Abstract
Background
Multiple randomized trials have established a favorable safety profile for aprotinin use during cardiac surgery, but recent database analyses suggest an increased risk of adverse thrombotic events. Our group previously demonstrated that off-pump coronary artery bypass (OPCAB) is linked to a postoperative hypercoagulable state. In this study, we tested whether aprotinin influences thrombotic events after OPCAB.
Methods
Patients randomly received saline (n = 61) or aprotinin (2 × 106 kallikrein inhibiting units (KIU) loading dose, 0.5 × 106 KIU/hour [n = 59]) during OPCAB. Aprotinin levels (KIU/mL) were analyzed before, and 30 minutes (peak) and 4 hours after the loading dose. Estimated glomerular filtration rate (eGFR) was calculated daily based on Cockcroft equation with acute kidney injury (AKI) defined as eGFR less than 75% of baseline. Major adverse cardiac and cerebrovascular events (MACCE) were monitored during the first year, including acute graft failure by predischarge computed tomographic angiography.
Results
Compared with placebo, the aprotinin group developed a significantly lower eGFR on day 3 (p < 0.006), but this difference resolved by day 5. Peak aprotinin level correlated with the degree of eGFR decline noted on day 3 (r = 0.56, p < 0.03) and independently predicted postoperative AKI (odds ratio 8.8, p < 0.008). The receiver operating characteristic analysis demonstrated that peak aprotinin level strongly predicts AKI (area under the curve = 0.86, 95% confidence interval 0.69 to 1.00). The percentage of patients reaching the composite MACCE endpoint was significantly reduced in the aprotinin versus placebo group (12 vs 34%, p = 0.01).
Conclusions
Compared with placebo, aprotinin use was associated with less MACCE but more AKI after OPCAB. The strong relationship between the peak aprotinin level and subsequent AKI suggests weight-based protocols for dosing aprotinin may reduce this risk.
Hemostatic agents have been used during coronary artery bypass grafting (CABG) in order to reduce bleeding and the risks associated with transfusion, reoperation, and tamponade. One of the most effective of these agents is the serine protease, aprotinin. In fact, aprotinin is so potent at promoting hemostasis that it raises concern for causing a hypercoagulable state. Recent observational studies suggest that intraoperative use of aprotinin increases the risk of adverse events such as renal insufficiency, saphenous vein graft (SVG) closure, stroke, and death due to thromboembolism [1].
A clinical definition of the “hypercoagulable state” is a change in coagulation and platelet function that leads to a greater prevalence of thrombosis in response to a given stimulus than in the normal population [2]. We have found that performing CABG “off-pump” (OPCAB) stimulates the production of thrombotic markers within SVG at a higher level than CABG done with conventional techniques [3]. Two separate meta-analyses have concluded that OPCAB is associated with a heightened risk of SVG failure compared with “on-pump” CABG [4,5]. Therefore, OPCAB provides a model for investigating whether intraoperative aprotinin use exacerbates this thrombotic risk, as illustrated by major adverse cardiac and cerebrovascular events (MACCE) and renal dysfunction. We hypothesized that, consonant with the results of prior randomized trials performed during on-pump CABG, aprotinin use would not have an unfavorable influence on these events after OPCAB.
Patients and Methods
Patient Selection and Enrollment
A randomized, double-blind, placebo-controlled study of aprotinin was completed in 120 OPCAB patients (University of Maryland IRB protocol #0902312). Because the use of aprotinin for OPCAB is “off-label,” a Food and Drug Administration (FDA) Investigational New Drug application (IND #67,890) was submitted and approved. All patients included in the study signed an individual letter of consent. Exclusion criteria included nonambulatory patients and those with creatinine greater than 2.0 mg/dL, active hepatitis or cirrhosis, allergy to radiographic contrast, prior exposure to aprotinin at any point, use of GPIIb/IIIa receptor inhibitors, or both aspirin and clopidogrel within 3 days of surgery.
Treatments
A modified “full dose” regimen was used: 10,000 kallikrein inhibiting units (KIU) intravenous test dose (or saline placebo) was followed by 2 million KIU aprotinin (Trasylol, Bayer Pharmaceuticals Corp) through a central line and then 500,000 KIU/hour until the end of the operation. Computer-generated randomization was based on permuted blocks of size 4. The study drug or saline placebo was delivered to the operating room in unlabeled bottles. Heparin was titrated using a Hepcon instrument (Medtronic, Inc, Minneapolis, MN) and kaolin-based activated clotting time (ACT) to maintain a heparin level greater than 2 mg/mL and ACT greater than 300 seconds. Heparin was reversed after OPCAB by half the recommended dose of protamine. Preoperative and postoperative aspirin (325 mg by mouth/day) was the sole platelet inhibitor used. Transfusions were based on complete blood count, coagulation profile, fibrinogen levels, and thromboelastography (TEG) after a previously described algorithm [6]. Intensive care unit (ICU) and hospital discharge followed established protocols.
Surgery
Four surgeons, experienced in OPCAB, enrolled patients. Internal mammary conduits were used in all patients. Saphenous veins were harvested using an endoscopic (Guidant Systems, Inc., Minneapolis, MN) approach and stored in PLASMA-LYTE (Baxter Healthcare Corporation, Deerfield, IL) solution until grafting. The distal anastomoses were created using suction-based exposure and stabilizing devices (Medtronic, Inc). The volume of shed blood collected using a cell saver device (Kobe Bratt II; Kobe Cardiovascular, Inc, Arvada, CO) was measured along with the amount of postoperative shed blood after 24 hours.
