Abstract
While it has been hypothesized that the adverse health effects associated with ambient particulate matter (PM) are due to production of hydroxyl radical (·OH), few studies have quantified ·OH production from PM. Here we report the amounts of ·OH produced from ambient fine particles (PM2.5) collected in northern California and extracted in a cell-free surrogate lung fluid (SLF). On average, the extracted particles produced 470 nmol ·OH mg−1-PM2.5 during our 15-month collection period. There was a clear seasonal pattern in the efficiency with which particles generated ·OH, with highest production during spring and summer and lowest during winter. In addition, nighttime PM was typically more efficient than daytime PM at generating ·OH. Transition metals played the dominant role in ·OH production: on average (± σ), the addition of desferoxamine (a chelator that prevents metals from forming ·OH) to the SLF removed (90 ± 5) % of ·OH generation. Furthermore, based on the concentrations of Fe in the PM2.5 SLF extracts, and the measured yield of ·OH as a function of Fe concentration, dissolved iron can account for the majority of ·OH produced in most of our PM2.5 extracts.
Keywords: Particulate Matter (PM), reactive oxygen species (ROS), transition metals
Introduction
Past studies have found that exposure to elevated levels of ambient particulate matter (PM) is associated with increased incidence of asthma, chronic obstructive pulmonary disease, lung cancer, and mortality (1-4). In the United States approximately 20,000 − 50,000 people die each year from PM exposure (5). Despite these impacts, the mechanisms by which PM causes adverse health effects are poorly understood. One hypothesis is that PM causes these effects via oxidative stress and cell damage through the production of reactive oxygen species (ROS) such as superoxide (•O2−), hydrogen peroxide (HOOH), and hydroxyl radical (·OH) (6-13).
A number of previous studies have measured the oxidizing capacity of ambient or source particles extracted in a buffer solution or other lung fluid surrogate (6, 8, 9, 11, 14-18). However, many past studies have used techniques that have two significant disadvantages: (1) they are non-specific (i.e., they respond to multiple ROS species), and (2) they are not absolutely quantitative. Despite these limitations, past studies have provided a number of useful insights into ROS generation from particles, including the observation that smaller particles typically have the highest mass-normalized generation of ROS (15).
One of the key objectives of many past ROS studies has been to understand the particle components that are responsible for oxidant generation. While several studies have found that quinones are found in ambient particles and can lead to ROS generation (10, 15, 19), most studies have focused on the contributions from transition metals. Transition metals appear to play a major role in ROS generation by ambient and source PM, since the addition of desferoxamine mesylate (DSF), a strong metal chelator that eliminates metal reactivity, suppresses most ·OH formation by particles (6, 8, 20, 21). Within the suite of transition metals, iron and copper are often identified as the most important metals for ROS generation (6, 7, 14, 18, 22, 23), and both can efficiently form ·OH in surrogate lung fluids (24). While a number of studies have found that iron was responsible for most of the generation of ·OH by ambient PM (21, 23, 25), other work has suggested that Cu is more important (14, 22).
Although ·OH is the strongest of the biological ROS, it has not been measured quantitatively from ambient particles, which makes it difficult to understand the potential role that ·OH might play in PM toxicity. In light of this, we had three goals for this current work: (1) to quantify the amounts of ·OH produced from ambient PM2.5 extracted in a surrogate lung fluid, (2) to examine whether there are seasonal or diurnal differences in ·OH production, and (3) to determine the contributions of transition metals in general, and iron and copper in particular, towards ·OH formation from fine particles.
Materials and Methods
Chemicals
Sodium citrate (A.C.S. reagent grade) and sodium bisulfite (A.C.S.) were from GFS and p-hydroxybenzoic acid was from TCI America. Desferoxamine mesylate (95%), 2,2,2-trifluoroethanol (Puriss, 99.0%) and ascorbic acid (Puriss p.a., 99.0%) were from Sigma. Chelex-100 molecular biology grade resin was from BioRad laboratories. Sodium benzoate (A.C.S.), potassium phosphate (HPLC grade), sodium phosphate (A.C.S.) and sodium chloride (A.C.S.) were from Fisher. Standard solutions of metals in 5% nitric acid were obtained from SPEX. Purified water was obtained from a Milli-Q Plus system (Millipore; ≥18.2 MΩ cm).
Surrogate Lung Fluid (SLF)
All experiments were performed in a cell-free surrogate lung fluid (SLF) consisting of 10 mM sodium benzoate as an ·OH probe, 114 mM NaCl, 10 mM total phosphate (7.8 mM Na2HPO4 and 2.2 mM KH2PO4) to buffer the solution at pH 7.4, 200 μM ascorbate and 300 μM citrate. SLF solutions (prior to addition of ascorbate and citrate) were treated by column chromatography with Chelex-100 resin to remove metals, stored in the refrigerator, and generally used within a month. Stock solutions of ascorbate and citrate were prepared fresh and added to the SLF on the day of each experiment.
PM2.5 Collection and Extraction
Ambient PM2.5 samples (i.e., particles with diameters ≤ 2.5 μm) were collected on the University of California — Davis campus using IMPROVE Version II Samplers (URG; 22.8 L min−1 flow rate) containing Teflo filters (25 mm diameter, pore size 3 μm, Pall). Approximately each month from May 2006 to August 2007 three consecutive 24-hour samples were collected, typically starting at 8:00 am each day. During each 24-hour sampling period we collected three replicate PM2.5 samples (two for ·OH measurements and one for metals analysis). We also collected three field blanks (filters kept in the samplers without air flow for the entire sampling period) for each 72-hour sampling episode. Filters were weighed on a CAHN 28 microbalance before and after sampling to determine the PM mass collected. All samples (and field blanks) were removed from the sampler at the end of the 3-day sampling period; thus the day 1 and 2 samples sat in the sampler until the end of day 3. However, control experiments showed that leaving the filters in the sampler for 24 or 48 hours after sampling did not cause a statistically significant change in ·OH production (< 6 % difference compared to the samples removed immediately after sampling was finished).
