 6MW Distance (meters) | <100 | 53.3/88.9 | 28.6/97.1 | 25.0/97.6 | 16.7/97.8 | 9.5/98.0 |
| <200 | 80.0/61.1 | 53.8/80.0 | 40.0/86.5 | 17.6/97.6 | 10.0/97.9 | |
| <300 | 86.7/52.8 | 61.5/75.0 | 46.7/82.9 | 23.5/94.9 | 10.0/97.9 |
Data represent the sensitivity and specificity (est. PH prevalence of 34.5%).
RVSPecho - Right Ventricular Systolic Pressure as estimated by echocardiography; 6MWD - The 6-minute walk test distance; RAsatrest -Oxygen saturation on room air at rest; and RAsatexercise - Room air oxygen saturation with exercise.
RVSPecho excluded = the physiologic parameters alone as predictors of PH in the patient population in whom there was an RVSPecho reported.
The parameter from the 6MWT that best predicted PH was exercise desaturation; specifically, desaturation to <85% had a 100% sensitivity and a 61.9% specificity for associated PH. ECHO RVSP improved the specificity of exercise desaturation, but this compromised the sensitivity. For example, desaturation to <85% during the 6MWT in conjunction with a RVSPecho > 40 mmHG increased the specificity to 89.5%, but with an associated sensitivity of only 41.7%. Lower thresholds for RAsatrest in the context of higher RVSPecho also had high specificities for underlying PH; for example, oxygen saturation <90% at rest in conjunction with a RVSPecho > 50 mmHg had specificity for PH of 96.9% (Table 2). The greatest value of using the tests in combination appears to be the high positive predictive values (PPV) seen with various combinations of ECHO and resting and/or exercise oxygen saturation.
Discussion
Pulmonary Hypertension frequently complicates the course of IPF patients and is associated with a worse survival.4,5 Recognition of PH is important in determining prognosis and the timing of listing for lung transplantation. RHC remains the gold standard test for the assessment of PH. At this time, the importance of detecting PH as a target of therapy remains uncertain.13,14 RHC is expensive, timeconsuming, invasive and impractical to assess in serial fashion. Therefore, a non-invasive diagnostic tool would be very helpful in the evaluation of IPF patients to enable the appropriate timing of RHC.
We describe the first comprehensive analysis of the RVSPecho as a screening tool for PH in a well-characterized population of patients with IPF, diagnosed as per the ATS/ ERS guidelines. Based on the results of our study, RVSPecho does not perform with sufficient accuracy to be relied upon as a stand-alone test for PH in IPF. These findings are in keeping with those of Arcasoy and associates, who described similar inaccuracies in patients with various forms of advanced lung disease referred for transplantation.14,15 In their interstitial lung disease (ILD) subgroup (n=106), they reported an accuracy rate of 48% compared to our 40% using the same definition. Further, utilizing a threshold RVSPecho > 45 mmHg as a predictor for the presence of PH, they reported a sensitivity, specificity, PPV and negative predictive value of 85%, 17%, 60% and 44%, respectively. However, their patient subgroup with ILD included connective tissue disease patients (with and without pulmonary vascular disease), various pneumoconioses as well as diverse idiopathic interstitial pneumonias, whereas we restricted our cohort to well-defined IPF patients. Further, we have expanded on their observations by reporting the performance characteristics of various threshold values of the RVSPecho as a predictor of PH alone and in combination with PFT and 6MWT data.
Nearly one-third of patients in whom there was no RVSPecho reported had PH by RHC. Therefore, although the sensitivity of ECHO was higher utilizing low threshold values for the RVSPecho, this cannot be relied upon as a screening tool for underlying PH. Further, this high sensitivity was associated with an unacceptably low specificity. For example, using an estimated RVSPecho > 35 mmHg as a predictor of PH yielded a sensitivity of 86.4%, but a specificity of only 28.9% (Table 2). On the other end of the spectrum, a high RVSPecho has very good specificity, but lacks sufficient sensitivity. This too is of limited clinical value, but does allow reasonable certainty as to the presence of PH.
