Abstract
Distortion-product otoacoustic emissions (DPOAEs) were weak or absent in about one-third of sheep (Ovis aries) of both sexes tested for otoacoustic emissions (OAEs) even though their click-evoked OAEs (CEOAEs) seemingly were typical of other sheep of the same sex. Various pieces of evidence suggest that the absence of measurable DPOAEs was unlikely to be attributable to anesthetic effects, a poorly located probe tip, a pressure differential between middle and outer ears, season of the year, body position during testing, temperature effects, or previous medical history. Sheep apparently can exhibit a marked dissociation between DPOAEs and CEOAEs. In those sheep having measurable DPOAEs, the DPOAEs were stronger in males than in females, which is the opposite direction of effect from the CEOAEs measured in these same sheep and in humans. In female sheep exposed to higher-than-normal levels of androgens during gestation, the measurable DPOAEs were stronger than in untreated females. Although this also was the opposite direction of effect from expected, it still was a shift in the male direction, in accord with past findings about the masculinizing effects of androgens on OAEs. In sheep, androgen exposure appears to have different effects on the mechanisms underlying DPOAEs from those underlying CEOAEs.
INTRODUCTION
Current thinking is that the different forms of otoacoustic emissions (OAEs) originate from somewhat different underlying mechanisms. Shera and Guinan (1999) argued that spontaneous OAEs (SOAEs) and click-evoked OAEs (CEOAEs) originate primarily from a linear, reflection-based mechanism whereas distortion-product OAEs (DPOAEs) depend on both the reflection-based mechanism and a nonlinear distortion mechanism. A number of lines of evidence led Shera and Guinan (1999) to this distinction; among them was the fact that some agents or manipulations that affect the strength of CEOAEs have either less effect on or a well-delayed effect on DPOAEs. For example, the administration of aspirin or quinine sulfate to humans rapidly diminished the strength of SOAEs and CEOAEs, but DPOAEs were diminished less, and more slowly (e.g., Wier et al., 1988; McFadden and Pasanen, 1994; also see Whitehead et al., 1996). In the few studies where both CEOAEs and DPOAEs have been measured in the same individuals (Smurzynski and Kim, 1992; Moulin et al., 1993; Gorga et al., 1993), the correlations between the two measures have been lower than the correlations typically seen between CEOAEs and SOAEs (e.g., McFadden et al., 2008a). These various partial dissociations are understandable if the crucial underlying mechanisms are different for the different forms of OAE (Shera and Guinan, 1999). Human DPOAEs also exhibit a considerably smaller sex difference from those exhibited by CEOAEs and SOAEs (reviewed by McFadden et al., 2008a).
Recently, while collecting OAE data on a sample of sheep, we encountered a number of animals having normal-appearing CEOAEs but only very weak or absent DPOAEs. This was not universal for the species, for either sex, nor for any specific treatment group (some animals had been given androgens or estrogens prenatally or postnatally). Indeed, many sheep of both sexes exhibited quite strong DPOAEs along with strong CEOAEs. Below we discuss a number of possible technical and procedural explanations for this dissociation, but none was adequate to explain our outcomes. Accordingly, we believe that these are true instances of CEOAEs existing in ears lacking DPOAEs, which is logically possible if the mechanisms underlying the two types of OAE are different (Shera and Guinan, 1999). The CEOAE data from sheep already have been reported (McFadden et al., 2008b); the DPOAE data are reported separately here because of their significance for research on underlying mechanisms. The implication is that the processes primarily responsible for producing DPOAEs can be weak without preventing that ear from producing CEOAEs.
METHODS
Only a brief summary of the subjects and procedures will be presented here; additional details can be found in McFadden et al. (2008b). All experimental procedures were performed in accordance with NIH guidelines and were approved by the University Committees on the Use and Care of Animals at both UM and UT. OAE measurements were obtained during three multiday visits of the UT team to Ann Arbor: in mid-November and mid-December 2006 and in mid-May 2007. The UM team created the various groups of treated animals to test an array of ideas about endocrine function and sex-typical behavior in sheep; they generously agreed to add the OAE measurements to their protocol long after the original planning and onset of their primary studies. The timing of the measurement sessions for OAEs was dictated by the schedule for the original projects.
Subjects
The sheep tested (Ovis aries) were of the Suffolk variety. At the time of OAE testing, the ages were approximately 20–26 months and approximately 8–14 months for the females and males, respectively. Because puberty begins at about 6 months for females and at about 2 months for males, all of the sheep tested can be considered young adults (Kosut et al., 1997). The nominal lifespan of sheep is 8–13 years.
The mothers of some of the sheep tested had been administered various hormones during their pregnancy. As a consequence, some of the sheep tested had been exposed to higher-than-normal levels of testosterone, dihydrotestosterone (DHT), or estradiol during prenatal development. The duration of gestation in sheep is about 147 days. The drug administrations occurred regularly beginning on day 30 of gestation and ending on day 90. The surge in androgen production that occurs normally in developing male sheep fetuses also begins about day 30 and lasts through about day 90 of gestation. Some sheep were gonadectomized (GDX) soon after birth, including sheep both treated and not treated with hormones prenatally. Thus, there were males and females that were untreated∕intact, untreated∕GDX, testosterone-treated∕GDX, etc. Because essentially all of the non-GDX males had been vasectomized early in life, here we will denote the untreated∕intact males as untreated∕VX.
Procedure
The sheep lived outdoors in a natural environment at the Reproductive Sciences Program Sheep Research Facility (Ann Arbor, MI; 42 deg 18 min north latitude). Two days before testing, sheep were isolated and solid food was withheld. Just prior to their test session, sheep were brought to the test location where they were injected with ketamine hydrochloride (4–6 mg∕kg, iv) and diazepam (0.2–0.3 mg∕kg, iv), intubated, and placed on a thick foam pad on a surgical table. In Fall 2006, the sheep were placed supine on the table, and in Spring 2007, they lay on their right side. For both body positions, the left ear was oriented upward. The sheep’s hindquarters were elevated several inches above its head to permit drainage from the rumen, throat, and mouth. Anesthesia was maintained with a mixture of halothane gas (approximately 1.5%) in nitrous oxide (1∕3) and oxygen (2∕3). Otoscopy was performed, the ear canals were cleaned as necessary (female ear canals required less cleaning than male’s), and a probe tip was fitted into the left ear canal. For most animals, CEOAEs and DPOAEs were collected for the left ear only.