Within 2 days of finishing each round of sampling, 2 filters from each sampling day were cut in half (with a stainless steel scalpel) and treated as follows: (a) one half was extracted in SLF, (b) 2 halves were extracted in SLF with the wetting agent 2,2,2-trifluoroethanol (TFE), and (c) the last half was extracted in SLF with desferoxamine (DSF) (and, typically, TFE). For procedure (a), the filter half was placed in a 125-mL Teflon bottle, 10 mL of SLF were added, the bottle was wrapped with aluminum foil, placed on a wrist-action shake table, and shaken for 24 hours. Procedure (b) was used to examine whether TFE enhanced PM extraction (and ·OH generation) from the filter. For these samples we added 30 μL of TFE directly to the half-filter to wet it, and then added SLF and treated as described above. Finally, in procedure (c) we examined the contribution of transition metals to ·OH production by adding 100 μL of 10 mM DSF, which removes transition metal reactivity (21), to the filter before adding SLF and shaking. Note that each reported ·OH value is the total amount produced in the SLF after the 24 hours of extraction.
For each procedure, within 10 minutes after finishing shaking, we added 100 μM DSF and 50 μM HSO3− to each bottle in order to stop the generation of ·OH. The extracts were then acidified to pH 2 by adding 100 μL of 1.0 M H2SO4, filtered using a syringe (3 ml Norm-Ject, Henke Sass Wolf Gmbh) with a 0.22 μm pore syringe filter (Millex GP, Millipore) and injected onto the HPLC described below.
Hydroxyl Radical Measurements
Hydroxyl radicals were quantitatively trapped and measured using benzoate (BA) as a chemical probe to produce p-hydroxybenzoate (p-HBA), which is measured using HPLC (24, 26). The concentration of ·OH in each solution was determined using
| (1) |
where [p-HBA] is the measured concentration of p-hydroxybenzoic acid, YpHBA is the molar yield of p-HBA produced from the reaction of ·OH with benzoate in SLF (0.215 ± 0.018; ref. (24, 26)) and fBA is the fraction of ·OH that reacts with benzoate in the SLF. Based on published rate constants for ·OH (27, 28), values of fBA in our SLF solutions are 0.99 without TFE or DSF, 0.85 with TFE (41.8 mM), 0.97 for DSF (100 μM), and 0.84 for DSF and TFE. Amounts of ·OH produced from the samples were blank corrected by using the corresponding filter blank containing DSF and/or TFE. On each experiment day we ran a calibration curve of p-HBA standards and also prepared and injected a control solution made by adding 200 μL of 1.0 mM FeSO4 to a Teflon bottle, adding 10 mL of SLF, and treating it as a sample. The average (± 1σ) response from these Fe controls was 340 ± 50 nmol ·OH.
Transition Metal Analysis
We made two measurements of light elements and transition metals for each sample: (1) total metal content using energy dispersive X-ray fluorescence (XRF) and (2) SLF-extractable metals using inductively coupled plasma - mass spectrometry (ICP-MS). For XRF analysis, we used a Mo-anode grounded X-ray tube system to quantify Ni, Cu, Zn, and As, and a Cu-anode grounded X-ray tube system operated under vacuum to quantify other elements (29). After analysis by XRF, filters were extracted in SLF in the same manner as for ·OH measurements (with TFE), except that after extraction the samples were not quenched with bisulfite and DSF, and were not acidified with sulfuric acid. Extracts in SLF were diluted by a factor of 10 using 3% HNO3 to reduce the amount of total dissolved salts to 0.1%. The diluted, acidified SLF extracts were then analyzed on an Agilent Technologies 7500ce ICP quadrupole mass spectrometer using SPEX CertiPrep (Memory Test 2 in 5% HNO3) standards in SLF for calibration. The SPEX CertiPrep standards were treated in the same manner as the PM samples (i.e., diluted to a 1:10 ratio SLF : 3% HNO3 solution). Field blanks and laboratory blanks (i.e., filters that had not been sampled) were extracted and analyzed in the same manner as the samples; for each metal the average field blank level was subtracted from the PM sample value.