Assays for Coagulation
Ex vivo coagulation and platelet function testing was done at four time points: preoperatively (ie, baseline prior to skin incision), postoperatively 30 minutes after protamine administration, and the mornings of postoperative days 1 and 3. Whole blood aggregometry was performed with thrombin (0.25, 0.5, 0.75, 1.0 U/mL) as the agonist. Thrombelastography was performed by adding calcium chloride (2 mM) to citrated whole blood followed by tissue factor (20 μM) ± tissue plasminogen activator (tPA, 40 IU). The degree of change in amplitude at 30 minutes after the maximum amplitude (% lysine 30) defined thrombolytic capacity. In vitro analyses of TEG and aggregometry were performed adding aprotinin (100, 150, 200, 250 KIU/mL, n = 3 for each concentration) to blood obtained from a normal volunteer.
Aprotinin Level
Aprotinin levels were determined at 30 minutes (peak) and 4 hours (trough) after the loading dose. Serum was diluted in 0.3M Tris-HCL, 0.15M NaCl, pH 7.3 by incubating with 10 U plasmin followed by the addition of chromogenic substrate (benzoyl-phe-val-arg-p-nitroanlide, Sigma) to determine plasmin activity. The concentration of aprotinin, reported in KIU/mL, was then determined by comparing the plasmin inhibitory activity in the sample against a standard curve of known aprotinin concentrations. The postoperative values were normalized against the baseline value [7].
Renal Function Assessment
The estimated glomerular filtration rate (eGFR) was calculated from the serum creatinine (sCr) level using the Cockcroft and Gault Formula: (186)(sCr mg/dL−1.154)(age year−0.203)(0.742 if female)(1.212 if African American)(1.73)/body surface area [8]. Urine output was monitored hourly in the first 24 hours and then daily for 4 days. We defined acute kidney injury (AKI) when postoperative eGFR was less than 75% of baseline and acute renal failure when combined with urine output less than 0.5 mL/kg/hour × 6 hours [9]. Changes in eGFR during the postoperative period were quantified by the slope of eGFR change on a given postoperative day as compared with preoperative eGFR using the equation: [(postop GFR − preop GFR)/preop GFR)] [10].
Postoperative Follow-Up
Stroke was assessed by daily physical examinations and confirmed by head computed tomographic (CT) examination. Noninvasive, 64 detector row, CT angiography (420 ms rotation, 100 to 150 mL contrast agent intravenously at 5 mL/second, retrospective electrocardiographic [ECG] gating) was obtained prior to hospital discharge. Patency of SVG, determined by a single, blinded, expert reviewer, was defined by evidence of any contrast within the length of the graft regardless of the presence of stenosis, and “nonpatent” if a stump or no graft was seen. Postoperative myocardial infarction (MI) was defined by cardiac troponin I (cTnI) equal to or greater than 5 times the upper limit of normal or new q-waves on ECG at 4, 12, or 72 hours. Mortality within 1 year of surgery was assessed using the Social Security Death Index with further review of the medical records used to classify whether the death was cardiac related. As a result, follow-up on cause of death for all patients was 100%. The combined incidence of MI, stroke, SVG failure, and cardiac-related death within 1 year served as our composite endpoint of MACCE.
Statistical Methods
The primary endpoint of this study was to compare the risk of developing MACCE in the first postoperative year for the aprotinin versus placebo groups. Prior analyses have demonstrated a 20% risk of these events occurring after CABG [1]. Power analysis indicated that 50 patients per group were required to demonstrate a 50% difference in events at p = 0.05 and power = 80%. A total of 60 patients per group were targeted for recruitment to allow for 20% attrition during follow-up. A secondary endpoint was to define the clinical impact of AKI that develops after aprotinin use. The value of determining the intra-operative aprotinin level as a means of predicting postoperative AKI was quantified by determining the area under the receiver operating characteristic (ROC) curve. Logistic regression was used to determine an interaction between the optimal cutoff value for aprotinin level and previously reported risk factors for AKI [11]. Variables with p less than 0.1 between groups with and without AKI were included in a stepwise fashion in the model. Analyses were performed with SPSS statistical software (SPSS version 13.0; SPSS, Chicago, IL) and SAS (SAS version 9.1; SAS, Cary, NC) with the assistance of a statistician. Comparisons were done by analysis of variance with subsequent pairwise comparisons according to the Duncan multiple range test and correlations determined by calculating a Pearson’s coefficient. Categoric data were compared using the Fischer exact test.
Results
Patient Population
During the enrollment period, 693 patients were screened and 130 were randomized, with 64 allocated to receive aprotinin and 66 allocated to placebo. A total of 563 CABG patients were excluded due to the requirement of cardiopulmonary bypass (CPB) (n = 410), not planning to use a SVG (n = 72), inability to obtain informed consent (n = 65), creatinine greater than 2.0 mg/dL (n = 14), and preoperative clopidogrel use (n = 2). Ten randomized patients (5 from each group) did not receive the study drug because of the intraoperative decision to use CPB. These patients were considered “inappropriately randomized” and were excluded from analysis as a justifiable exception to the “intention to treat” principle [12], leaving a total of 120 patients (n = 59 aprotinin, 61 placebo). After enrollment, CT angiographic follow-up was not obtained in 4 patients because of a heart rate greater than 100 bpm or creatinine greater than 2.0 mg/dL (n = 3) and patient withdrawal of consent (n = 1).