We then assessed the performance characteristics of RVSPecho in relation to patients' PFTs and 6MWT data to assess if a step-wise approach incorporating two independent diagnostic tests, would improve the accuracy of detection. We have previously shown that the presence of underlying PH does not correlate with lung volumes but does have an association with a low DLco.16,17 As shown in Table 2,we assessed whether lower levels of the RVSPecho could yield similar high sensitivities, but with greater specificities when assessed in conjunction with patients' PFTs or 6MWT data. Although the specificity was increased with this approach, it was at the expense of the sensitivity. For example, the sensitivity of a RVSPecho > 30 mmHg decreased from 86.4% to 66.7% when it was evaluated in conjunction with a DLCO < 40% predicted (Table 2). The value of this combined approach was realized with the specificity, where lower threshold levels for the RVSPecho yielded high values when assessed in conjunction with PFT or 6MWT data. For example, a RVSPecho> 30 mmHg in conjunction with a DLCO< 30%, resulted in a specificity of 78.9%. This is in comparison to specificities of 13.2% and 66.7% for each of these variables alone. RVSPecho did not add much to the predictive abilities of 6MWT parameters, which by themselves have good performance characteristics for the detection of PH. For example, desaturation to <85% while on room air during the walk test was associated with a sensitivity and specificity for PH of 100% and 61.9%, respectively.
There are certain limitations to our study. Although RVSPecho was estimated using standard methodology at both institutions, the estimations were collated from multiple technicians and interpreters potentially increasing the variability of the measurements. This, however, is also a strength of the study as it is more reflective of the circumstances in clinical practice. ECHOs were not performed concurrently with RHCs but we do not believe that this impacted our findings. Although there can be serial change in PA pressures in patients with IPF, these are likely to be more significant towards the later stages of the disease.18 In addition, our analysis of the subgroup with ECHOs and RHCs within a 1-month timeframe was very similar to the group as a whole. Further in 51/60 cases, the ECHO was performed concurrently or prior to the RHC. Most of the cases of inaccuracy were due to ECHO overestimating the PASPcath. Since pressures are unlikely to decrease with time, this, therefore, lends further support to the inaccuracy of ECHO. The measurements of the RVSPecho were reported in only 55% of the patients. There are likely multiple reasons for this including the absence of a tricuspid regurgitant jet, or the technician not looking for or unable to identify a jet. Rather than being a limitation of our study, this is a limitation of ECHO and/or the experience, methodology and tenacity of the technicians in detecting and accurately assessing the peak velocity of the regurgitant jet. One of the important messages of our analysis is that the lack of a reported RVSPecho does not infer the absence of PH, since about one-third of these patients did indeed have PH as measured by RHC. Also, other ancillary ECHO features of right ventricular function, such as the tricuspid annular plane systolic excursion, which might have indicated the possible presence of PH were not routinely assessed for.18,19 Use of Doppler ultrasound flow assessment of the internal jugular vein has also been proposed as a indirect measure of the mPAP.20 Lastly, the patients who underwent both ECHO and RHC were generally a sicker, but robust subgroup of patients and whether our data can be extrapolated to all IPF patients will require further study.
In conclusion, our study demonstrates that RVSPecho might not be an accurate tool for the assessment of PH in IPF. Even when used in conjunction with standard PFT measures and 6MWT data, we were unable to define the optimal combination of parameters to provide sufficient accuracy to diagnose PH in IPF. However, our study does provide valuable information, especially, with regards to the high specificity for PH with the various combinations of parameters. When assessed in conjunction with PFTs or 6MWT data, lower threshold values of the RVSP do perform with sufficient specificity to implicate the likely presence of associated PH. Awareness of the performance characteristics of RVSPecho with and without PFTs and 6MWT data might enable the optimal timing of RHC in selected patients. A non-invasive tool that provides both a high sensitivity and specificity for PH in IPF remains to be identified and validated. The measurement of brain natriuretic peptide might have a role in this regard, but remains to be validated.21,22 The final determination of the role of ECHO in assessing for PH in IPF will require a prospective study, inclusive of a broader range of disease severity, with experienced echocardiographers focusing on the right side of the heart. Until such time, RHC remains the gold standard test for PH in IPF.