Results and Discussion
Filter Wetting with 2,2,2-Trifluoroethanol
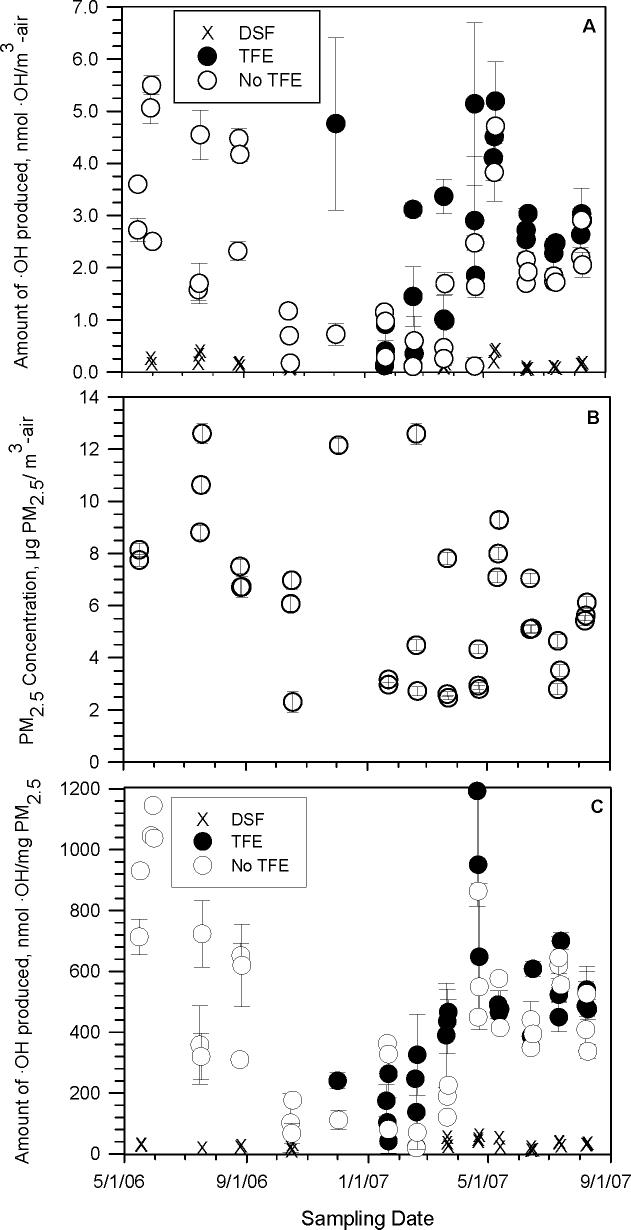
Because the Teflon filters used to collect the PM are hydrophobic, the SLF solution might not be able to reach all of the embedded PM during shaking. To test this, for many of the samples we added the wetting agent 2,2,2-trifluoroethanol (TFE) to a replicate filter so that the SLF might better penetrate the Teflon matrix and access the particles. Ethanol, a typical wetting agent, could not been used in our samples since it reacts rapidly with ·OH (k = 1.9 × 109 L mol−1 s−1; (28)). In contrast, the reaction of TFE with ·OH is relatively slow (2.3 × 108 L mol−1 s−1; (27)), thus allowing most of the ·OH to react with benzoate. As shown in Figures 1a and 1c, the addition of TFE generally increased the amount of ·OH produced by a given PM sample, consistent with our picture of the role of filter wetting. Compared to the same sample extracted by SLF in the absence of TFE, on average (± 1σ) the addition of TFE enhanced ·OH production by (80 ± 40) %.
Figure 1.
(a) Formation of hydroxyl radical by PM2.5 in SLF, normalized by the air volume sampled for each filter. “TFE” (filled circles) refers to sampled filters that were first wetted with 2,2,2-trifluoroethanol, while data labeled “No TFE” (open circles) were PM2.5 samples extracted without TFE. “DSF” (×) refers to PM2.5 samples extracted in the presence of desferoxamine, a chelator that inhibits the reactivity of transition metals. Error bars are 1σ from replicate PM2.5 samples. (b) PM2.5 mass concentrations for each sample. (c) Formation of hydroxyl radical by PM2.5 in SLF, normalized by the mass of particulate matter collected for each sample. Legend is the same as in Fig. 1a.
Hydroxyl Radical Production by Particulate Matter
There are two main ways to express the formation of ·OH by PM2.5 in the surrogate lung fluid. The first is to normalize the amount of ·OH generated by the volume of air sampled for each PM filter (i.e., units of nmol ·OH m−3-air), as shown in Figure 1a. In these units, there is no apparent seasonal pattern in the amount of ·OH produced from the PM, and there is significant day-to-day variability, with values ranging from 0.1 to 6.0 nmol ·OH m−3-air. On average (± 1 σ), the amount of ·OH produced from fine particulate matter normalized by air volume is 2.3 ± 1.5 nmol ·OH m−3-air. Thus, at least at the Davis collection site, inhalation exposure to PM-generated ·OH can vary tremendously from one day to the next and there are no trends throughout the year. Figure 1a also shows that adding DSF, the transition metal chelator, removes most of the ·OH generated by the PM samples, as discussed below.
Figure 1b shows the 24-hour average mass concentrations for our PM2.5 samples over the course of the 15-month sampling period. There is large day-to-day variability in the particle mass loadings (range = 2.3 − 12.6 μg PM2.5 m−3-air; mean = 6.0 ± 2.9 μg PM2.5 m−3-air) and no apparent seasonal variation, in agreement with values measured in Davis by the California Air Resources Board (30). The high day-to-day variability in the PM2.5 mass concentrations (Figure 1b) is probably a major reason why there is so much variability in the air-volume-normalized amount of ·OH shown in Figure 1a.
To eliminate the effect of variability in particle mass concentrations on ·OH generation, in Figure 1c we show ·OH generation normalized by PM mass (i.e., nmol ·OH mg−1-PM2.5). This graph shows that the efficiency with which particles generate ·OH in the surrogate lung fluid has a clear seasonal pattern: Davis PM2.5 is generally most efficient at producing ·OH during the spring and summer (May — August) and is least efficient during the winter (December — February). On average, there is a 3.3-fold difference in the amount of ·OH produced between the spring/summer and winter months (with average values of 560 ± 80 and 170 ± 50 nmol ·OH mg−1 PM2.5, respectively). Examining the data as monthly averages shows even greater differences: the maximum monthly average amount of ·OH produced in our samples, in May 2006 (1150 ± 160 nmol ·OH mg−1 PM2.5), is nearly 6 times higher than the minimum monthly average amount of ·OH production, in January 2007 (200 ± 40 nmol ·OH mg−1 PM2.5). Our results are consistent with past studies in which fine and ultrafine particles collected during summer usually were more efficient at producing ROS compared to those collected during winter (14, 31), although we see a much more pronounced seasonal effect.