Intraoperatively collected data, such as ejection fraction (0.439 ± 0.151 vs 0.402 ± 0.89, p = not significant [NS]), number of grafts per patient (3.05 ± 0.89 vs 2.83 ± 0.93, p = NS), conduit diameter (3.89 ± 0.62 mm vs 4.07 ± 0.78 mm, p = NS), and endothelial integrity (49.2 ± 35.8% vs 43.9 ± 35.7% luminal surface positive for CD31, p = NS), average target size (1.89 mm vs 1.91 ± 0.34 mm, p = NS), and quality for the SVG and inotropic requirements were all similar between groups.
Effects on Renal Function
Although mean eGFR declined significantly over the first 5 postoperative days in both groups (Fig 1), the incidence of postoperative AKI was more frequent in patients receiving aprotinin (27 of 59) versus placebo (15 of 61) (45.8 vs 24.6%, p < 0.03). The aprotinin group showed a significantly greater reduction in eGFR compared with placebo on day 2 (slope of eGFR change: −8.35 ± 7.58 vs −4.59 ± 10.93, p < 0.05) and day 3 (slope −8.86 ± 8.60 vs −4.39 ± 6.71, p = 0.006). However, eGFR differences between groups resolved by day 5 (slope −6.18 ± 5.79 vs −9.09 ± 9.04, p = NS). Postoperative elevations in eGFR (serum creatinine rise of >0.5 mg/dL) were more frequent in the aprotinin group (20 of 59) versus placebo (9 of 61) (33.9 vs 14.8%, p < 0.04). Acute renal failure was noted within the first 6 months after surgery in 4 patients (2 in each group) but resolved without dialysis in all cases. In the placebo group, AKI was associated with longer intubation time (12.8 ± 8.3 vs 8.3 ± 7.3 hours, p = 0.09) and longer hospital stay (11.0 ± 6.4 vs 7.4 ± 4.9 days, p = 0.11), though the differences were not statistically significant. In aprotinin-treated patients, the onset or absence of postoperative AKI had no detectable effect on intubation time (15.3 ± 9.4 vs 17.9 ± 15.9, p = NS) or hospital stay (9.4 ± 4.3 vs 7.9 ± 5.9, p = NS).
Fig 1.
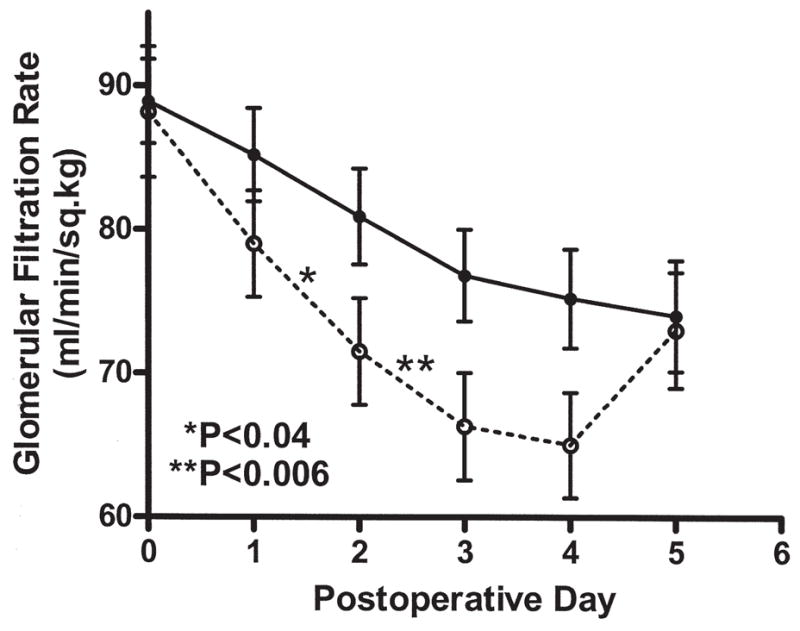
Glomerular filtration rate was estimated (eGFR) from the serum creatinine daily prior to hospital discharge in all subjects. While preoperative eGFR was similar between groups, the aprotinin group (○) showed a significant reduction in GFR on day 2 (*p < 0.04) and day 3 (**p < 0.006) compared with the placebo group (●). Differences between groups resolved by day 5.
Urine output was not significantly different between the aprotinin and placebo groups over the first 24 hours (1,463 ± 647 vs 1,518 ± 846 mL/24 hours, p = NS), on day 2 (1,400 ± 822 vs 1,369 ± 648 mL/24 hours, p = NS), day 3 (1,461 ± 728 vs 1,460 ± 951, p = NS), or day 4 (1,456 ± 568 vs 1,384 ± 708, p = NS).
Aprotinin Levels and Nephrotoxicity
For the aprotinin group, the average aprotinin level was 275 ± 56 KIU/mL after the loading dose (ie, peak) and 237 ± 25 KIU/mL at case completion. There was a significant correlation noted between peak serum aprotinin level and decline in eGFR on day 3 (r = 0.56, p < 0.03) and day 4 (r = 0.50, p < 0.05), as well as the greatest absolute eGFR decline at any time point (r = 0.47, p < 0.05) (Fig 2). Peak aprotinin levels were significantly higher in patients with AKI (292.6 ± 9.0 vs 219.7 ± 26.72 KIU/mL, p = 0.007). The ROC analysis demonstrated that peak aprotinin level was a highly effective assay for predicting postoperative AKI (area under the curve = 0.86, 95% confidence interval [CI] 0.69 to 1.00). A cutoff aprotinin level of 271 KIU/mL provided a sensitivity of 100% and specificity of 76.9% for predicting postoperative AKI.
Fig 2.
