Abstract
Objective
To examine histopathologic and immunohistochemical features of human corneal buttons from patients who developed keratectasia after laser in situ keratomileusis (LASIK).
Methods
Five corneal buttons were obtained during penetrating keratoplasty from patients who developed keratectasia after LASIK. Histologic features were examined by hematoxylin-eosin staining using paraffinembedded sections and by transmission electron microscopy. Immunostaining for α1-proteinase inhibitor, Sp1, and matrix metalloproteinases 1, 2, and 3 was performed with 2 healthy corneas and 2 corneas with keratoconus as controls.
Results
Central stromal thinning was observed after hematoxylin-eosin staining in all corneas with keratectasia. No histologic features specific to keratoconus, including Bowman layer disruption, were identified in the corneas with keratectasia. By transmission electron microscopy, collagen fibril thinning and decreased interfibril distance were observed in the stromal bed. Immunostaining intensity and/or pattern for α1-proteinase inhibitor and Sp1 in the corneas with keratectasia was comparable to that of healthy corneas and differed from that in the corneas with keratoconus. No significant staining with anti-matrix metalloproteinases 1, 2, and 3 antibodies was observed in either the corneas with keratectasia or the healthy corneas.
Conclusions
Histologic findings suggest that post-LASIK keratectasia results in collagen fibril thinning and decreased interfibril distance within the residual stromal bed. Discrepant results between keratectasia and keratoconus suggest that the pathogenesis of the 2 conditions differ.
Keratectasia remains one of the most troublesome complications that can arise after laser in situ keratomileusis (LASIK). Patients with this complication present with an increase in myopia and astigmatism, loss of uncorrected visual acuity, and often a loss of best-corrected visual acuity1-3 due to progressive corneal steepening that takes place centrally or inferiorly.4-6 Ectatic changes can occur as early as 1 week after LASIK,7 or they can be delayed up to several years after the initial procedure.8,9 In many cases, penetrating keratoplasty is eventually performed to manage this complication. The incidence of keratectasia after LASIK has been estimated to be 0.04% to 0.6%3,10,11; however, accurate data based on large-scale clinical studies are not available.1,5,12-14 Although a number of clinical risk factors have been reported,2,5-9,13-17 the mechanisms of post-LASIK keratectasia remain unclear.
Studies of the histologic changes in post-LASIK keratectasia have demonstrated variable degrees of corneal thinning in the stromal bed and the flap. Although disruption in the Bowman layer typically observed in keratoconus has not been shown in most cases of post-LASIK keratectasia,2,8,18-20 other pathologic findings, such as macrostriae in the stromal bed, thinning of the stromal collagen lamellae, minimal scarring at the flap-stromal bed interface and lack of inflammation, and the presence of an iron ring around the steepening, have been reported.2,16,18-23 An immunohistochemical study24 on wound healing in ectatic corneas after LASIK also reported no scar tissue formation, indicated by a lack of collagen type III expression, even at the flapstromal bed interface.
Keratectasia after LASIK shares similar topographic and clinical characteristics with keratoconus, a noninflammatory disease characterized by thinning of the corneal stroma, defects in the Bowman layer, and the eventual protrusion of the central cornea.25-28 Although some of the keratoconus-like histologic changes have been detected in post-LASIK keratectasia, the biochemical abnormalities found in keratoconus have not been studied in keratectasia. These biochemical alterations include decreased expression of the protease inhibitor α1-proteinase inhibitor (α1-PI) and upregulation of transcription factor Sp1.29-31 In addition, matrix metalloproteinases (MMPs), a group of enzymes that are involved in tissue remodeling, have recently been examined in keratectasia. Studies have detected MMP-3 in rare keratocytes, and MMP-10 expression was elevated in the epithelium of ectatic corneas.32 The MMP expression patterns in keratoconus remain controversial, although over-expression of MMP-1 and MMP-2 has been recently reported in keratoconus corneas.33,34 In addition, MMPs have been implicated in various other corneal conditions, including wound repair and healing, ulcerations, and diabetic corneas.35
In this study, we examined the histopathologic and biochemical characteristics of keratectasia after LASIK using light microscopy and transmission electron microscopy (TEM). Immunohistochemical analysis was also performed with antibodies against α1-PI and transcription factor Sp1. In addition, immunolocalization of major members of the MMP family, MMP-1, MMP-2, and MMP-3, was performed to determine if an MMP-mediated degradation process was involved in keratectasia after LASIK.
METHODS
The protocol was approved by the University of Illinois at Chicago institutional review board. Five keratectasia corneal buttons were obtained from patients during penetrating keratoplasty from the University of Illinois Medical Center, Chicago, Evanston Northwestern Healthcare, Evanston, Illinois, and Armed Forces Institute of Pathology, Washington, DC. Two healthy human corneal buttons from donors (aged 29 and 69 years) were obtained from the Illinois Eye Bank, Chicago, within 24 hours of death. None of the donors had known ocular diseases, and their corneas were clear, with unremarkable gross and microscopic appearance. Two corneal buttons from patients with typical keratoconus features (aged 40 and 41 years) were obtained after transplantation from the cornea service at the University of Illinois at Chicago as another set of controls. The corneas excised from healthy human eyes and the keratectasia and keratoconus buttons were fixed in 10% buffered formalin, processed, and embedded in paraffin.