Two Etymotic ER-2 earphones (Etymotic Research, Elk Grove Village, IL) were used to present the two primary tones needed to evoke DPOAEs, and an Etymotic ER-10B+ microphone was used to record the sounds from the ear canal. Typically, rubber tips originally made for otoadmittance measurements were fitted over the end of the Etymotic probe tip, but for some especially large canals, special oversized foam plugs were fitted. The probe tip was traversed by two silicon sound-delivery tubes attached to the two earphones.
Stimuli were presented and responses collected using a Macintosh laptop computer (Power Macintosh G3, Apple Computer, Cupertino, CA) connected to a data-acquisition board (PCI-4451, National Instruments, Austin, TX) that was installed in a PCI-bus-extension chassis. All stimulus presentation and data collection was accomplished using custom-written LabView software (National Instruments, Austin, TX) running on the Macintosh laptop computer. A 50-kHz sampling rate (16-bit precision) was used both for digitizing the output from the microphone system and for generating the acoustic stimuli for presentation to the ear.
Typically, the DPOAE data were collected after CEOAE data had been collected. (For the first few sheep tested, CEOAEs were collected only with clicks at 75 dB peak-equivalent sound-pressure level (peSPL), but for the majority of animals, CEOAEs were collected with both 75- and 81-dB clicks.) The procedure used to measure DPOAEs was a variant of CUBDIS (Allen, 1990); details of our version have been published (McFadden et al., 2006a). Briefly, two primary tones, f1 and f2 (f2>f1), of equal sound-pressure level and having a frequency ratio (f2∕f1) of approximately 1.21, were presented for 4-s intervals while the frequency region centered on 2f1−f2 was monitored.
The levels of the primaries initially were set to 50 dB each, and if a distortion product of −10 dB SPL or stronger was measured, then the levels of the primaries were diminished by 7 dB. If the distortion product still was at least −10 dB SPL, then the levels of the primaries were reduced again by 7 dB, and so on until the distortion product was less than −10 dB SPL. Then the levels of the primaries were set to 57 dB each, the distortion product was measured, and the primary levels were increased again until either the levels reached 71 dB each or the magnitude of the distortion product increased less than 3 dB for a 7-dB increase in the primaries. The result was a gain function relating the strength of the distortion product to the level of the primaries. After that sequence was complete, the frequencies of the two primaries were increased by approximately 100 Hz, their levels both were set to 50 dB, and the sequence just described was implemented again until another gain function was obtained. This procedure was repeated until six gain functions were obtained in a localized frequency region. A similar set of six gain functions was obtained in two other frequency regions; the three frequency regions for the DPOAEs themselves were approximately 2.0–2.5, 3.3–3.8, and 5.0–5.5 kHz. (These correspond to frequency ranges of about 3.06–3.83, 5.05–5.82, and 7.66–8.42 kHz, respectively, for the f2 tone.) The data for the three frequency regions were collected in a different order for each subject. The six gain functions within each frequency region were combined to produce one composite gain function for each frequency region for each ear (for the details see McFadden et al. 2006a), and those composite gain functions were used to obtain estimates of the strength of the primary tones necessary to generate a distortion product of 0 dB SPL. Thus, in this context, the presence of a strong distortion product is revealed by small values of the estimated primary levels. In order to avoid confusion, we generally will discuss the results in terms of the relative strength of the DPOAEs themselves, not the levels of the primaries necessary to elicit a constant DPOAE strength.
The Ns for all of the groups studied were small even before the loss of the no-DPOAE animals. The usefulness of statistical tests for placing individual comparisons in perspective therefore was diminished. As a supplement to statistical tests, we provide effect sizes, which are calculated as the difference between the means of the two groups of interest divided by the square root of the weighted mean of the two variances for those two groups. According to Cohen (1992), effect sizes of 0.2, 0.5, and 0.8 correspond to small, medium, and large effects, respectively. All t-tests conducted were equal variance, unmatched, and two tailed.
RESULTS
The absence of measurable DPOAEs in a large fraction of both the male and female sheep means that our ability to reach confident conclusions about treatment effects was reduced. Accordingly, the primary emphasis here is on the characteristics of the sheep having no measurable DPOAEs and on possible procedural details that might have contributed to this anomalous outcome. Analyses for sex differences and treatment effects also are reported, but considerable caution is necessary when interpreting those outcomes because of the possibility of a selection bias existing in the attrition process. For background, we note that the CEOAEs of sheep having, and not having, DPOAEs were not noticeably different, so those two groups were pooled for the presentation of those data (McFadden et al., 2008b).
Characteristics of sheep not having DPOAEs
The absence of DPOAEs in the presence of apparently typical CEOAEs seemingly had no relationship to the various treatment groups in either sex. In Table 1 are shown both the number of sheep having DPOAEs (the numerators) and the total number of sheep from which OAE measures were obtained in each of the various treatment categories (the denominators). Also, one additional untreated∕intact female had extremely weak DPOAEs in all three frequency regions, three of the four untreated∕GDX females had no measurable DPOAEs in two frequency regions and very weak DPOAEs in the 3.5-kHz region, and two other males had no measurable DPOAEs in one of the three frequency regions. Thus, slightly more than one-third of the animals had no, or weak, DPOAEs across both the sexes and the treatment groups. As noted, this loss of animals substantially reduced our ability to make trustworthy statistical comparisons between groups, and consequently we emphasize effect sizes.1
Table 1.