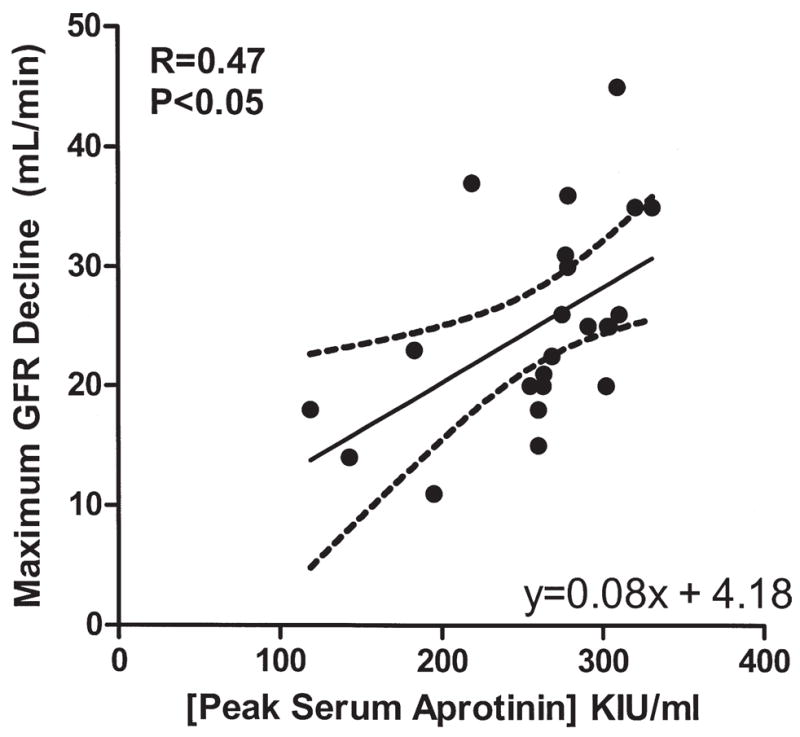
Peak serum aprotinin level was compared to the maximum degree of glomerular filtration rate (GFR) change for days 1 to 5 after off-pump coronary artery bypass. For patients receiving aprotinin, the peak level of aprotinin showed a linear relationship to the decline in GFR (r = 0.47, p < 0.05). Aprotinin-treated patients with acute kidney injury showed significantly higher peak aprotinin levels than patients who maintained normal renal function throughout the course of the study. (KIU = kallikrein inhibiting units.)
Logistic regression also showed that an aprotinin level greater than 271 KIU/mL was an independent predictor of AKI (odds ratio [OR] 8.8, 95% CI 2.45 to 31.56, p = 0.0008) after adjusting for potential confounders. None of the other clinical variables that were analyzed (variables selected based on reference 11) were found to be significant predictors of AKI in this cohort.
Aprotinin Level and Efficacy
Comparison of patients with peak aprotinin levels above or below the 271 KIU/mL cutoff showed no difference in blood loss intraoperatively (867 ± 413 mL vs 870 ± 383, p = NS) or postoperatively (415 ± 330 mL vs 427 ± 171 mL/24 hours, p = NS). In comparison, the placebo group showed significantly higher intraoperative (1,252 ± 380 mL, p < 0.02) and postoperative (716 ± 336 mL, p < 0.003) bleeding.
In vitro testing (n = 3 for each aprotinin dose) confirmed that an aprotinin level of 200 KIU/mL was sufficient for inhibition of thrombin-mediated platelet aggregation. Inhibition of fibrinolysis was noted at levels greater than 50 KIU/mL (Fig 3 A;B).
Fig 3.
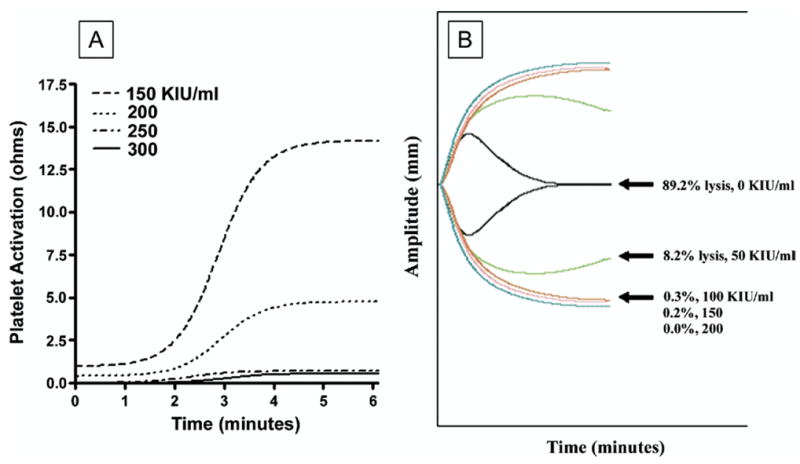
(A) Representative impedance traces obtained during whole blood aggregometry performed on blood obtained from a normal volunteer after the in vitro addition of aprotinin. Without aprotinin added, there was a strong aggregation response to thrombin 1 U/mL, as illustrated by a change in ohms of 12.5 over 6 minutes. No platelet response to thrombin was noted when aprotinin levels ranged from 200 to greater than 300 kallikrein inhibiting units [KIU]/mL. A modest response (ie, 5 ohms at 6 minutes) was noted for aprotinin levels less than 200 KIU/mL. (B) Fibrinolytic capacity was assayed using thrombelastography (TEG) to demonstrate clot lysis in response to a low dose of tissue plasminogen activator (tPA, 40 IU) after the in vitro addition of varying doses of aprotinin (n = 3 per dose). As illustrated in these representative traces, the addition of tPA results in near complete lysis of the clot that formed within the TEG cup in the absence of aprotinin. At aprotinin concentrations greater than 50 KIU/mL, the amplitude of the TEG trace shows very minimal change compared with a TEG trace without tPA added, indicating near complete resistance to fibrinolysis.