Paraffin sections 5 μm in size were deparaffinized, rehydrated, and stained with hematoxylin-eosin (H&E). Prussian blue staining was used to detect the presence of iron deposits. The thickness of the total stroma, flap, and posterior stromal bed for LASIK was measured in H&E-stained sections (AxioVision, version 4.4.1.0 software; Carl Zeiss MicroImaging Inc, Göttingen, Germany) after photographing under a Zeiss Axioskop 2 plus microscope using a Zeiss AxioCam HR camera.
Immunohistochemical studies were also conducted. The sections were incubated at 4°C with primary antibodies for 16 hours. The primary antibodies used in the study included (1) a polyclonal goat antibody to α1-PI (1:200; MP Biomedicals, Aurora, Ohio), (2) a polyclonal rabbit antibody to Sp1 (PEP 2, 1:100; Santa Cruz Biotechnology, Santa Cruz, California), (3) a polyclonal rabbit anti-MMP-1 antibody (1:100; Lab Vision, Fremont, California), (4) a monoclonal mouse antibody specific to MMP-2 (A-Gel VC2, 1:50; Lab Vision), and (5) a monoclonal mouse antibody to MMP-3 (SL-1 IID4, 1:20; Lab Vision). Before primary antibody incubation for MMPs, an antigen retrieval method was applied on each section with 8-mol/L buffered urea (pH 3.5) at room temperature for 2 hours. Biotinylated donkey anti-goat IgG (1: 250; Jackson ImmunoResearch Laboratories, Inc, West Grove, Pennsylvania), donkey anti-rabbit IgG (1:250; Jackson ImmunoResearch Laboratories, Inc), and donkey anti-mouse IgG (1: 250; Jackson ImmunoResearch Laboratories, Inc) were used as secondary antibodies at room temperature for 45 minutes. The color reaction for the anti-Sp1 was performed with fast red TR/naphthol AS-MX phosphate (Sigma-Aldrich, St Louis, Missouri) after sections were incubated with alkaline phosphatase conjugated Extravidin (1:50; Sigma-Aldrich). For α1-PI, MMP-1, MMP-2, and MMP-3, sections were incubated with avidin-biotin complex (Vectastain; Vector Laboratories, Burlingame, California) for 45 minutes followed by incubation with 3,3-diaminobenzidine tetrahydrochloride (Sigma-Aldrich). After mounting in Permount (α1-PI, MMP-1, MMP-2, and MMP-3) or aqueous mounting fluid (Sp1), the staining intensity in each experiment was scored by 3 masked observers (D.P.E., B.Y., H.N.) on a scale from 0 to 3, with 0 indicating no staining and 3 the most intense staining. Experiments were repeated twice.
Ultrastructural examination of 2 corneal specimens with keratectasia (right eye in patient 2 and left eye patient 4) and 2 healthy corneal specimens was performed by TEM. The areas of study were obtained from the midperipheral cornea in the healthy specimen and the specimen with keratectasia. The central area was not included because most of the ectatic area was included in the paraffin block. The specimens were fixed in 2% glutaraldehyde at 4°C, processed, and embedded in epoxy resin. Photographs were taken using a Gatan Digital CCD camera and Gatan Digital Micrograph 2.5 version acquisition software (Gatan Inc, Pleasanton, California). Corneal collagen fibril diameter and interfibril distance were measured using ImageJ 1.36b software (National Institutes of Health, Bethesda, Maryland). The fibrils were measured from the central cornea in the area of thinning and in areas where the fibrils were well oriented, which accounts for the disparity in the number of fibrils measured. The number of fibrils measured for diameter in each specimen was 21 in patient 2, 40 in patient 4, and 80 in the healthy controls. The number of interfibril distances measured were 39 in patient 2, 52 in patient 4, and 104 in the healthy controls. Statistical analysis was performed using the Mann-Whitney U test to compare the fibril diameter and interfibril distance of each of the keratectasia corneas with healthy controls. P<.05 was considered statistically significant.
RESULTS
CLINICAL HISTORY
All patients in this study were women, ranged in age from 44 to 57 years, and had varying degrees of myopia. Preoperative pachymetry was available only in patient 3 and revealed corneal thicknesses of 532 μm in the right eye and 515 μm in the left eye. The salient findings at the time of penetrating keratoplasty for post-LASIK keratectasia are summarized in Table 1 and Figure 1.
Table 1.