Number of female and male sheep exhibiting DPOAEs in at least one frequency region (numerator) and number of sheep measured in that category (denominator). For this study, the untreated and untreated∕GDX sheep were combined within sex to create the two control groups, and the non-obese and obese females were combined to create a single female prenatal-testosterone group. GDX represents gonadectomized; DHT represents dihydrotestosterone; untreated males were vasectomized (VX).
| Group | Female | Male |
|---|---|---|
| Untreated | 6∕8 | 5∕9 |
| Untreated∕GDX | 4∕4 | 5∕6 |
| Prenatal testosterone | ||
| Non-obese | 8∕12 | 3∕5 |
| Obese | 5∕6 | 0∕0 |
| Prenatal estradiol (E2) | 4∕4 | 0∕0 |
| Prenatal DHT | 0∕0 | 3∕4 |
CEOAE strength in sheep not having DPOAEs
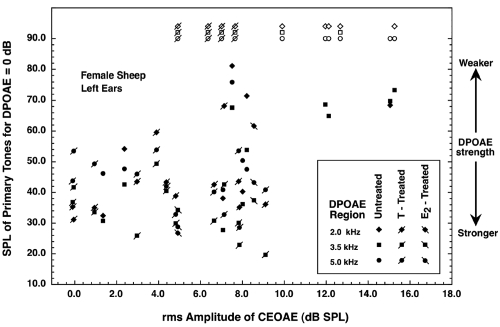
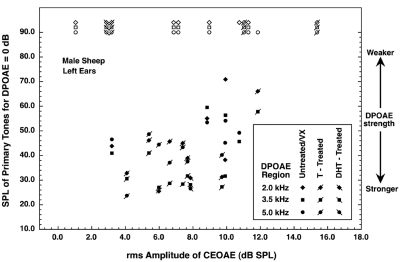
The CEOAEs exhibited by the sheep having no DPOAEs varied widely in strength. As a way of demonstrating that fact, special scattergram plots were prepared. Figures 12 show the scattergrams for females and males, respectively. For these figures, each sheep contributed three DPOAE values (one for each frequency region), all plotted at a single CEOAE value. In both figures, ordinate values greater than 90 dB are dummy values assigned when a sheep lacked DPOAEs in one or more of the three frequency regions measured. (Three additional female sheep and two additional male sheep had usable DPOAE data, but they do not appear in Figs. 12 because CEOAE data were not collected from them with the 81-dB click level; their CEOAEs for the 75-dB click were unremarkable.) Different symbols were used for the data from treated and untreated sheep. Recall that large ordinate values in Figs. 12 correspond to weak emissions at the 2f1−f2 frequency.
Figure 1.
Scattergram plot for the CEOAE (abscissa) and DPOAE (ordinate) data obtained from the female sheep. The CEOAE values were obtained using the 81-dB click. For each abscissa value (each individual animal), three ordinate values are possible, one for each frequency region in which DPOAEs were measured. DPOAE values of 90 dB or higher (the open symbols) are dummy values assigned for conditions in which DPOAEs could not be measured. The results for female sheep exposed to testosterone or estradiol during gestation are designated with symbols having a diagonal slash. Large ordinate values correspond to weak emissions at the 2f1−f2 frequency. Omitted here are data for three female sheep who had usable DPOAE values but no CEOAE data collected at this click level. Some data points were displaced slightly for clarity.
Figure 2.
Scattergram plot for the CEOAE (abscissa) and DPOAE (ordinate) data obtained from the male sheep. The CEOAE values were obtained using the 81-dB click. For each abscissa value (each individual animal), three ordinate values are possible, one for each frequency region in which DPOAEs were measured. DPOAE values of 90 dB or higher (the open symbols) are dummy values assigned for conditions in which DPOAEs could not be measured. The results for male sheep exposed to testosterone or DHT during gestation are designated with symbols having a diagonal slash. Large ordinate values correspond to weak emissions at the 2f1−f2 frequency. Omitted here are data for two male sheep who had usable DPOAE values but no CEOAE data collected at this click level. Some data points were displaced slightly for clarity.
For the females (Fig. 1), there was a seemingly paradoxical tendency for the absence of DPOAEs to be associated with relatively strong CEOAEs and for strong DPOAEs to be associated with relatively weak CEOAEs. For the males (Fig. 2), however, the absence of DPOAEs occurred over the full range of CEOAE strength. An interesting fact is that the two males having the strongest and weakest CEOAEs both lacked measurable DPOAEs. The correlations between the CEOAE values and the primary levels necessary for a DPOAE of 0 dB at 3.5 kHz were 0.58 and 0.43 for the females and males, respectively (with the dummy values omitted). These both actually are “negative relationships” in that strong CEOAEs tended to be associated with weaker DPOAEs.
Possible technical problems
Because an absence of DPOAEs in ears having CEOAEs is not a common outcome in human ears, some assurances are needed that a technical problem or procedural error was not to blame. The shortage of time available for additional study of these ears necessarily limits our knowledge, but this is what can be said.
-
(1)
The dissociation was unlikely to be attributable simply to the order of collection of the data. Although CEOAE data typically were collected prior to the DPOAE data, dissociations also were seen when the order was reversed.
-
(2)
The dissociation was unlikely to be attributable to the choice of stimulus parameters used for DPOAE collection. The f2∕f1 ratio used (approximately 1.21) is in the range of optimal ratios reported for an array of different mammalian species (reviewed by Valero et al., 2008), and small variations from the optimal ratio for an individual animal have never been shown to produce large changes in DPOAE magnitude.
-
(3)
The dissociation could not be attributed to one of the earphones failing to present its primary tone because those tones were calibrated in the ear canal using the Etymotic microphone, and their actual levels in the canal at the time of testing were recorded along with the level of the DPOAE.