Effect of Aprotinin on MACCE
Prior to discharge, postoperative MI developed in 1 (1.7%) patient in the aprotinin group versus 4 (6.6%) placebo patients (p = NS). No additional infarcts were reported. The CT angiography showed acute occlusion in 3 of 80 SVG (3.8%) in the aprotinin group (1 prior to discharge, 2 at six-month follow-up) and 8 of 90 SVG (8.9%) in the placebo group (3 prior to discharge, 5 at six-month follow-up) (p = NS). Prior to discharge, one postoperative stroke was noted in a placebo patient; none after aprotinin. No additional strokes were reported. At one year, death was noted in 3 (5.1%) patients in the aprotinin group (no deaths prior to discharge, 1 within 30 days, 2 within 1 year) compared with 8 (13.1%) in the placebo group (no deaths prior to discharge, 2 within 90 days, 6 within 1 year) (p = NS). All patients in the study had comprehensive follow-up to ensure death was cardiac related. The composite endpoint, MACCE, was less common in the aprotinin group (11.8 vs 34.4%, p < 0.005). The risk of MACCE by 1 year was significantly increased in the placebo group (HR 2.871, 95% CI 1.252 to 5.570, p < 0.01). The protective effect of aprotinin on MACCE was not influenced by the onset of postoperative AKI (hazard ratio [HR] 1.103, 95% CI 0.248 to 4.906, p = NS) (Fig 4).
Fig 4.
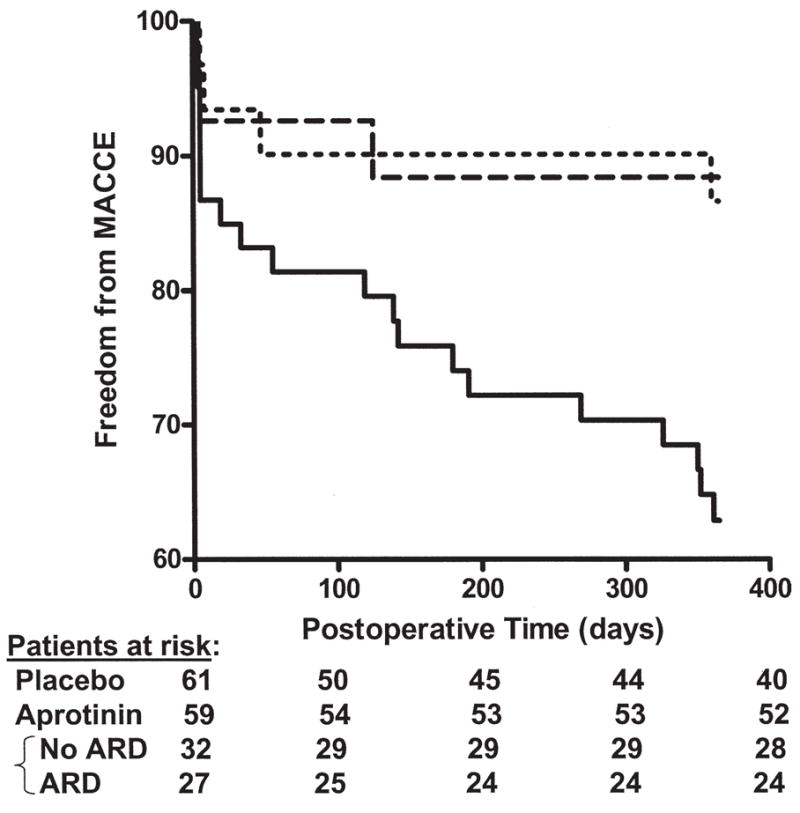
The combination of graft failure, stroke, myocardial infarction, and death over the first postoperative year was used to generate a composite endpoint, major adverse cardiac, and cerebrovascular events (MACCE). The aprotinin group showed a significant reduction in MACCE after the first year (11.8% vs 34.4% of the placebo patients, p < 0.005), and did not appear to be related to the development of postoperative AKI (hazard ratio [HR] 1.103, 95% CI 0.248 to 4.906, p = NS). In contrast, the risk of MACCE was increased in the placebo group (hazard ratio 2.871, 95% CI 1.252 to 5.570, p < 0.01) compared with the aprotinin group, an effect that did not appear to be confined to the perioperative period. (ARD = acute respiratory distress.)
Comment
There has been considerable concern recently that aprotinin increases the risk of adverse events due to thrombosis. Because endogenous fibrinolysis is an important mechanism for preventing unwanted vascular occlusion [13, 14], blockade of fibrinolysis by aprotinin after CABG theoretically removes an important protective mechanism for vessels at risk, such as the SVG. Recent observational studies have linked aprotinin use during OPCABG surgery to an increased risk of thrombotic events, using the rates of postoperative AKI and MACCE to illustrate this point [1, 15]. However, observational studies cannot establish cause and effect and statistical assumptions to correct for physician bias (eg, aprotinin use in higher risk cases) are not always accurate [16]. This randomized study of aprotinin use during on-pump CAB provides a unique look at the safety of aprotinin by analyzing this issue in a cohort already at increased risk for thrombotic events due to a postoperative hypercoagulable state [3]. Instead of finding more adverse events, the use of aprotinin during OPCAB led to a significant reduction in the risk of MACCE compared with placebo within the first postoperative year. These findings corroborate the safety of aprotinin use during on-pump CABG previously suggested by meta-analyses of randomized placebo controlled trials [17,18] and a recent exhaustive review of all available data on this topic by the FDA [16].