Patient Clinical Data After LASIK
| Patient No. (Eye) | No. of Enhancement Procedures | Onset Delay | Corneal Thickness, μm | Keratometry, D | Condition of Other Eye |
|---|---|---|---|---|---|
| 1 (Right) | 1 | 10 mo | 260 | 59.5 × 90/greater 65 × 35 | UCVA 20/50 after LASIK |
| 2 (Right) | 2 | 4 y | 320 | NA | -2.00 + 2.75 × 55 after LASIK |
| 3 (Right) | 0 | 18 mo | NA | 45.50/49.00 × 118 | Penetrating keratoplasty |
| 3 (Left) | 0 | 18 mo | NA | 47.50/52.00 × 73 | Penetrating keratoplasty |
| 4 (Left) | 1 | 3 y | 318 | 63.9/56.5 × 147 | BCVA 20/30, -3.75 + 0.75 × 050 refraction after LASIK |
Abbreviations: BCVA, best-corrected visual acuity; LASIK, laser in situ keratomileusis; NA, clinical data were not available; UCVA, uncorrected visual acuity.
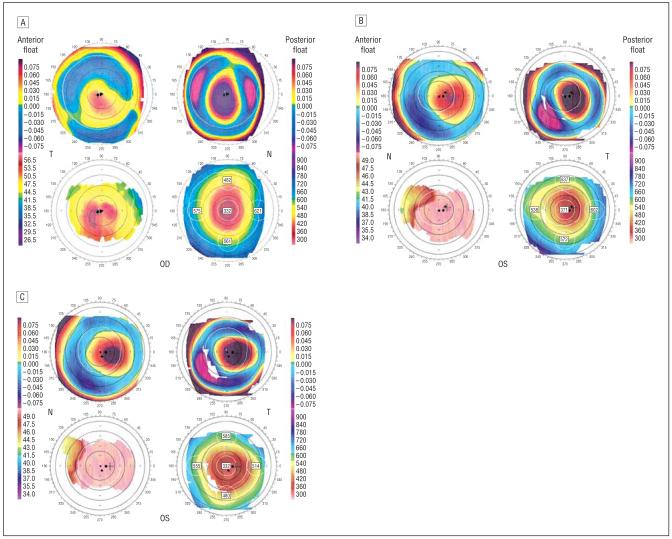
Figure 1.
Orbscan images of study cases. Orbscan image of patient 2 shows central corneal thinning, increased posterior float, and inferocentral corneal steepening (A). Orbscan images of patient 4 at 3 years (B) and 5 years (C) after laser in situ keratomileusis. Both show central corneal thinning, increased posterior float, and central corneal steepening.
HISTOLOGIC ANALYSIS
Measurements of corneal stromal thickness were performed on histologic sections of all 5 keratectasia corneas. Full stromal, residual stromal bed, and flap thicknesses were measured centrally and peripherally. Peripheral measurements were taken just central to the microkeratome incision point on all specimens. Mean (SD) central and peripheral full stromal corneal thickness measurements in keratectasia were 293(69.4) μm and 440 (36.7) μm, respectively. In comparison, healthy corneal thickness was 399 (5.7) μm centrally and 431 (7.8) μm peripherally. Additional results of thickness measurements (Table 2) showed stromal thinning in the flap and the residual bed.
Table 2.
Corneal Thickness Measurements by Histologic Analysis
| Patient No. (Eye) | Full Stroma, μma |
Residual Stromal Bed, μm |
Stromal Flap, μm |
|||
|---|---|---|---|---|---|---|
| Central | Peripheral | Central | Peripheral | Central | Peripheral | |
| 1 (Right) | 218 | 395 | 129 | 343 | 60 | 78 |
| 2 (Right) | 229 | 434 | 126 | 269 | 104 | 101 |
| 3 (Right) | 317 | 452 | 244 | 356 | 71 | 76 |
| 3 (Left) | 319 | 427 | 264 | 353 | 77 | 69 |
| 4 (Left) | 384 | 495 | 242 | 348 | 136 | 135 |
Mean (SD) central and peripheral full stromal corneal thickness measurements in keratectasia were 293 (69.4) μm and 440 (36.7) μm, respectively. In comparison, the mean (SD) for healthy corneal thickness was 399 (5.7) μm centrally and 431 (7.8) μm peripherally.
The 5 H&E-stained specimens had similar histopathologic features, which are summarized in Table 3. These features included the presence of an epithelial iron ring, a lack of Bowman layer disruption that is typically seen in keratoconus, a lack of inflammation or scarring, a faint demarcation line between the flap and residual stromal bed, and an unremarkable Descemet membrane and endothelium. In addition, areas of peripheral epithelial ingrowth at the site of the microkeratome incision were observed in patients 2 (right eye) and 4 (left eye). The central corneal epithelium showed thinning on patient 3 (left eye) and patient 4 (left eye); macrostriae were seen in the residual stromal bed of patients 3 (right eye) and 4 (left eye). Patients 2 and 3 are depicted in Figure 2 and Figure 3; these images are representative of the spectrum of histologic changes observed in our series of keratectasia cases.
Table 3.