-
(4)
The dissociation seems unlikely to be attributable to the anesthetic used because the CEOAEs obtained from the ears without DPOAEs covered a wide range (see Figs. 12). Also, in some animals, DPOAEs were absent even though the attempt to measure DPOAEs began immediately after starting the anesthetic, and in no individual animal was there evidence of DPOAEs getting weaker with time spent under anesthetic. Within a single approximately 60-min session, some sheep first showed strong CEOAEs at both click levels, then no DPOAEs, and then strong CEOAEs again when retested. For one male sheep having strong DPOAEs, especially in the 3.5-kHz region, the halothane dose was increased at the end of the test session with no evident effect on DPOAE strength. Many of the sheep having no DPOAEs had never before received gas anesthetic; some others had received halothane once, months before, for about 20 min. Guven et al. (2006) concluded that halothane has no effect on human CEOAEs (compare Kettembeil et al., 1995, for birds), and ketamine long has been used when recording OAEs in nonhumans (e.g., Martin et al., 1999; Torre and Fowler, 2000; Hatzopoulos et al., 2002). Bergevin et al. (2008) also concluded that anesthesia often has minimal effects on OAEs.
-
(5)
The dissociation was unlikely to be attributable to occlusions in the ear canal or to a poorly located probe tip because we continued to observe the dissociation in several sheep even after removing and repositioning the probe tip in the ear canal. Also, the majority of the sheep without DPOAEs exhibited no DPOAEs in any of the three frequency regions tested; generally it was not localized to just one frequency region as might be expected if the probe tip had shifted. Finally, is not clear how occlusions or a misplaced probe tip could affect only the DPOAEs.
-
(6)
The dissociation was unlikely to be attributable to a gradual build-up of a pressure differential in the middle and external ears (Kettembeil et al., 1995; Zheng et al., 1997). In several sheep, after the probe tip had been securely sealed into the ear-canal for many minutes, the silicone tube connecting one ER-2 earphone to the ear-canal space was pulled free on its proximal end, allowing the air pressure on the distal side of the probe tip to become equal to that in the test room, yet the OAEs measured afterward were the same as before. This demonstration does not rule out a lower pressure in the middle-ear space than in the ear canal, but in either case, it is unclear how such a pressure differential could affect DPOAEs without also affecting CEOAEs.
-
(7)
The dissociation was not simply attributable to season of the year, nor body position during testing. Of the six females with no measurable DPOAEs in any of the three frequency regions, two were tested in Fall 2006 (supine testing position) and four were tested in Spring 2007 (on-the-side testing position). Of the nine males with no measurable DPOAEs in any of the three frequency regions, eight initially were tested in Fall 2006 and one was tested in Spring 2007. Of those eight males tested in Fall 2006, four were retested in May 2007 using the other body position, and no DPOAEs were obtained in either case. Additionally, one sheep was tested both supine and on its side in the same test session, and the DPOAEs were of similar magnitudes in both positions. In a few sheep having no measurable DPOAEs in the left ear, the right ear also was tested, and the same result was obtained.
-
(8)
The dissociation was unlikely to be attributable to temperature effects. Although no systematic manipulations of body temperature were attempted, we observed no evident relationship with temperature. Each sheep lay on the same foam mat of about 1.5-in. thickness covered with a heavy blanket, all of which rested on the top of the metal surgical table. A second blanket was laid over the top of some animals. The sheep were brought into the heated test room within minutes of having been outside. The outside temperature was approximately 10°F cooler in November and December than in May, yet we observed no seasonal effect on DPOAE absence. Animals with no DPOAEs were chronologically interleaved with those having DPOAEs, even though all animals were coming from the same outside temperature into the same indoor temperature. Also, there was no evidence of changes in DPOAE strength with time since leaving the outdoors and coming indoors. Finally, we find it unlikely that temperature could affect only DPOAEs, leaving CEOAEs normal.
-
(9)
An idea that we were not able to test was that perhaps the animals exhibiting no DPOAEs had extremely strong middle-ear muscles that were well activated by the long-duration primary tones used to elicit DPOAEs but were not activated by the brief click stimuli used to elicit CEOAEs. A strong acoustic reflex would impair transmission into and out of the cochlea and could possibly lead to the appearance of an absence of DPOAEs. Besides being rather far-fetched, this idea is offset by the fact that our maximum-length-sequence (MLS) click procedure involved relatively short interclick intervals (the shortest being about 10 ms). Thus, this imaginary hyper-effective acoustic reflex should have been activated when measuring both CEOAEs and DPOAEs.
-
(10)
A search of the medical histories of the individual sheep revealed no evident differences between those animals showing DPOAEs and those not. If any of the deworming, antifungal, or other drugs routinely administered to these sheep did have cumulative ototoxic side-effects, or if aging were somehow related to the dissociation, it is not clear why DPOAEs would be affected and CEOAEs not. (The sheep were highly homogeneous in age within each sex; the males were approximately one year younger than the females yet more males lacked measurable DPOAEs.) We can say, anecdotally, that numerous old sheep of both sexes who have been separated from the main flock for one reason or another have been observed to respond to sheep calls from animals located at considerable distances, which at least suggests good hearing.
-
(11)
A number of sheep were tested multiple times within sessions or across the different seasons. Some never showed DPOAEs, and some showed DPOAEs during November or December 2006 and then showed no DPOAEs in May 2007. However, we did not observe a single instance of DPOAEs returning after having been absent, either across seasons or within measurement sessions. In fact, one sheep was tested during all three measurement sessions. In November 2006, the DPOAEs were relatively strong; in December they were significantly weaker; and in May 2007 they were completely unmeasurable.