Our findings corroborate reports that aprotinin administration increases the risk of postoperative AKI [1, 15]. Because thromboembolism is a known mechanism of severe renal injury after CABG, a higher rate of AKI after aprotinin administration is often assumed to be evidence of the prothrombotic potential of this drug [1]. However, it is important to discriminate between renal failure, characterized by low urine output ± the need for dialysis, from renal insufficiency, characterized by a rise in creatinine. Through the use of radiolabeled aprotinin, it has been illustrated that this drug is actively reabsorbed in the proximal convoluted tubule [19]. The resulting aprotinin deposits are thought to saturate mechanisms responsible for creatinine reabsorption, thereby leading to a transient change in creatinine clearance. Physicians noticing a rise in creatinine in the postoperative period might be tempted to conclude that aprotinin increases the risk for renal failure. However, analysis of data from randomized trials [16], retrospective reviews of databases [20], and our study demonstrate a return to normal renal function and no long-term risk of renal failure or dialysis after aprotinin use. Finally, AKI developing in the absence of aprotinin increases mortality after CABG [21]. Although this OPCAB study was underpowered to fully characterize the clinical consequences of AKI in our patients, we found no adverse impact on the clinical course of patients with AKI in the aprotinin group. Furthermore, AKI after aprotinin use was not found to be associated with other adverse events to suggest an ischemic-thrombotic syndrome. For these reasons, we do not interpret AKI after aprotinin treatment as a thrombotic event.
Despite its safety when used during OPCAB, our evidence suggests a fairly narrow therapeutic window for aprotinin. Peak aprotinin level greater than 271 KIU/mL was an independent risk factor for AKI, while a threshold of 200 KIU/mL appears to be required to maintain full hemostatic effects by the inhibition of fibrinolysis and thrombin-mediated platelet aggregation. Prior analyses have revealed an increased risk of AKI with the full dose compared with the half dose regimen [20], which corroborates our finding about the importance of peak aprotinin levels. The standard regimen of fixed bolus dosing (ie, modified Hammersmith protocol) that we used was inconsistent at achieving target concentrations of aprotinin, erring toward excessively high levels. Our findings suggest that an alternative strategy such as weight-based dosing should be investigated as a method for reducing the risk of AKI [22].
Our trial has several limitations. The outcome for our OPCAB cohort differs from that of a large multicenter trial of on-pump CABG, which found a significant reduction in SVG patency after aprotinin use [23]. Targets of aprotinin such as PAR-1 and plasmin are activated differently during on-pump versus off-pump CABG [3]. It is possible that these differences influence the therapeutic effect of aprotinin. Additional unique effects of OPCAB, such as reduced risk of perioperative renal injury [24, 25] and less intraoperative hemodilution [26, 27], may have influenced the pharmacodynamics and safety profile of aprotinin. As a result, our findings cannot be extrapolated to on-pump CABG.
In conclusion, our data suggest that aprotinin does not increase the risk of MACCE after OPCAB, thereby removing some of the trepidations about the use of this drug in appropriate candidates undergoing this procedure. Aprotinin increased the risk of AKI in the postoperative period, but this effect appeared transient and did not increase the risk of perioperative complications. The optimal benefit of this drug appears to occur within a relatively narrow therapeutic window that avoids AKI (>271 KIU/mL) but is sufficient to inhibit PAR-1 (>200 KIU/mL) [28]. Although FDA-approved regimens follow a fixed-dose strategy, our findings strongly support alternative dosing strategies such as those based on weight.
Acknowledgments
Dr Poston is supported by a phase IV grant from Bayer Pharmaceuticals Corp, a Scientist Development Grant from the American Heart Association, an Intramural Grant from the University of Maryland, a faculty pilot grant from the Tobacco Restitution Fund at the University of Maryland, and by the National Institutes of Health (RO1 HL084080-01A1).
DISCUSSION
DR HERBERT B. WARD (Minneapolis, MN): You conclude that aprotinin is effective. You base this conclusion on improved graft patency in the aprotinin group instead of the more common reasons such as less blood usage or blood loss. It seems illogical to me that aprotinin would improve graft patency. Can you postulate a mechanism for this result? Did the patients who received placebo get more platelets?
DR GRANT: Well, I think that’s an excellent hypothesis right there. But another potential concept is that aprotinin may not be prothrombotic, but it could actually be antithrombotic.
PAR-1 is a very important mechanism through which intra-vascular thrombosis occurs. And at the doses that we’re using this drug, between 200 and 250 KIU/mL, it has a nearly complete inhibition of PAR-1. So I don’t think it’s inconceivable that aprotinin could, indeed, have antithrombotic effects in the patients that tend to be more hypercoagulable. It may not show up in on-pump surgery, because you don’t have as much platelets that have the PAR-1 available for activation and so the antifibrinolytic effect might be a more important clinical factor in those patients and so they have an increased tendency to clot. So it depends on the clinical application you’re applying it to. That’s why I focus my conclusions on the off-pump group.
With regards to efficacy, I’ve presented that in other avenues and I didn’t have time to get into it today. But it showed the same things that you expect; 50% reduction in blood loss, reduction in transfusions, and those kinds of things.
DR J. LANCELOT LESTER (West Palm Beach, FL): Did you prep these patients with aspirin or Plavix or anything else before?
DR GRANT: Aspirin.