Summary of Histopathologic Findingsa
| Patient No. (Eye) | Epithelium | Bowman Layer Disruption | Residual Stromal Bed |
|---|---|---|---|
| 1 (Right) | Central artifact | No | No scar or inflammation |
| 2 (Right) | Iron ring, ingrowth at microkeratome incision | Yes, in area of epithelial ingrowth | No scar or inflammation |
| 3 (Right) | Iron ring | No | No scar or inflammation, macrostriae |
| 3 (Left) | Iron ring, central thinning | No | No scar or inflammation |
| 4 (Left) | Central thinning, ingrowth at microkeratome incision | No | No scar or inflammation, macrostriae |
Note that in all patients, the interface between the corneal flap and stromal bed was faintly delineated. Also, the Descemet membrane and corneal endothelim show no changes.
Figure 2.
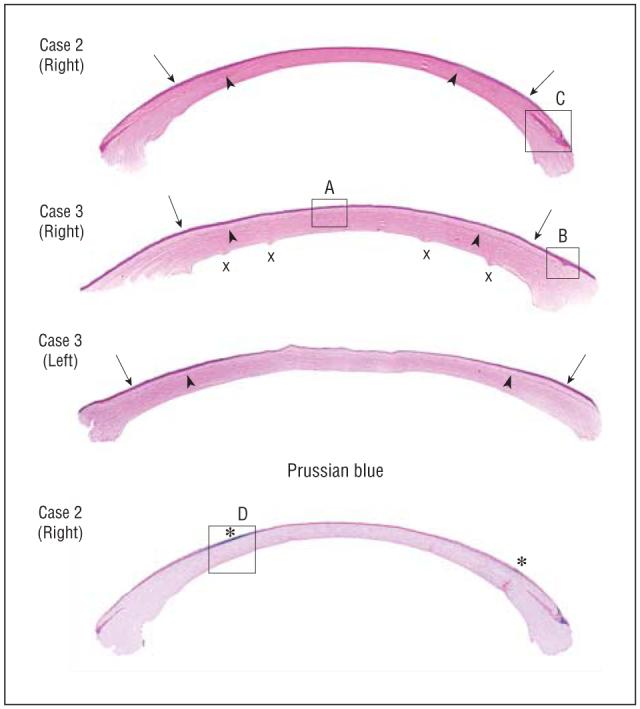
Histopathologic analysis of keratectasia cases. Note the central corneal thinning and macrostriae (x) in patient 3. Arrows point to where peripheral thickness measurements were taken. Arrowheads indicate flap-residual stromal bed interface (hematoxylin-eosin, original magnification ×63). The bottom of the figure demonstrates the peripheral epithelial iron ring (asterisk) (Prussian blue, original magnification ×63). Areas enclosed in boxes (labeled A-D) are shown at a higher magnification (×252 for A and B and ×126 for C and D) in Figure 3.
Figure 3.
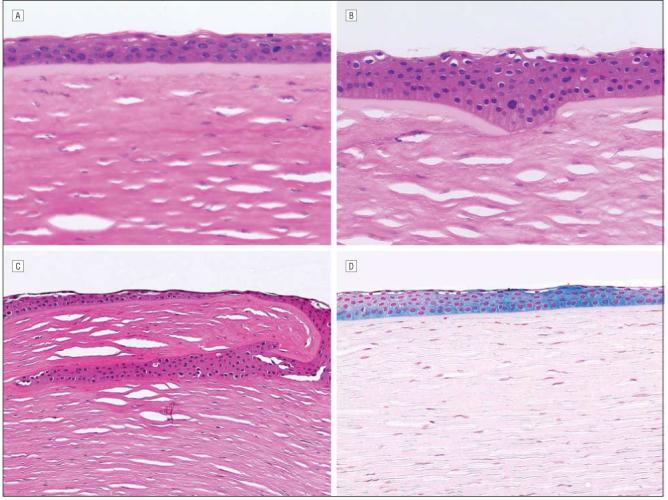
Cornea with keratectasia from Figure 2 at a higher magnification. A, Central cornea of patient 3 showing intact Bowman layer (hematoxylin-eosin, original magnification ×252). B, Peripheral cornea in patient 3 showing disruption of the Bowman layer at the site of microkeratome incision (hematoxylin-eosin, original magnification ×252). C, Epithelial ingrowth in patient 2 at the point of microkeratome incision (hematoxylin-eosin, original magnification ×126). D, Epithelial iron deposits visualized with Prussian blue stain in patient 2 (original magnification ×126).
TRANSMISSION ELECTRON MICROSCOPY
Examination of patients 2 (right eye) and 4 (left eye) by TEM revealed ultrastructural changes in the midperipheral corneal stroma (Figure 4). The mean (SD) diameter of the collagen fibrils in the residual stromal bed was 27.6 (2.7) nm in patient 2 and 27.5 (2.3) nm in patient 4, and these collagen fibrils were significantly thinner than collagen fibrils of healthy controls (32.9 [2.8] nm, P<.001). Interfibril distances observed in keratectasia patients 2 and 4 were 23.4 (9.6) nm and 23.5 (7.0) nm, respectively, and were also significantly less than interfibril distances seen in healthy controls (25.9 [6.1] nm, P=.031).