Sex differences in DPOAEs
Although OAEs are routinely reported to exhibit sex and ear differences (e.g., Bilger et al., 1990; Talmadge et al., 1993; McFadden, 1993; McFadden et al., 1996; McFadden and Pasanen, 1998, 1999; McFadden and Shubel, 2003), those differences typically are smaller for DPOAEs than for CEOAEs or SOAEs (for a review, see McFadden et al., 2008a). Because only one ear was measured for most of the sheep, ear differences cannot be reported here. In order to calculate sex differences for the DPOAE data, the subjects were pooled in the same way as for the CEOAE calculations (McFadden et al., 2008b). Namely, the untreated∕intact females were pooled with the untreated∕GDX females to form a female control group, and the untreated∕VX males were pooled with the untreated∕GDX males to form a male control group.
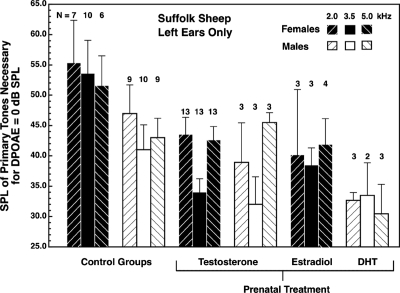
As can be seen in Fig. 3, control females required stronger primary tones than control males to produce the same DPOAE magnitude. That is, the females had weaker DPOAEs than the males and that was true in each of the three frequency regions tested for DPOAEs. This is opposite to the direction of effect previously reported for DPOAEs in humans (McFadden et al., 2008a) and other species (e.g., McFadden et al., 2006a; Valero et al., 2008). This sex difference also differs in direction from the sex difference exhibited by these same sheep for CEOAEs (McFadden et al., 2008b). The effect sizes for the sex differences in the DPOAEs are shown separately for each frequency region in Table 2. All three effect sizes were moderate to large in size (Cohen, 1992), but none of the three comparisons was statistically significant for these small samples.2
Figure 3.
Strength of the primary tones necessary to produce a 2f1−f2 DPOAE of constant magnitude in those sheep that did exhibit DPOAEs. Approximately one-third of the sheep having CEOAEs did not have measurable DPOAEs. The primary tones always were of equal level. Large ordinate values here correspond to weak DPOAEs. For both sexes, the control groups consisted of the untreated∕intact animals (males were untreated∕VX) plus the untreated∕GDX animals. Error flags designate standard errors of the mean.
Table 2.
Effect sizes for various pair-wise comparisons of groups in three DPOAE frequency regions. Effect sizes were calculated as second group listed minus first group to compensate for inverse relationship. T represents testosterone injections during days 30–90 of gestation; GDX represents gonadectomized within 2 weeks of birth; a 0.01>p>0.001; unpaired, two-tailed t-test, equal variance; control females include untreated∕intact group pooled with untreated∕GDX group; control males include untreated∕VX group and untreated∕GDX group; prenatal-T females include obese group pooled with non-obese group.
| Comparison | N | 2.0 kHz | N | 3.5 kHz | N | 5.0 kHz |
|---|---|---|---|---|---|---|
| 1. Control females vs control males | 7∕9 | −0.500 | 10∕10 | −0.803 | 6∕9 | −0.585 |
| 2. Control females vs prenatal-T females | 7∕13 | −0.834 | 10∕13 | −1.472a | 6∕13 | −0.903 |
| 3. Control males vs prenatal-T, GDX males | 9∕3 | −0.597 | 10∕3 | −0.754 | 9∕3 | 0.296 |
Treatment effects
In order to assess the effects of prenatal hormone treatment on DPOAEs in females, the obese∕testosterone-treated females were pooled with the nonobese∕testosterone-treated females and that group was compared with the female control group described above. These two groups of testosterone-treated females were pooled in this same way when analyzing their CEOAE data (McFadden et al., 2008b). Figure 3 reveals that the females treated with testosterone prenatally required substantially weaker primary tones than the control females to produce a DPOAE of the same magnitude; that is, the testosterone-treated females had stronger DPOAEs than the control females. Again, this is the opposite direction of effect from that shown by these same animals for CEOAEs (McFadden et al., 2008b). Note, however, that the DPOAE data for these testosterone-treated females were shifted in the male direction just as their CEOAE data were (McFadden et al., 2008b). That is, the testosterone-treated females did exhibit masculinized DPOAEs, in accord with expectations based on their treatment. Table 2 reveals that the effect sizes for these comparisons were large for all three frequency regions tested, and the comparison at 3.5 kHz achieved statistical significance, unpaired t(21)=3.51, p=0.002, two-tailed test, equal variance. These testosterone-treated females are also masculinized in other ways. They are born with no vaginal opening, with an empty scrotal sac, and with a pseudopenis that is used for urination (no erections ever have been observed); they also exhibit various neuroendocrine and ovarian differences from untreated females (see Padmanabhan et al., 2006) as well as behavioral differences (Roberts et al., 2008). So the prenatal treatment was effective at masculinizing both the body and the cochlea.
An interesting outcome is that the two females treated with estradiol prenatally had DPOAEs that were masculinized to about the same extent as the DPOAEs of the females treated with testosterone prenatally. This may seem paradoxical, but testosterone is aromatized to estradiol in mammals, so individuals high in testosterone are likely to be high in estradiol as well. The implication is that estradiol, not androgen, may be the relevant agent for masculinizing the cochlea. However, considerable caution must accompany this implication because the N was extremely small.
Figure 3 also shows the differences in DPOAE strength between the control males and those males treated with testosterone propionate or DHT prenatally. However, no effect sizes or statistical tests are reported because of the extremely small N remaining in these groups after attrition. For comparison, the CEOAEs of the testosterone-treated males were about the same as the CEOAEs of the control males, and the CEOAEs of the DHT-treated males were somewhat stronger than those of the control males, just as was true for the DPOAEs shown in Fig. 3.
Slopes of the gain functions
Examination of the slopes of the lines fitted to the linear ranges of the gain functions revealed that the strength of the DPOAE grew slowest with increases in primary levels in the lowest frequency region tested and grew increasingly more rapidly for each move to a higher frequency region. This trend was evident in both sexes, with the three slopes for the control females being slightly steeper than the three corresponding slopes for the control males. Also, the slopes for the testosterone-treated females were masculinized. When the slopes were pooled for all 15 males and all 23–26 females, the sex difference disappeared, and the mean slopes were about 0.85, 0.96, and 1.16 for the 2.0-, 3.5-, and 5.0-kHz frequency regions, respectively.