DR LESTER: The second thing is: I’m not a fan of aprotinin because my experience was that it produced renal failure in high-risk patients, particularly right after cardiac catherization (perhaps this subset was not randomized), at a time in which the randomized studies were saying that it didn’t happen—but it strikes me that the study in the New England Journal, as compared to the randomized studies of aprotinin, showed it was a terrible thing, but indicts study design. Propensity matching in the study would suggest that those patients had the equivalence of randomization, yet they had a dramatic difference in their results than the randomized studies that have been published.
I think it suggests that propensity matching was not as good as prospective randomized analysis. Perhaps of greater concern is that prospective randomized analysis continues to be limited, as in some studies of surgery versus angioplasty, by the patient selection utilizing a very low percentage of patients. So I think your studies and the other studies speak to the limitations of propensity matching, whereas some of the recent studies speak to the limitation of randomizing small, select populations and then generalizing to the larger population.
DR GRANT: I agree with you and I appreciate your comment. I would like to add that a significant problem with the Mangano data was the lack of transparency of the data. Until very lately, just last September, the FDA didn’t have access to that data. It didn’t make sense to them, either, that they did propensity matching and yet the groups looked so drastically different, the aprotinin-treated patients versus the patients that didn’t receive aprotinin.
When they finally did get the data, and this is on the Web at the FDA website, they rejected all the conclusions other than the fact that it does, indeed, cause renal failure, according to Mangano’s own blinded, you know, the data set that they had access to. His propensity-score matching methods were not accurate and they didn’t appropriately adjust for the patients. That’s the FDA’s conclusion.
So I think that propensity matching and the database analyses do have an important role for events that are unusual and they can’t be captured in randomized trials because you just don’t have the funds to do a large enough powered study, but it has to be done in a way that’s accurate and transparent.
DR LESTER: I just think it’s critical. Our colleagues believe in the randomized trials so strongly, particularly in Medicine. And I remember one striking phenomena, which was the original VA [Veterans Administration] studies on unstable angina showed that surgery made no difference, but they only randomized 2 out of every 50 patients in each hospital. And yet we knew that the untreated mortality—this was prior to the aggressive stenting age, or prior to stenting probably—was 20% and 30% over the next 2 to 3 months.
And there is another area of concern where surgeons have hurt ourselves. No one in surgery is discussing the subsets of patients with the lowest mortality. We’re busy trying to indict ourselves by being sure that we collect our entire series in each hospital, or in the STS [Society of Thoracic Surgeons]. We should point out the low-risk patients who, for instance, have coronary bypass, that have incredibly low mortalities and compare favorably to stenting or any other management.
I think that the issue of trials and patient selection and how they’re conducted is so critical to, really, the future of our specialty if nothing else.
DR ROBERT S. D. HIGGINS (Chicago, IL): I did have a question about your final conclusion; you did not present data to address the safety issue or that aprotinin was effective. Taking into account the recent concerns about mortality as an outcome, how do you interpret your study and think about where mortality is in the spectrum of problems that we have to think about with aprotinin?
DR GRANT: So the randomized trials and FDA’s analysis of the postmarketing database have not shown a difference in mortality. So I mean, I think that –
DR HIGGINS: But the FDA has responded to prospective data out of the Canadian study that suggests that there is a significant mortality risk and that’s why the use of drug has been temporarily suspended until further analysis.
DR GRANT: That’s really hard to comment on because we have a very limited understanding of exactly what that showed. If you look at the website for the BART study [biomarkers that can be used to predict acute rejection in transplantation], it says that it increased mortality due to bleeding. And I think sometimes randomized trials, even if they’re appropriately powered, can give us answers that just don’t make sense. And if that was, indeed, the mechanism through which aprotinin increased bleeding, which wasn’t statistically significant, actually, the difference in mortality was a trend toward significant. So I just don’t know what to do with that. Until we have the manuscript to look at, and that came out in November, and I was hoping that would have been here by now, by the day of this presentation, to be able to comment on that, but I just can’t at this point.
DR GUS J. VLAHAKES (Boston, MA): If you look at the original pivotal trial that the vendor conducted for final approval, there were four groups: there was a control; there was a pump load only; and there were two dose regimens, a full Hammersmith dose and the half-dose regimen. And if you look at the data, which is all in the package insert and in the PDR, the information about the potential role in creating renal dysfunction is in that data. And so the relationship between the amount of drug given, and hence, the blood levels, was already known. I think your presentation really has quantitated this issue and brought it to light.
Footnotes
Dr Poston discloses that he has a financial relationship with Bayer Pharmaceuticals Corp.
Presented at the Forty-fourth Annual Meeting of The Society of Thoracic Surgeons, Fort Lauderdale, FL, Jan 28–30, 2008.