Figure 4.
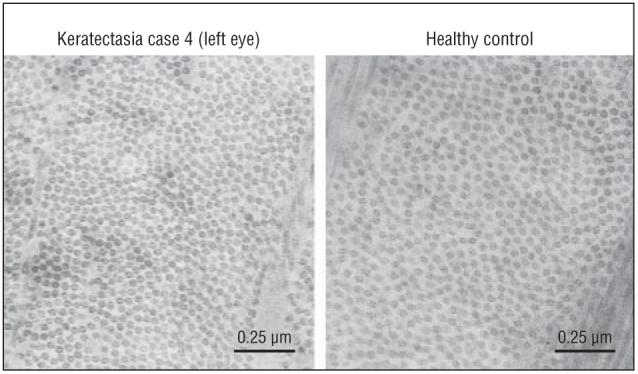
Transmission electron micrographs from individuals after laser in situ keratomileusis. A, Keratectasia patient 4. B, A healthy control. Mean (SD) collagen fibril diameters were 27.5(2.3) nm (number of fibrils measured=40) in patient 4 and 32.9(2.8) nm (number of fibrils measured=80) in the healthy control. Mean (SD) interfibril distance was 23.5(7.0) nm (number of interfibril distances measured=52) in patient 4 and 25.9(6.1) nm (number of interfibril distances measured=104) in the healthy control (original magnification ×100 000).
IMMUNOHISTOCHEMICAL ANALYSIS
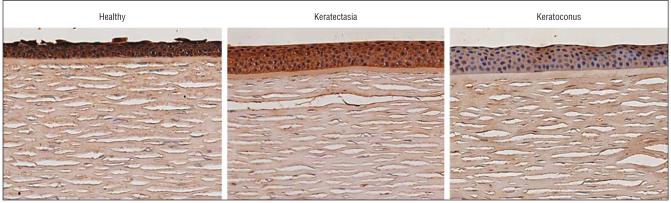
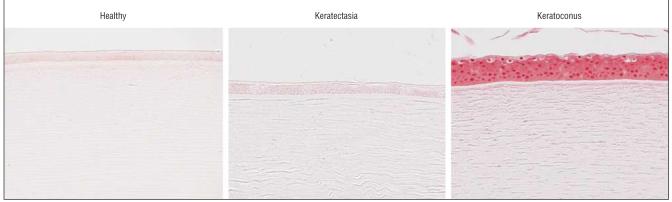
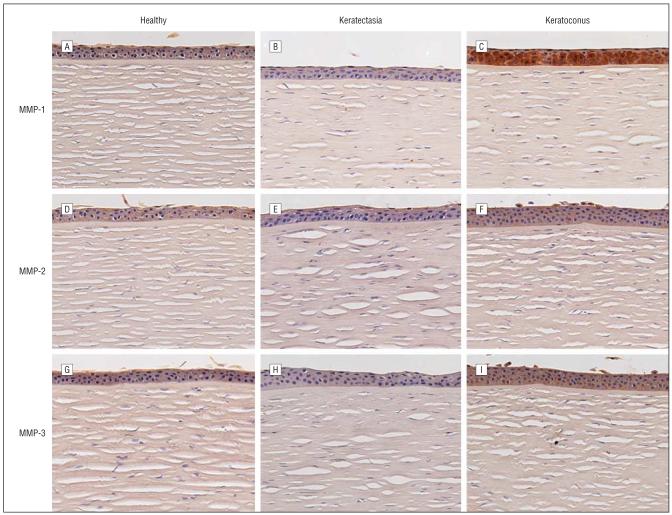
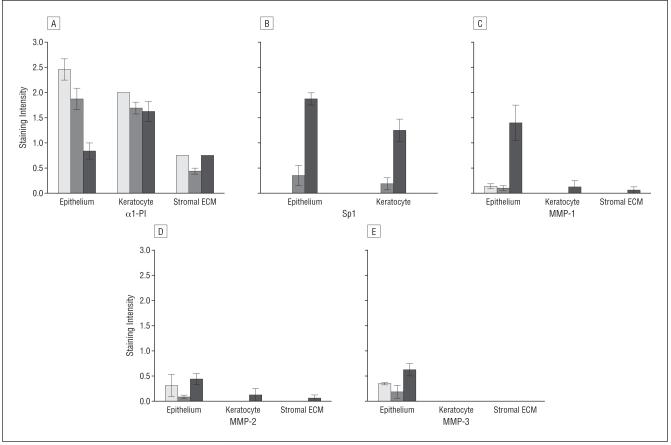
Immunohistochemical experiments demonstrated positive staining of α1-PI in the epithelium of keratectasia corneas. The intensity of the staining was similar to that found in healthy human corneas. The epithelial staining of α1-PI in corneas with keratoconus, in the meantime, was markedly decreased as expected (Figure 5).29-31 Immunohistochemical analysis for Sp1 in corneas with keratectasia and healthy human corneas revealed much weaker staining than that in corneas with keratoconus (Figure 6). The epithelium of healthy human corneas and corneas with keratectasia displayed nearly absent staining for MMP-1, whereas strong staining was observed in the corneal epithelium of keratoconus cases. Both MMP-2 and MMP-3 immunolabeling were at the background level or absent in all corneal specimens. However, the positive controls (placenta) showed strong positive staining (data not shown). In general, the MMP immunostaining pattern in the keratectasia corneas resembled that seen in healthy controls (Figure 7). Semiquantitative immunostaining results for all antibodies are displayed in Figure 8.