Relative strength of DPOAEs
One way to demonstrate differences across individuals or across species is to compare the strength of the primary tones used to evoke DPOAEs to the strength of the 2f1−f2 component produced by those primary tones. That is, how many decibels weaker than the primaries was the DPOAE? For sheep, the measurable DPOAEs averaged about 45 dB (males) to 50 dB (females) weaker than the primary tones. This is very similar to what we have obtained from spotted hyenas (McFadden et al., 2006b), rhesus monkeys (McFadden et al., 2006a), and lemurs (unpublished). The DPOAEs of marmosets were a bit weaker, averaging about 50 dB (females) to 57 dB (males) weaker than the primaries (Valero et al., 2008). All of these values are considerably stronger than those for humans, which average about 60–68 dB weaker than the primary tones for both sexes (Harris et al., 1989; McFadden et al., 2008a). Furthermore, the gain functions for DPOAEs in sheep typically asymptoted at about 25–30 dB SPL whereas the gain functions for humans we have tested with similar procedures and in comparable frequency ranges typically asymptoted at ∼5–15 dB SPL.
The considerable strength of the measurable DPOAEs in sheep is notable in light of both the dissociation between DPOAEs and CEOAEs seen in some animals and the fact that hearing sensitivity in sheep is about 18 dB worse than in humans over the range of DPOAEs and primary tones tested here (Wollack, 1963). For comparison, our past measurements reveal that the CEOAEs of sheep are weaker than those of rhesus monkeys, which in turn are weaker than those of humans.
DISCUSSION
To our knowledge, these are the first measurements of OAEs in a ruminant species. The DPOAEs measured had some similarities with DPOAEs measured in humans and some differences. Surely the most interesting discovery was the large fraction of sheep having apparently normal CEOAEs but no, or quite weak, DPOAEs. Several possible technical problems or procedural errors that might have contributed to this marked dissociation were considered, but none seemed to be plausible explanations. Until a fully explanatory problem or error emerges, we believe that this species tentatively ought to be viewed as unusual and worthy of further study. Sheep appear to offer considerable potential for learning more about the cochlear mechanisms responsible both for DPOAEs and the other forms of OAE.
Shera and Guinan (1999) explained DPOAEs by assuming that the distortion mechanism that rides the displacement envelope in the vicinity of f2 gives rise to a wave at the 2f1−f2 frequency that travels both back to the stapes and forward to the 2f1−f2 location on the basilar membrane. The forward-propagating wave is thought to encounter one or more discontinuities along its path, leading to a reflection-based wave also traveling backward toward the stapes. Thus, the DPOAE measured in the ear canal is a mix of two responses, one directly from the distortion mechanism and the other indirectly from the distortion mechanism. Accordingly, a simple explanation for how some sheep can have apparently normal CEOAEs (a reflection-based form of OAE) but no DPOAEs (a mix of two forms of OAE) would be that their distortion mechanism is damaged or inactive. Exactly what that would mean mechanistically or anatomically is far from clear. Because DPOAEs often were absent over a substantial range of f2 frequencies, the implication is that the distortion mechanism had to be unable to function over a substantial segment of the basilar membrane even though the reflection mechanism and the cochlear-amplification process apparently were intact over that same segment of basilar membrane. Under this Shera and Guinan (1999) explanation, it is not clear how a localized lesion could account for the marked wideband dissociation between CEOAEs and DPOAEs in some sheep.
Although rarely stated explicitly, there appears to exist an implicit assumption among hearing scientists that each of the two mechanisms underlying the various forms of OAE is highly similar, if not identical, across all mammalian species (Bergevin et al., 2008). The results from sheep appear to contradict this assumption of a universal mammalian plan.
-
(1)
It appears to be reasonably common for individual sheep to have perfectly normal-appearing CEOAEs even though they have no measurable DPOAEs (see Figs. 12), a unique finding in mammals so far.
-
(2)
While the (small) sex difference for CEOAEs did favor the females (McFadden et al., 2008b), as in other mammals, the sex difference for DPOAEs favored the males (see Fig. 3 and Table 2). In humans (Bilger et al., 1990; Talmadge et al., 1993; McFadden, 1993; McFadden et al., 1996; McFadden and Pasanen, 1998, 1999; McFadden and Shubel, 2003) and rhesus monkeys (McFadden et al., 2006a), the sex difference for CEOAEs is moderate in size and also favors the females, whereas the sex difference for DPOAEs typically is smaller than for CEOAEs (McFadden et al., 2008a) but still favors the females.
-
(3)
Female sheep exposed to higher-than-normal levels of androgens during the middle part of gestational development exhibited weaker CEOAEs than untreated females, which is in accord with a considerable array of outcomes from various species and special populations of humans (McFadden, 2002, 2008). However, those same androgen-treated females exhibited DPOAEs that were stronger than those in untreated females—the opposite direction of effect for CEOAEs. What is fascinating about this outcome is that the testosterone-treated females still were exhibiting a masculinized cochlea; their DPOAEs were shifted in the male direction of stronger DPOAEs than in the females. Thus, both the CEOAEs and DPOAEs of sheep offer confirmation of the prenatal-androgen-exposure explanation (McFadden, 2002, 2008) even though their DPOAEs exhibited an anomalous sex difference. To our knowledge, the only other report of a reversal in the direction of effect for the sex difference is for both the CEOAEs and DPOAEs of spotted hyenas (McFadden et al., 2006b), where an absence of sex difference was predicted because female spotted hyenas are naturally exposed to high levels of androgens prenatally.