Diurnal Changes in Hydroxyl Radical Production
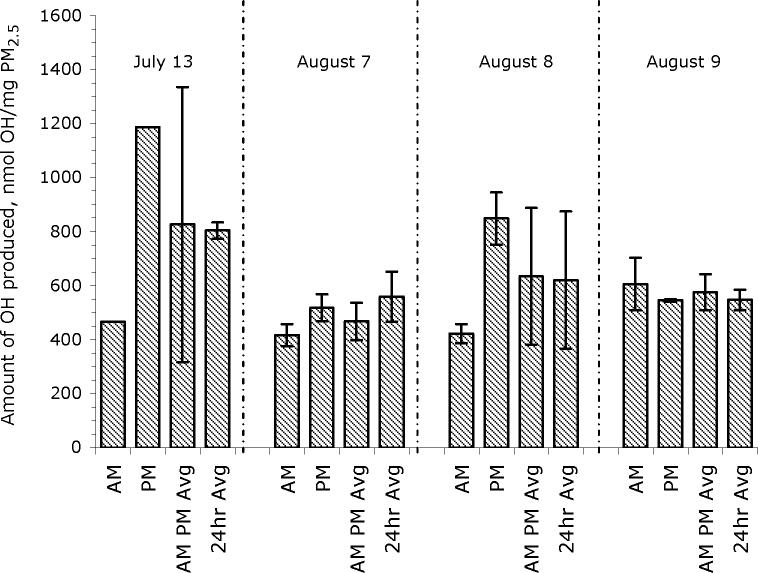
To investigate whether there are diurnal changes in ·OH formation by ambient aerosol, for several periods we collected both a 24-hour sample (8:00 am - 8:00 am) as well as daytime (8:00 am - 8:00 pm) and nighttime (8:00 pm - 8:00 am) sub-samples. As expected, within a given 24-hr period the average of the AM and PM 12-hr values was very similar (within 3 − 17%) to the corresponding 24-hr sample (Figure 2). More significantly, Figure 2 shows that the nighttime PM typically is more efficient at generating ·OH compared to the daytime PM. The night/day ratio of ·OH production for the four periods tested ranged from 0.81 to 2.6, with an average value of 1.6 ± 0.6; i.e., on average the nighttime particles were 60% more efficient at generating ·OH. This difference in ·OH production efficiency might be due to diurnal differences in meteorology, which would change the sources of PM arriving at the collection site. For example, on August 8 (where daytime PM2.5 was approximately half as efficient at generating ·OH compared to nighttime PM2.5; Figure 2), the winds were approximately 2 m s−1 from the SSW and W during the day, but then changed to 4 − 5 m s−1 from the ESE and S during the night (Figure S1 in Supplemental Info).
Figure 2.
Differences in PM2.5-mass-normalized formation of hydroxyl radical between day and night within a given 24-hour period. Daytime samples (AM) were collected from 8:00 am — 8:00 pm, while nighttime samples (PM) were collected during the next 12 hours (8:00 pm — 8:00 am). Listed dates represent the start of sample collection. AM and PM bars represent mean values with error bars of 1σ from replicate filters (n = 3); there are no error bars on the July 13 data because replicate filters were not available. “AM PM Avg” represents the average (± 1σ) value of the AM and PM samples in a given 24-hour period. “24hr Avg” is the average (± 1σ) value from the 24-hour PM2.5 samples collected on that date as part of our normal collection (i.e., data from Figure 1c).
Role of Transition Metals in Generating Hydroxyl Radical
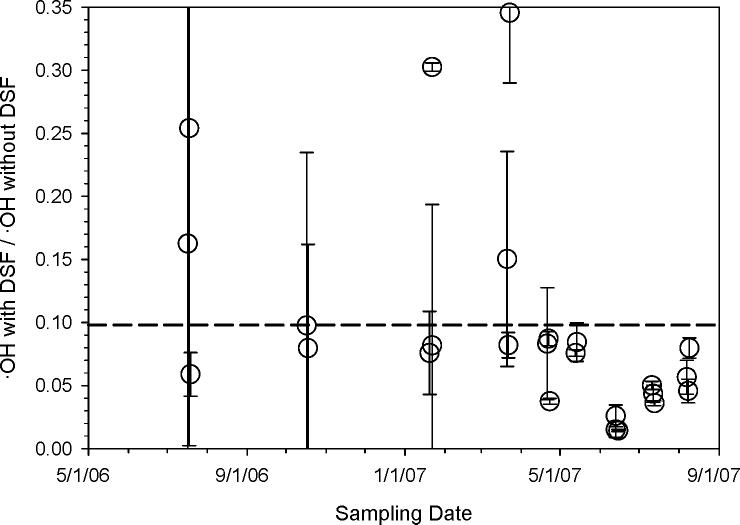
In order to determine the overall contribution of transition metals to ·OH production in our SLF solutions, we added desferoxamine (DSF) to a portion of each PM2.5 sample prior to SLF extraction. Figure 3 shows the ratio of the amount of ·OH produced in a sample with DSF to the amount of ·OH produced in the same PM sample without DSF. This figure (along with Figures 1a and 1c) shows that transition metals play a criticial role in ·OH production: the addition of DSF, on average, removes (90 ± 5) % of ·OH productivity (n = 25). Previous researchers have observed similar results, such as addition of 1 mM DSF inhibiting 90% of ROS generation by urban PM2.5 in a phosphate buffer solution (32). Our DSF results are also consistent with previous studies suggesting that metals such as iron and copper are mostly responsible for ·OH generation from PM2.5 (6-8, 18, 23).