Figure 5.
Immunostaining results for α1-protease inhibitor. A, Healthy control. B, Cornea with keratectasia. C, Cornea with keratoconus. Note the intense staining in the epithelium of the healthy cornea and the cornea with keratectasia. In comparison, epithelial staining in the cornea with keratoconus is nearly absent (chromagen 3,3-diaminobenzidine tetrahydrochloride, original magnification ×126).
Figure 6.
Immunostaining for Sp1. A, Healthy control. B, Cornea with keratectasia. C, Cornea with keratoconus. Note that the intensity of the Sp1 nuclear staining is much lower in the epithelium and keratocytes of the healthy cornea and the cornea with keratectasia compared with the cornea with keratoconus (chromagen fast red TR/naphthol AS-MX phosphate, original magnification ×126).
Figure 7.
Immunostaining results for the matrix metalloproteinases (MMPs). A-C, MMP-1 in healthy corneas, corneas with keratectasia, and corneas with keratoconus, respectively. D-F, MMP-2 in healthy corneas, corneas with keratectasia, and corneas with keratoconus, respectively. G-I, MMP-3 in healthy corneas, corneas with keratectasia, and corneas with keratoconus, respectively. Note that the intensity of the MMP-1 staining is very low in the epithelium of the healthy corneas and the corneas with keratectasia. By contrast, strong epithelial staining of MMP-1 is present in the keratoconus case. The MMP-2 and MMP-3 staining was at the background level or absent in healthy corneas, the corneas with keratoconus, and the corneas with keratectasia (chromagen 3,3-diaminobenzidine tetrahydrochloride, original magnification ×126).
Figure 8.
Semiquantitative immunostaining results for all antibodies. A, Staining intensity for α1-proteinase inhibitor (α1-PI). B, Nuclear staining intensity for Sp1 in corneal epithelial cells and keratocytes in the corneas with keratectasia (open bar, n=5), healthy human corneas (gray bar, n=2), and corneas with keratoconus (black bar, n=2) as scored by 3 masked observers. Staining intensity for matrix metalloproteinase (MMP) 1 (C), MMP-2 (D), MMP-3 (E) in corneal epithelial cells, keratocytes, and stromal extracellular matrix (ECM). Error bars indicate SEM.
COMMENT
The continuously growing popularity of refractive surgery procedures, namely LASIK, has caused increased concern regarding the serious complication of keratectasia. Little is known about the mechanism of this rare complication. In this study, we analyzed 5 corneal buttons from patients who developed this complication using histologic analysis, immunohistochemical analysis, and TEM to better understand the disease process of keratectasia. In addition, we sought to compare keratectasia with keratoconus given the similarities, both clinically and topographically, between the 2 conditions.
The histologic findings from H&E staining of all 5 of our specimens of post-LASIK keratectasia were consistent with those reported in the literature2,8,16,18-23, including stromal thinning of both the residual bed and flap and the lack of signs of inflammation or corneal scarring in the posterior stroma. Furthermore, Seitz et al2 reported macrostriae in the residual stroma of a case of keratectasia after LASIK; similar macrostriae were observed in patient 3 (right eye). Also in patient 3 (right eye) epithelial thinning was observed in the central cornea; a similar finding was reported by Kim et al.36 The presence of an iron ring after LASIK-related keratectasia observed in our cases was likely due to tear pooling at the edge of the ectatic cornea; this finding has also been previously reported.23 The Bowman layer in all 5 of our corneas did not show disruptions that are typically seen in keratoconus. Most previous histologic studies on post-LASIK keratectasia also reported a lack of Bowman layer disruption.2,8,18-20
Immunohistochemical analysis performed on corneas with keratectasia, corneas with keratoconus, and healthy corneas disclosed that the expression pattern of α1-PI and Sp1 in keratectasia was similar to that of healthy corneas. The previously reported decrease in α1-PI expression and upregulation of Sp1 that occur in corneas with keratoconus were not observed in our cases of post-LASIK keratectasia.30,31,36 Therefore, LASIK-related keratectasia and keratoconus, despite similar clinical presentations, appear to be biochemically different, suggesting that the mechanisms by which progressive post-LASIK keratectasia develops differ from those in keratoconus.