-
(4)
DPOAEs can exhibit considerable fluctuation in strength as the primary tones are varied incrementally in frequency. This is sometimes called the microstructure of the DPOAE. DPOAE microstructure appeared to be substantially smaller in these sheep than in a sample of humans we have tested recently. As noted above, our DPOAE procedure involves changing the frequencies of the primary tones in steps of approximately 100 Hz, which means that some microstructure easily could be missed. However, that same step size was used for both sheep and humans, so the apparent difference in microstructure cannot be attributed to that factor.
A curious aspect of this dissociation between DPOAEs and CEOAEs in sheep is that it is the mechanisms underlying DPOAEs that appear to be more vulnerable (to some unknown agent or process) than are the mechanisms underlying CEOAEs. In the previously known dissociations, DPOAEs were altered less, or more slowly, by aspirin or quinine than were other forms of OAE (Wier et al., 1988; McFadden and Pasanen, 1994; Whitehead et al., 1996). Thus, this dissociation of OAEs in sheep is peculiar both in its extent and in its direction.
ACKNOWLEDGMENTS
This research was supported in part by Research Grant No. DC00153 from the National Institute on Deafness and Other Communication Disorders (NIDCD) awarded to one of the authors (D.M.) and in part by Research Grant No. HD44232 from the National Institute of Child Health and Human Development (NICHD) awarded to another author (T.M.L.) as part of a Program Project Grant entitled Prenatal Programming of Reproductive Health and Disease. We thank Mr. Douglas D. Doop for expert technical advice and assistance with animal care. In addition, we thank Michael Zakalik and Allie Spencer for assistance with data collection. M.M. Maloney prepared the figures. A preliminary report of this work was presented at a meeting of the Acoustical Society of America (McFadden et al., 2008c).
Footnotes
Note that the procedures used by the UM team unavoidably also created some females treated prenatally with DHT and some males treated prenatally with estradiol, but because these animals could not contribute to the experimental questions initially of interest to the UM team, those animals were not retained, and thus were not available at the time of the OAE measurements.
The standard procedure in this laboratory is to calculate effect sizes as female minus male, so that a positive value indicates stronger OAEs in females. Here, however, the large values for the primaries needed by the females correspond to weak DPOAEs. For easy comparison with our past publications, the signs of all the effect sizes in Table 2 have been reversed to offset the inverse relationship between primary level and DPOAE strength.
References
- Allen, J. B. (1990). User manual for the CUBDIS distortion product measurement system (AT&T Bell Labs).
- Bergevin, C., Freeman, D. M., Saunders, J. C., and Shera, C. A. (2008). “Otoacoustic emissions in humans, birds, lizards, and frogs: Evidence for multiple generation mechanisms,” J. Comp. Physiol., A 194, 665–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger, R., Matthies, M. L., Hammel, D. R., and Demorest, M. E. (1990). “Genetic implications of gender differences in the prevalence of spontaneous otoacoustic emissions,” J. Speech Hear. Res. 33, 418–432. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1992). “A power primer,” Psychol. Bull. 10.1037//0033-2909.112.1.155 112, 155–159. [DOI] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Bergman, B. M., Beauchaine, K. L., Kaminski, J. R., Peters, J., Schulte, L., and Jesteadt, W. (1993). “A comparison of transient-evoked and distortion product otoacoustic emissions in normal-hearing and hearing-impaired subjects,” J. Acoust. Soc. Am. 10.1121/1.407348 94, 2639–2648. [DOI] [PubMed] [Google Scholar]
- Guven, S., Tas, A., Adali, M. K., Yagiz, R., Alagol, A., Uzun, C., Koten, M., and Karasalihoglu, A. R. (2006). “Influence of anaesthetic agents on transient evoked otoacoustic emissions and stapedius reflex threshold,” J. Laryngol. Otol. 120, 10–15. [DOI] [PubMed] [Google Scholar]
- Harris, F. P., Lonsbury-Martin, B. L., Stagner, B. B., Coats, A. C., and Martin, G. K. (1989). “Acoustic distortion products in humans: Systematic changes in amplitude as a function of f2∕f1 ratio,” J. Acoust. Soc. Am. 10.1121/1.397728 85, 220–229. [DOI] [PubMed] [Google Scholar]
- Hatzopoulos, S., Petruccelli, J., Laurell, G., Finesso, M., and Martini, A. (2002). “Evaluation of anesthesia effects in a rat animal model using otoacoustic emission protocols,” Hear. Res. 170, 12–21. [DOI] [PubMed] [Google Scholar]
- Kettembeil, S., Manley, G. A., and Siegl, E. (1995). “Distortion-product otoacoustic emissions and their anaesthesia sensitivity in the European Starling and the chicken,” Hear. Res. 10.1016/0378-5955(95)00053-7 86, 47–62. [DOI] [PubMed] [Google Scholar]
- Kosut, S. S., Wood, R. I., Herbosa-Encarnacion, D., and Foster, D. L. (1997). “Prenatal androgens time neuroendocrine puberty in the sheep: Effect of testosterone dose,” Endocrinology 138, 1072–1077. [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Stagner, B. B., Jassir, D., Telischi, F. F., and Lonsbury-Martin, B. L. (1999). “Suppression and enhancement of distortion-product otoacoustic emissions by interference tones above f2. I. Basic findings in rabbits,” Hear. Res. 10.1016/S0378-5955(99)00119-7 136, 105–123. [DOI] [PubMed] [Google Scholar]