Figure 3.
Effect of transition metal chelator (DSF) on the generation of hydroxyl radical from fine particles extracted in surrogate lung fluid. Each point represents the ratio of the amount of ·OH formed from a PM2.5 sample extracted in SLF in the presence of desferoxamine (DSF) over the amount of ·OH formed from the same PM sample in SLF in the absence of DSF. Error bars represent 1σ, propagated from the errors on the values used to calculate each point. The horizontal dashed line (0.10) is the average value of the points.
To further examine the role that Fe, Cu, and other transition metals play in ·OH production, we measured the amounts of total and SLF-soluble metal in our PM2.5 samples using, respectively, (a) XRF analysis on whole PM filters and (b) ICP-MS analysis of SLF extracts of PM (Table S1). Iron is by far the dominant particulate transition metal measured by XRF: the average total iron amount was 218 ± 11 nmol Fe mg−1-PM2.5, while the sum of the other redox-active transition metals measured (V, Cr, Mn, Ni, Cu, and Zn) was 21 ± 2 nmol mg−1-PM2.5. The ICP-MS data shows that Fe still dominated the soluble transition metal pool, but it was not as dominant as in the total (i.e., soluble + insoluble) pool: on average, the ratio of soluble iron to the sum of the other soluble redox active metals was 2.4 ± 2.8. Despite iron's dominance, over the entire collection period we see no correlation between ·OH production and total, or SLF-soluble, Fe (R2 = 0.13 and 0.11, respectively; Table S2). If we examine this ·OH-Fe relationship as a function of season, winter has the highest correlation for both total and SLF-soluble iron (R2 = 0.97 and 0.55, respectively), but there are only weak relationships in the other seasons (R2 ≤ 0.3; Table S2). Similarly, during winter ·OH is correlated with both total and SLF-soluble copper (R2 = 0.99 and 0.40, respectively), but the relationship is weak in the other seasons (R2 ≤ 0.18; Table S2).
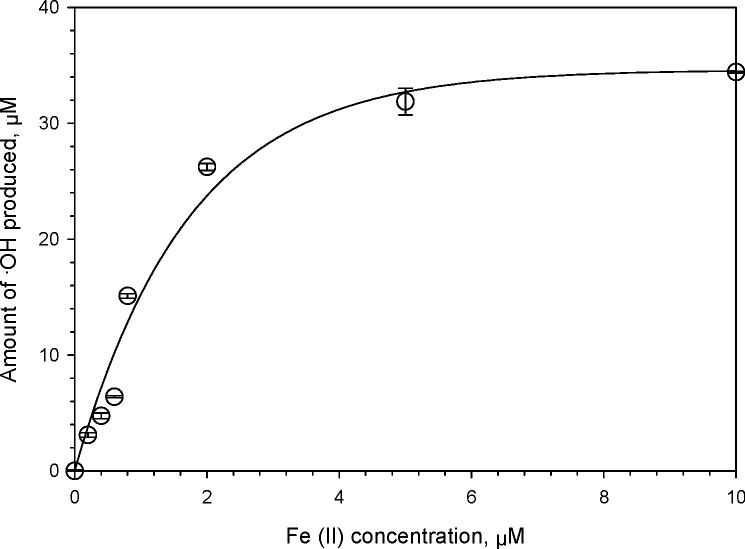
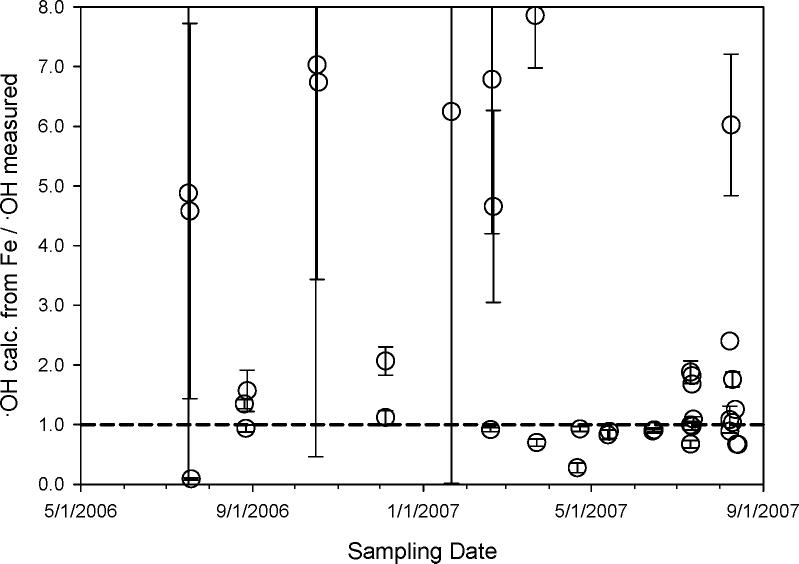
Although the correlation between ·OH and Fe over the entire sampling period is weak, this does not mean that iron was not a significant source of ·OH. In order to quantify the role of SLF-extractable iron in ·OH generation, we first measured hydroxyl radical production in SLF by dissolved iron. As shown in Figure 4, the ·OH concentration increases nearly linearly with Fe at low iron concentrations, but reaches a plateau at Fe concentrations above approximately 5 μM. To calculate the amount of ·OH that can be produced from dissolved iron in each PM extract, we used the measured ICP-MS Fe amount in the SLF extract in conjunction with Figure 4. Figure 5 shows what fraction of the measured ·OH in each sample can be attributed to iron, i.e., the ratio of the calculated amount of ·OH from iron over the actual ·OH measured in each PM2.5 sample. While there are a wide range of values, this figure shows that Fe can account for most or all of the ·OH produced in the majority of the samples. Considering all points, the average (±1 σ) value of the ratio (i.e., (calculated ·OH from Fe) / (measured ·OH)) is 2.5 ± 1.0; removing 10 outliers (with ratios above 4.0) gives an average ratio of 1.1 ± 0.07. In a number of samples, iron can account for more ·OH than was measured from the PM (i.e., points with values above 1 in Figure 5); this suggests that there are species in the PM extracts that reduce the amount of ·OH produced by iron compared to in our Figure 4 experiment. These antagonist species could include copper (24) and possibly other transition metals. In addition, some of the organic compounds in the PM might reduce the reactivity of Fe, either by binding to iron (as done by DSF) or by inhibiting the electron transfer reactions that convert dissolved O2 to, sequentially, superoxide, hydrogen peroxide, and finally hydroxyl radical. Our finding, that soluble Fe is generally the dominant source of ·OH in our SLF extracts, is broadly consistent with many past studies that have shown iron often plays a critical role in ROS generation by ambient and source particles (7, 8, 33).