The delayed onset and progressive nature of the development of keratectasia after LASIK led us to postulate that the condition may be related to alteration in protein degradation by the various MMPs. Our results showed that the MMP-1, MMP-2, and MMP-3 immunostaining in corneas with keratectasia was weak and similar to that observed in healthy corneas. A recent study,32 however, showed an increased expression of MMP-3 in the keratocytes of 2 corneas with post-LASIK keratectasia using an alternative antibody on cryofrozen sections and the immunofluorescence procedure. Despite a lack of immunolabeling for MMP-3 in our keratectasia specimens, immunostaining for MMP-3 in antigen-retrieved paraffin-embedded human placenta sections (positive control) consistently showed obvious positive staining. Interestingly, immunostaining for MMP-1 in keratoconus corneas showed a higher intensity in our study. This finding differs from that reported in a previous article.37 The disparity may be related to the adoption of antigen retrieval techniques specific to MMPs in the present study. Further research with a larger sample size and possibly zymography would be required to investigate the expression of MMP-1 and MMP-3 in corneas with post-LASIK keratectasia.
Two of the 5 corneas with keratectasia (patient 2 [right eye] and patient 4 [left eye]) were examined by TEM in the midperipheral cornea (close to the edge of the area of central thinning) and compared with the healthy controls. A statistically significant decrease in the diameter of collagen fibrils within the residual stromal bed was demonstrated in comparison to collagen fibril diameter measurements conducted on healthy controls. In addition, interfibril distances in the cases of keratectasia were significantly decreased when compared with interfibril distances in healthy corneal specimens. These new findings imply that the development of keratectasia after LASIK may be due to stretching of the collagen fibrils in addition to compression of the cornea, which is evident even in corneal segments away from the area of ectasia. The thinning of the actual collagen fibrils along with depletion of interfibril space may suggest that the fibril and surrounding extracellular matrix in keratectasia cannot resist ectatic changes due to intraocular pressure. Currently, it is recommended that at least 250 μm of residual stroma should be left after laser ablation to provide the cornea with sufficient mechanical strength to resist ectatic changes.6,10 This value is not an absolute cutoff point because several reports have described corneas with a residual stromal bed thickness of more than 300 μm that developed ectasia, and conversely other reports have described corneas with a residual stromal bed thickness of less than 250 μm after laser ablation that did not develop keratectasia.1 This leads us to believe that each cornea has its own threshold of residual stromal thickness needed to resist deformation by healthy intraocular pressure. A biomechanical instability, however, does not explain the delayed onset and progressive nature of the disorder in some cases. Although we did not find an increase in degradative enzymes in our cases of keratectasia, Maguen and colleagues32 reported an increased expression of MMP-3 in keratocytes and MMP-10 in the epithelium of 2 ectatic corneas after LASIK. This finding suggests that biochemical changes may occur in the corneal stromal matrix before biomechanical failure in some cases. Further studies with a larger number of LASIK-related ectatic corneas are needed to further strengthen this idea.
In summary, the 5 corneas with keratectasia showed histologic changes consistent with those reported previously, including stromal thinning in the residual bed and flap, lack of inflammation or corneal scarring, macrostriae in the residual bed, presence of an iron ring, and a lack of Bowman layer disruption typically seen in keratoconus.2,16,18-23 Immunohistochemical studies revealed that corneas with keratectasia and healthy corneas showed similar staining patterns for α1-PI and Sp1, distinctly different from those seen in corneas with keratoconus. The expression of MMP-1, MMP-2, and MMP-3 in keratectasia was also demonstrated to be similar to that in healthy corneas. In addition, we demonstrated the thinning of the collagen fibrils with depletion of the interfibril space in keratectasia by TEM. These results may imply that after LASIK the collagen fibrils in the residual stromal bed of corneas with keratectasia may be mechanically stretched while the surrounding extracellular matrix is concurrently compressed because of loss of structural resistance to intraocular pressure.