- McFadden, D. (1993). “A masculinizing effect on the auditory systems of human females having male co-twins,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.90.24.11900 90, 11900–11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden, D. (2002). “Masculinization effects in the auditory system,” Arch. Sex Behav. 31, 93–105. [DOI] [PubMed] [Google Scholar]
- McFadden, D. (2008). “What do sex, twins, spotted hyenas, ADHD, and sexual orientation have in common?” Perspect. Psychol. Sci. 3, 309–323. [DOI] [PubMed] [Google Scholar]
- McFadden, D., Loehlin, J. C., and Pasanen, E. G. (1996). “Additional findings on heritability and prenatal masculinization of cochlear mechanisms: Click-evoked otoacoustic emissions,” Hear. Res. 10.1016/0378-5955(96)00065-2 97, 102–119. [DOI] [PubMed] [Google Scholar]
- McFadden, D., Martin, G. K., Stagner, B. B., and Maloney, M. M. (2008a). “Sex differences in distortion-product and transient-evoked otoacoustic emissions compared,” J. Acoust. Soc. Am. (to be published). [DOI] [PMC free article] [PubMed]
- McFadden, D., and Pasanen, E. G. (1994). “Otoacoustic emissions and quinine sulfate,” J. Acoust. Soc. Am. 10.1121/1.410022 95, 3460–3474. [DOI] [PubMed] [Google Scholar]
- McFadden, D. and Pasanen, E. G. (1998). “Comparison of the auditory systems of heterosexuals and homosexuals: Click-evoked otoacoustic emissions,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.95.5.2709 95, 2709–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden, D., and Pasanen, E. G. (1999). “Spontaneous otoacoustic emissions in heterosexuals, homosexuals, and bisexuals,” J. Acoust. Soc. Am. 10.1121/1.426845 105, 2403–2413. [DOI] [PubMed] [Google Scholar]
- McFadden, D., Pasanen, E. G., Raper, J., Lange, H. S., and Wallen, K. (2006a). “Sex differences in otoacoustic emissions measured in rhesus monkeys (Macaca mulatta),” Horm. Behav. 50, 274–284. [DOI] [PubMed] [Google Scholar]
- McFadden, D., Pasanen, E. G., Valero, M. D., Roberts, E. K., and Lee, T. M. (2008b). “Effect of prenatal androgens on click-evoked otoacoustic emissions in male and female sheep (Ovis aries),” Horm. Behav. (in press). [DOI] [PMC free article] [PubMed]
- McFadden, D., Pasanen, E. G., Valero, M. D., Roberts, E. K., and Lee, T. M. (2008c). “Otoacoustic emissions in sheep (Ovis aries): Sex differences and prenatal androgen effects,” J. Acoust. Soc. Am. 123, 3855–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden, D., Pasanen, E. G., Weldele, M. L., Glickman, S. E., and Place, N. J. (2006b). “Masculinized otoacoustic emissions in female spotted hyenas (Crocuta crocuta),” Horm. Behav. 50, 285–292. [DOI] [PubMed] [Google Scholar]
- McFadden, D. and Shubel, E. (2003). “The relationships between otoacoustic emissions and relative lengths of fingers and toes in humans,” Horm. Behav. 43, 421–429. [DOI] [PubMed] [Google Scholar]
- Moulin, A., Collet, L., Veuillet, E., and Morgon, A. (1993). “Interrelations between transiently evoked otoacoustic emissions, spontaneous otoacoustic emissions and acoustic distortion products in normally hearing subjects,” Hear. Res. 10.1016/0378-5955(93)90215-M 65, 216–233. [DOI] [PubMed] [Google Scholar]
- Padmanabhan, V., Manikkam, M., Recabarren, S., and Foster, D. (2006). “Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female,” Mol. Cell Endocrinol. 246, 165–174. [DOI] [PubMed] [Google Scholar]
- Roberts, E. K., Padmanabhan, V., and Lee, T. M. (2008). “Differential effects of prenatal testosterone on phenotypic and behavioral masculinization and defeminization of female sheep,” Biol. Reprod. 79, 43–50. [DOI] [PubMed] [Google Scholar]
- Shera, C. A. and Guinan, J. J., Jr. (1999). “Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs,” J. Acoust. Soc. Am. 10.1121/1.426948 105, 782–798. [DOI] [PubMed] [Google Scholar]
- Smurzynski, J. and Kim, D. O. (1992). “Distortion-product and click-evoked otoacoustic emissions of normally-hearing adults,” Hear. Res. 10.1016/0378-5955(92)90132-7 58, 227–240. [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Long, G. R., Murphy, W. J., and Tubis, A. (1993). “New off-line method for detecting spontaneous otoacoustic emissions in human subjects,” Hear. Res. 10.1016/0378-5955(93)90032-V 71, 170–182. [DOI] [PubMed] [Google Scholar]
- Torre, P., III, and Fowler, C. G. (2000). “Age-related changes in auditory function of rhesus monkeys (Macaca mulatta),” Hear. Res. 142, 131–140. [DOI] [PubMed] [Google Scholar]
- Valero, M. D., Pasanen, E. G., McFadden, D., and Ratnam, R. (2008). “Distorion-product otoacoustic emissions in the common marmoset (Callithrix jacchus): Parameter optimization,” Hear. Res. 243, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead, M. L., Lonsbury-Martin, B. L., Martin, G. K., and McCoy, M. J. (1996). “Otoacoustic emissions: Animal models and clinical observations,” in Clinical Aspects of Hearing, Springer Handbook of Auditory Research Vol. 7, edited by van de Water T. R., Popper A. N., and Fay R. R. (Springer-Verlag, New York: ), pp. 199–257. [Google Scholar]
- Wier, C. C., Pasanen, E. G., and McFadden, D. (1988). “Partial dissociation of spontaneous otoacoustic emissions and distortion products during aspirin use in humans,” J. Acoust. Soc. Am. 10.1121/1.396970 84, 230–237. [DOI] [PubMed] [Google Scholar]
- Wollack, C. H. (1963). “The auditory acuity of the sheep (Ovis aries),” J. Aud Res. 3, 121–132. [Google Scholar]
- Zheng, Y., Ohyama, K., Hozawa, K., Wada, H., and Takasaka, T. (1997). “Effect of anesthetic agents and middle ear pressure application on distortion product otoacoustic emissions in the gerbil,” Hear. Res. 10.1016/S0378-5955(97)00118-4 112, 167–174. [DOI] [PubMed] [Google Scholar]