Figure 4.
Amount of hydroxyl radical formed in a surrogate lung fluid (SLF) from various concentrations of aqueous Fe(II) from FeSO4. Fe solutions were treated as samples (e.g., 24 hr of shaking) without the addition of TFE. Error bars represent 1 σ, while the solid line is a regression fit to all the experimental data: [•OH] = 34.61 × (1- exp (−0.58×[Fe(II)])), where concentrations are in units of μM.
Figure 5.
Contribution of iron to ·OH formation in the PM2.5 extracts. Each point represents the ratio of the calculated amount of hydroxyl radical formed from iron over the amount of ·OH measured in the SLF extract of PM2.5 (Figure 1c), i.e., ·OH calculated from Fe / ·OH measured from PM2.5. Error bars represent 1σ. One point is not shown: 10.1 ± 9.6 on 10/16/2006. The average value (± σ) of all points is 2.5 ± 0.9; excluding the 10 points with values above 4, the average is 1.1 ± 0.07.
Interestingly, while our results in Figure 5 indicate that SLF-extractable Fe is mostly responsible for ·OH generation, we see no correlation between these variables (R2 = 0.11; Table S2). Apparently this is because of two reasons: first, the efficiency of ·OH generation by Fe varies over approximately an order of magnitude in our samples (Figure 5). And, second, in a small number of our samples (7 out of 39), extractable iron cannot explain all of the production of ·OH (i.e., samples with values below 1 in Figure 5), which indicates that mechanisms other than Fe contribute to ·OH formation. Copper is, at most, responsible for only (5 ± 9) % of this unexplained ·OH, based on the dissolved Cu concentrations in our SLF extracts and assuming that Cu has half the reactivity shown by Fe in Figure 4 (24). Copper played a similarly negligible role in ·OH formation overall: averaged over all samples, Cu accounted for only approximately (5 ± 5) % of ·OH formation. In the approximately 20% of samples where Fe cannot account for all ·OH formed, other transition metals might account for the missing reactivity, but organic species such as quinones might also be important as sources of hydrogen peroxide (19), which is the likely precursor for ·OH.
Implications and Uncertainties
In this section we first estimate the ·OH burden from inhalation of PM2.5. To a first approximation, the amount of ·OH generated in human lungs from particulate matter can be estimated by:
| (2) |
Using an average ·OH production of 2.3 nmol m−3 (Figure 1a; recall this is the amount produced after 24 hours of PM extraction), a volume of air inhaled by an adult of 20 m3 day−1, and assuming 45% of particles deposit in the lungs (34), we calculate an average lung burden of 21 nmol ·OH d−1 in Davis. Our previous estimate of 8 nmol ·OH d−1 (24) is significantly lower because it was based on ·OH production in solutions containing 20 μM Fe(II); because ·OH plateaus at Fe(II) ≥ 5 μM (Figure 4), we had previously underestimated the amount of ·OH that could be produced from low levels of Fe.
Lung burdens of hydroxyl radical in more polluted locations are likely to be even higher than in Davis since the average PM2.5 concentration during our sampling was quite low, at 6.0 μg m−3 (Figure 1b). If we scale the calculated, average ·OH burden in Davis to other locations using annual average PM masses (i.e., assuming PM in other locations can generate ·OH with a similar efficiency), we estimate average ·OH lung burdens of 60 − 90 nmol ·OH d−1 in Fresno, Riverside and Burbank, California, which have annual average PM2.5 concentrations of 16.9, 21.0, and 25.0 μg m−3, respectively (30). Furthermore, based on peak 24-hr average PM2.5 concentrations for these locations over the last three years (30), daily maximum lung burdens of ·OH will be on the order of 690 − 1210 nmol ·OH d−1.