Acknowledgments
Dr. Zisman is funded by the National Institutes of Health IPF Clinical Research Network, which includes participation in a pulmonary hypertension study with sildenafil. This work was supported, in part, by grants from the NIH (5U10HL080411 to DAZ; HL080206 and HL086491 to JAB).
Abbreviations
- DLCO
Diffusing Capacity for Carbon Monoxide
- DLCO%
Diffusing Capacity for Carbon Monoxide Percent Predicted
- ECHO
Echocardiography
- FVC
Forced Vital Capacity
- FVC%
Forced Vital Capacity Percent Predicted
- IPF
Idiopathic Pulmonary Fibrosis
- mPAP
Mean Pulmonary Artery Pressure
- PAP
Pulmonary Artery Pressure
- PASPcath
Pulmonary Artery Systolic Pressure obtained via right-heart catheterization
- PFTs
Pulmonary Function Tests
- PH
Pulmonary Hypertension
- RHC
Right-Heart Catheterization
- RVSPecho
Right Ventricular Systolic Pressure as estimated by echocardiography
- 6MWT
The six-minute walk test
- 6MWD
The 6-minute walk test distance
- RAsatrest
Oxygen saturation on room air at rest
- RAsatexercise
Room air oxygen saturation with exercise
References
- 1.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 2.Latsi PI, du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–7. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 3.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164:1171–81. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 4.Nadrous HF, Pellikka PA, Krowka MJ, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:2393–9. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 5.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in idiopathic pulmonary fibrosis. Chest. 2006;129(3):746–52. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 6.Zisman DA, Karlamangla AS, Ross DJ, et al. High-resolution chest computed tomography findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2007;132:773–9. doi: 10.1378/chest.07-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan KL, Currie PJ, Seward JB, Hagler DJ, Mair DD, Tajik AJ. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol. 1987;9:549–54. doi: 10.1016/s0735-1097(87)80047-5. [DOI] [PubMed] [Google Scholar]
- 8.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–6. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed SN, Syed FM, Porembka DT. Echocardiographic evaluation of hemodynamic parameters. Crit Care Med. 2007;35:S323–9. doi: 10.1097/01.CCM.0000270242.03536.D3. [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society Guidelines for the six-minute walk test. Consensus statement. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 12.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update—a Consensus Report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–55. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Nathan SD, Noble P, Tuder R. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med. 2007;175:875–80. doi: 10.1164/rccm.200608-1153CC. [DOI] [PubMed] [Google Scholar]
- 14.Collard HR, Anstrom KJ, Schwarz MI, Zisman DA. Sildenafil improves walk distance in idiopathic pulmonary fibrosis. Chest. 2007;131(3):897–9. doi: 10.1378/chest.06-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–40. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 16.Nathan SD, Shlobin OA, Ahmad S, Urbanek S, Barnett SD. Pulmonary hypertension and pulmonary function testing in idiopathic pulmonary fibrosis. Chest. 2007;131:657–63. doi: 10.1378/chest.06-2485. [DOI] [PubMed] [Google Scholar]
- 17.Zisman DA, Ross DJ, Belperio JA, et al. Prediction of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med. 2007;101:2153–9. doi: 10.1016/j.rmed.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan SD, Ahmad S, Koch J, Barnett S, Ad N, Burton N. Serial measures of pulmonary artery pressures in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:168S. [Google Scholar]
- 19.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–41. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 20.Vich IB, Ramirez JB, Clara PC. Noninvasive, indirect measurement of pulmonary artery pressure. Arch Bronconeumol. 2007;43:267–71. [PubMed] [Google Scholar]
- 21.Leuchte HH, Neurohr C, Baumgartner R, et al. Brain natriuretic peptide and exercise capacity in lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med. 2004;170:360–5. doi: 10.1164/rccm.200308-1142OC. [DOI] [PubMed] [Google Scholar]
- 22.Leuchte HH, Baumgartner RA, Nounou ME, et al. Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am J Respir Crit Care Med. 2006;173:744–50. doi: 10.1164/rccm.200510-1545OC. [DOI] [PubMed] [Google Scholar]