References
- 1.Mangano DT, Tudor IC, Dietzel C Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 2.Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22:1381–9. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- 3.Kon ZN, Kwon MH, Collins MJ, et al. Off-pump coronary artery bypass leads to a regional hypercoagulable state not detectable using systemic markers. Innovations Tech Cardiac Surg. 2006;1:232–8. doi: 10.1097/01.imi.0000242160.21278.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim E, Drain A, Davies W, Edmonds L, Rosengard BR. A systematic review of randomized trials comparing revascularization rate and graft patency of off-pump and conventional coronary surgery. J Thorac Cardiovasc Surg. 2006;132:1409–13. doi: 10.1016/j.jtcvs.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Takagi H, Tanabashi T, Kawai N, Kato T, Umemoto T. Off-pump coronary artery bypass sacrifices graft patency: meta-analysis of randomized trials. J Thorac Cardiovasc Surg. 2007;133:e2–3. doi: 10.1016/j.jtcvs.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 6.Brown JM, Poston RS, Gammie JS, et al. Off-pump versus on-pump coronary artery bypass grafting in consecutive patients: decision-making algorithm and outcomes. Ann Thorac Surg. 2006;81:555–61. doi: 10.1016/j.athoracsur.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 7.Beath SM, Nuttall GA, Fass DN, Oliver WC, Jr, Ereth MH, Oyen LJ. Plasma aprotinin concentrations during cardiac surgery: full-versus half-dose regimens. Anesth Analg. 2000;91:257–64. doi: 10.1097/00000539-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Greene T, Kusek J, Beck G. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 9.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wali RK, Mohanlal V, Ramos E, et al. Early withdrawal of calcineurin inhibitors and rescue immunosuppression with sirolimus-based therapy in renal transplant recipients with moderate to severe renal dysfunction. Am J Transplant. 2007;7:1572–83. doi: 10.1111/j.1600-6143.2007.01825.x. [DOI] [PubMed] [Google Scholar]
- 11.Mehta RH, Grab JD, O’Brien SM, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–16. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- 12.Fergusson D, Aaron SD, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325:652–4. doi: 10.1136/bmj.325.7365.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Páramo JA, Orbe J, Fernández J. Fibrinolysis/proteolysis balance in stable angina pectoris in relation to angiographic findings. Thromb Haemost. 2006;86:636–9. [PubMed] [Google Scholar]
- 14.Nordenhem A, Leander K, Hallqvist J, de Faire U, Sten-Linder M, Wiman B. The complex between tPA and PAI-1: risk factor for myocardial infarction as studied in the SHEEP project. Thromb Res. 2005;116:223–32. doi: 10.1016/j.thromres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Mangano DT, Miao Y, Vuylsteke A, et al. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA. 2007;297:471–9. doi: 10.1001/jama.297.5.471. [DOI] [PubMed] [Google Scholar]
- 16.Mark Levenson., PhD Post-market safety of aprotinin (Trasylol). Statistical review of the observational studies of aprotinin safety part I: Methods, Mangano and Karkouti Studies. CRDAC and DSaRM Meeting; September 12, 2007; [Accessed October 3, 2007]. Available at: http://www.fda.gov/OHRMS/DOCKETS/AC/07/slides/2007-4316s1-12-FDA-Levenson_files/frame.htm. [Google Scholar]
- 17.Brown JR, Birkmeyer NJ, O’Connor GT. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation. 2007;115:2801–13. doi: 10.1161/CIRCULATIONAHA.106.671222. [DOI] [PubMed] [Google Scholar]
- 18.Sedrakyan A, Treasure T, Elefteriades JA. Effect of aprotinin on clinical outcomes in coronary artery bypass graft surgery: a systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg. 2004;128:442–8. doi: 10.1016/j.jtcvs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 19.Rustom R, Grime JS, Maltby P, Stockdale HR, Critchley M, Bone JM. Observations on the early renal uptake and later tubular metabolism of radiolabelled aprotinin (Trasylol) in man: theoretical and practical considerations. Clin Sci (Lond) 1993;84:231–5. doi: 10.1042/cs0840231. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich W, Busley R, Kriner M. High-dose aprotinin in cardiac surgery: is high-dose high enough? An analysis of 8281 cardiac surgical patients treated with aprotinin. Anesth Analg. 2006;103:1074–81. doi: 10.1213/01.ane.0000238446.30034.c8. [DOI] [PubMed] [Google Scholar]
- 21.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. Epub 2006 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuttall GA, Fass DN, Oyen LJ, Oliver WC, Jr, Ereth MH. A study of a weight-adjusted aprotinin dosing schedule during cardiac surgery. Anesth Analg. 2002;94:283–9. doi: 10.1097/00000539-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Alderman EL, Levy JH, Rich JB, et al. Analyses of coronary graft patency after aprotinin use: results from the International Multicenter Aprotinin Graft Patency Experience (IMAGE) trial. J Thorac Cardiovasc Surg. 1998;116:716–30. doi: 10.1016/S0022-5223(98)00431-0. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa M, Yaku H, Doi K, et al. Does off-pump coronary artery bypass grafting beneficially affect renal function? ANZ J Surg. 2005;75:675–9. doi: 10.1111/j.1445-2197.2005.03490.x. [DOI] [PubMed] [Google Scholar]
- 25.Sellke FW, DiMaio JM, Caplan LR, et al. Comparing on-pump and off-pump coronary artery bypass grafting: numerous studies but few conclusions: a scientific statement from the American Heart Association council on cardiovascular surgery and anesthesia in collaboration with the interdisciplinary working group on quality of care and outcomes research. Circulation. 2005;111:2858–64. doi: 10.1161/CIRCULATIONAHA.105.165030. [DOI] [PubMed] [Google Scholar]
- 26.Royston D. Aprotinin prevents bleeding and has effects on platelets and fibrinolysis. J Cardiothorac Vasc Anesth. 1991;5(6 suppl 1):18–23. doi: 10.1016/1053-0770(91)90082-5. [DOI] [PubMed] [Google Scholar]
- 27.Puskas JD, Williams WH, Duke PG, et al. Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: a prospective randomized comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:797–808. doi: 10.1067/mtc.2003.324. [DOI] [PubMed] [Google Scholar]
- 28.Day JR, Landis RC, Taylor KM. Aprotinin and the protease-activated receptor 1 thrombin receptor: antithrombosis, inflammation, and stroke reduction. Semin Cardiothorac Vasc Anesth. 2006;10:132–42. doi: 10.1177/1089253206288997. [DOI] [PubMed] [Google Scholar]