Are these estimated lung burdens of ·OH significant for human health? Over long averaging times, the answer initially appears to be “no”, since the total amount of antioxidants in lung lining fluid, approximately 15000 nmol (24), is much greater than the estimated annual average ·OH lung burdens in these three California cities (60 − 90 nmol d−1). However, on shorter time scales, the peak ·OH burdens in these cities (690 − 1210 nmol ·OH d−1) represent a non-negligible fraction of the total antioxidant pool in the lung lining fluid. In addition, several factors increase the significance of PM-generated ROS, regardless of the time scale of exposure: (1) The total amount of oxidation associated with ·OH is approximately 4 times the amount of ·OH formed, since converting dissolved O2 to ·OH requires 3 electrons (24) and each ·OH can initiate an oxidation; (2) Because we extract PM in a cell-free SLF solution, we are likely underestimating the amount of ·OH produced in human lungs since PM exposure stimulates cellular release of additional ROS (35); (3) While our technique specifically measures hydroxyl radical, other ROS species (e.g., •O2− and H2O2) will also be formed by PM in the lung; and (4) susceptible populations such as the elderly and asthmatics can have much smaller antioxidant pools than the estimated 15000 nmol for healthy adults (36).
While our results indicate that ·OH formation could be a significant component in the health effects of PM, and that Fe can account for much of this ·OH, there are a number of uncertainties that make it difficult to extrapolate to adverse effects in vivo. For example, our cell-free assay will not reflect biological responses that might ameliorate the oxidative potential of inhaled PM, such as PM-induced synthesis of antioxidants. In addition, our results — both in terms of ·OH generation and the role of iron — are dependent upon the composition of our extraction fluid; while our SLF is a reasonable, simple surrogate for actual lung fluid, it certainly does not capture its full chemical or biological complexity.
Supplementary Material
Acknowledgements
We thank Brian Perley, Krystyna Trzepla-Nabaglo, and Hege Indresand of Crocker Nuclear Laboratory for the XRF analyses; Jessie Charrier for assisting with correlation analyses; Mike Kleeman for suggesting the use of a filter wetting agent; and the reviewers for their insightful comments. This research was supported by Grant Number P42ES004699 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the NIH. Additional support was provided by the California Agricultural Experiment Station (Project CA-D*-LAW-6403-RR) and the University of California Toxic Substances Research & Teaching Program (TSR&TP) through the Atmospheric Aerosols & Health Lead Campus Program (aah.ucdavis.edu).
Footnotes
Supporting Information Available
Supporting information — containing two figures and three tables — is available free of charge via the Internet at http://pubs.acs.org.
Brief:
Hydroxyl radical generation by fine particles in a surrogate lung fluid varies seasonally and diurnally, and can largely be explained by aqueous-extractable iron.
References
- 1.Slaughter JC, Kim E, Sheppard L, Sullivan JH, Larson TV, Claiborn C. Association between particulate matter and emergency room visits, hospital admissions and mortality in Spokane, Washington. J. Expos. Sci. Environ. Epidem. 2005;15:153–159. doi: 10.1038/sj.jea.7500382. [DOI] [PubMed] [Google Scholar]
- 2.Dockery DW, Pope CA, Xu XP, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An association between air pollution and mortality in 6 United-States cities. N. Engl . J. Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 3.Pope CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang MY, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Jama. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 6.Shi T, Knaapen AM, Begerow J, Birmili W, Borm PJA, Schins RPF. Temporal variation of hydroxyl radical generation and 8-hydroxy-2′-deoxyguanosine formation by coarse and fine particulate matter. Occup. Environ. Med. 2003;60:315–321. doi: 10.1136/oem.60.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmour PS, Brown DM, Lindsay TG, Beswick PH, MacNee W, Donaldson K. Adverse health effects of PM10 particles: Involvement of iron in generation of hydroxyl radical. Occup. Environ. Med. 1996;53:817–822. doi: 10.1136/oem.53.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prahalad AK, Inmon J, Dailey LA, Madden MC, Ghio AJ, Gallagher JE. Air pollution particles mediated oxidative DNA base damage in a cell free system and in human airway epithelial cells in relation to particulate metal content and bioreactivity. Chem. Res. Tox. 2001;14:879–887. doi: 10.1021/tx010022e. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson K, Stone V, Borm PJA, Jimenez LA, Gilmour PS, Schins RPF, Knaapen AM, Rahman I, Faux SP, Brown DM, MacNee W. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Rad. Biol. Med. 2003;34:1369–1382. doi: 10.1016/s0891-5849(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 10.Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hedge V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem. Res. Tox. 2001;14:1371–1377. doi: 10.1021/tx010050x. [DOI] [PubMed] [Google Scholar]
- 11.Hasson AS, Paulson SE. An investigation of the relationship between gas-phase and aerosol-borne hydroperoxides in urban air. J. Aerosol Sci. 2003;34:459–468. [Google Scholar]
- 12.Imlay JA. Pathways of oxidative damage. Ann. Rev. Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 13.Risom L, Moller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res. 2005;592:119–137. doi: 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Baulig A, Poirault JJ, Ausset P, Schins R, Shi TM, Baralle D, Dorlhene P, Meyer M, Lefevre R, Baeza-Squiban A, Marano F. Physicochemical characteristics and biological activities of seasonal atmospheric particulate matter sampling in two locations of Paris. Environ. Sci. Technol. 2004;38:5985–5992. doi: 10.1021/es049476z. [DOI] [PubMed] [Google Scholar]
- 15.Cho AK, Sioutas C, Miguel AH, Kumagai Y, Schmitz DA, Singh M, Eiguren-Fernandez A, Froines JR. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 2005;99:40–47. doi: 10.1016/j.envres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16.de Kok T, Driece HAL, Hogervorst JGF, Briede JJ. Toxicological assessment of ambient and traffic-related particulate matter: A review of recent studies. Mut. Res. - Rev. Mut. Res. 2006;613:103–122. doi: 10.1016/j.mrrev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Venkatachari P, Hopke PK, Brune WH, Ren XR, Lesher R, Mao JQ, Mitchel M. Characterization of wintertime reactive oxygen species concentrations in Flushing, New York. Aerosol Sci. Tech. 2007;41:97–111. [Google Scholar]
- 18.Kunzli N, Mudway IS, Gotschi T, Shi TM, Kelly FJ, Cook S, Burney P, Forsberg B, Gauderman JW, Hazenkamp ME, Heinrich J, Jarvis D, Norback D, Payo-Losa F, Poli A, Sunyer J, Borm PJA. Comparison of oxidative properties, light absorbance, and total and elemental mass concentration of ambient PM2.5 collected at 20 European sites. Environ. Health Perspect. 2006;114:684–690. doi: 10.1289/ehp.8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Squadrito GL, Cueto R, Dellinger B, Pryor WA. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Rad. Biol. Med. 2001;31:1132–1138. doi: 10.1016/s0891-5849(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 20.Alaghmand M, Blough NV. Source-dependent variation in hydroxyl radical production by airborne particulate matter. Environ. Sci. Technol. 2007;41:2364–2370. doi: 10.1021/es061902o. [DOI] [PubMed] [Google Scholar]
- 21.Smith KR, Aust AE. Mobilization of iron from urban particulates leads to generation of reactive oxygen species in vitro and induction of ferritin synthesis in human lung epithelial cells. Chem. Res. Tox. 1997;10:828–834. doi: 10.1021/tx960164m. [DOI] [PubMed] [Google Scholar]
- 22.Valavanidis A, Fiotakis K, Bakeas E, Vlahogianni T. Electron paramagnetic resonance study of the generation of reactive oxygen species catalysed by transition metals and quinoid redox cycling by inhalable ambient particulate matter. Redox Report. 2005;10:37–51. doi: 10.1179/135100005X21606. [DOI] [PubMed] [Google Scholar]
- 23.Valavanidis A, Salika A, Theodoropoulou A. Generation of hydroxyl radicals by urban suspended particulate air matter. The role of iron ions. Atmos. Environ. 2000;34:2379–2386. [Google Scholar]
- 24.Vidrio E, Jung H, Anastasio C. Generation of hydroxyl radicals from dissolved transition metals in surrogate lung fluid solutions. Atmos. Environ. 2008;42:4369–4379. doi: 10.1016/j.atmosenv.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song HS, Bang WG, Chung N, Cho YS, Kim YS, Cho MH. Effect of chelators and reductants on the mobilization of metals from ambient particulate matter. Environ. Sci. Technol. 2003;37:3531–3536. doi: 10.1021/es025981p. [DOI] [PubMed] [Google Scholar]
- 26.Jung H, Guo B, Anastasio C, Kennedy IM. Quantitative measurements of the generation of hydroxyl radicals by soot particles in a surrogate lung fluid. Atmos. Environ. 2006;40:1043–1052. [Google Scholar]
- 27.Walling C, El-Taliawi GM, Johnson RA. Fenton's reagent. IV. Structure and reactivity relations in the reactions of hydroxyl radicals and the redox reactions of radicals. J. Am. Chem. Soc. 1974;96:133–139. [Google Scholar]
- 28.Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O-) in aqueous solution. J. Phys. Chem. Ref. Data. 1988;17:513–886. [Google Scholar]
- 29.Trzepla-Nabaglo K. Data report for elemental analysis of IMPROVE samples collected during January, February and March, 2005. 2006 http://vista.cira.colostate.edu/improve/Data/QA_QC/UCD_QAQC/XRFQA_2005_Jan_to_Mar.pdf.
- 30.California Air Resources Board, Air Quality Data Statistics 2007 http://www.arb.ca.gov/adam/php_files/aqdphp/sc8start.php.
- 31.Becker S, Dailey LA, Soukup JM, Grambow SC, Devlin RB, Huang YCT. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ. Health Perspect. 2005;113:1032–1038. doi: 10.1289/ehp.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aust AE, Ball JC, Hu AA, Lighty JS, Smith KR, Straccia AM, Veranth JM, Young WC. Particle characteristics responsible for effects on human lung epithelial cells. Research Report/Health Effects Institute. 2002:1–65. discussion 67−76. [PubMed] [Google Scholar]
- 33.Donaldson K, Brown DM, Mitchell C, Dineva M, Beswick PH, Gilmour P, MacNee W. Free radical activity of PM10: Iron-mediated generation of hydroxyl radicals. Environ. Health Perspect. 1997;105:1285–1289. doi: 10.1289/ehp.97105s51285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarangapani R, Wexler AS. The role of dispersion in particle deposition in human airways. Toxicol. Sci. 2000;54:229–236. doi: 10.1093/toxsci/54.1.229. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Flecha B. Oxidant mechanisms in response to ambient air particles. Molec. Aspects Med. 2004;25:169–182. doi: 10.1016/j.mam.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Kelly FJ, Dunster C, Mudway I. Air pollution and the elderly: oxidant/antioxidant issues worth consideration. Europ. Resp. J. 2003;21:70S–75S. doi: 10.1183/09031936.03.00402903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.