Acknowledgments
Funding/Support: This study was supported by gifts from Doris Semler and Sandra Schild EY03890 (BYJTY) and grants EYO1792 (Core) from the National Eye Institute, Bethesda, Maryland.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Binder PS. Ectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2003;29(12):2419–2429. doi: 10.1016/j.jcrs.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Seitz B, Rozsival P, Feuermannova A, Langenbucher A, Naumann GO. Penetrating keratoplasty for iatrogenic keratoconus after repeat myopic laser in situ keratomileusis: histologic findings and literature review. J Cataract Refract Surg. 2003;29(11):2217–2224. doi: 10.1016/s0886-3350(03)00406-1. [DOI] [PubMed] [Google Scholar]
- 3.Randleman JB, Russell B, Ward MA, Thompson KP, Stulting RD. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110(2):267–275. doi: 10.1016/S0161-6420(02)01727-X. [DOI] [PubMed] [Google Scholar]
- 4.Comaish IF, Lawless MA. Progressive post-LASIK keratectasia: biomechanical instability or chronic disease process? J Cataract Refract Surg. 2002;28(12):2206–2213. doi: 10.1016/s0886-3350(02)01698-x. [DOI] [PubMed] [Google Scholar]
- 5.Kohnen T. Iatrogenic keratectasia: current knowledge, current measurements. J Cataract Refract Surg. 2002;28(12):2065–2066. doi: 10.1016/s0886-3350(02)01879-5. [DOI] [PubMed] [Google Scholar]
- 6.Seiler T, Koufala K, Richter G. Iatrogenic keratectasia after laser in situ keratomileusis. J Refract Surg. 1998;14(3):312–317. doi: 10.3928/1081-597X-19980501-15. [DOI] [PubMed] [Google Scholar]
- 7.Rao SN, Epstein RJ. Early onset keratectasia following laser in situ keratomileusis: case report and literature review. J Refract Surg. 2002;18(2):177–184. doi: 10.3928/1081-597X-20020301-13. [DOI] [PubMed] [Google Scholar]
- 8.Geggel HS, Talley AR. Delayed onset keratectasia following in situ keratomileusis. J Cataract Refract Surg. 1999;25(4):582–586. doi: 10.1016/s0886-3350(99)80060-1. [DOI] [PubMed] [Google Scholar]
- 9.Lifshitz T, Levy J, Klemperer I, Levinger S. Late bilateral keratectasia after LASIK in a low myopic patient. J Refract Surg. 2005;21(5):494–496. doi: 10.3928/1081-597X-20050901-12. [DOI] [PubMed] [Google Scholar]
- 10.Rad AS, Jabbarvand M, Saifi N. Progressive keratectasia after laser in situ keratomileusis. J Refract Surg. 2004;20(5suppl):S718–S722. doi: 10.3928/1081-597X-20040903-18. [DOI] [PubMed] [Google Scholar]
- 11.Pallikaris IG, Kymionis GD, Astyrakakis NI. Corneal ectasia induced by laser in situ keratomileusis. J Cataract Refract Surg. 2001;27(11):1796–1802. doi: 10.1016/s0886-3350(01)01090-2. [DOI] [PubMed] [Google Scholar]
- 12.McLeod SD, Kisla TA, Caro NC, McMahon TT. Iatrogenic keratoconus: corneal ectasia following laser in situ keratomileusis for myopia. Arch Ophthalmol. 2000;118(2):282–284. [PubMed] [Google Scholar]
- 13.Binder PS, Lindstrom RL, Stulting RD, et al. Keratoconus and corneal ectasia after LASIK. J Cataract Refract Surg. 2005;31(11):2035–2038. doi: 10.1016/j.jcrs.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Amoils SP, Deist MB, Gous P, Amoils PH. Iatrogenic keratectasia after in situ keratomileusis for less than -4.0 to -7.0 diopters of myopia. J Cataract Refract Surg. 2000;26(7):967–977. doi: 10.1016/s0886-3350(00)00434-x. [DOI] [PubMed] [Google Scholar]
- 15.Randleman JB. Post-laser in-situ keratomileusis ectasia: current understanding and future directions. Curr Opin Ophthalmol. 2006;17(4):406–412. doi: 10.1097/01.icu.0000233963.26628.f0. [DOI] [PubMed] [Google Scholar]
- 16.Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg. 1998;24(7):1007–1009. doi: 10.1016/s0886-3350(98)80057-6. [DOI] [PubMed] [Google Scholar]
- 17.Klein SR, Epstein RJ, Randleman JB, Stulting RD. Corneal ectasia after laser in situ keratomileusis in patients without apparent preoperative risk factors. Cornea. 2006;25(4):388–403. doi: 10.1097/01.ico.0000222479.68242.77. [DOI] [PubMed] [Google Scholar]
- 18.Spadea L, Palmieri G, Mosca L, Fasciani R, Balestrazzi E. Iatrogenic keratectasia following laser in situ keratomileusis. J Refract Surg. 2002;18(4):475–480. doi: 10.3928/1081-597X-20020701-12. [DOI] [PubMed] [Google Scholar]
- 19.Argento C, Cosentino MJ, Tytiun A, Rapetti G, Zarate J. Corneal ectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2001;27(9):1440–1448. doi: 10.1016/s0886-3350(01)00799-4. [DOI] [PubMed] [Google Scholar]
- 20.Ou RJ, Shaw EL, Glasgow BJ. Keratectasia after laser in situ keratomileusis (LASIK): evaluation of the calculated residual stromal bed thickness. Am J Ophthalmol. 2002;134(5):771–773. doi: 10.1016/s0002-9394(02)01656-2. [DOI] [PubMed] [Google Scholar]
- 21.Spirn MJ, Dawson DG, Rubinfeld RS, et al. Histopathological analysis of post-laser assisted in situ keratomileusis corneal ectasia with intrastromal corneal ring segments. Arch Ophthalmol. 2005;123(11):1604–1607. doi: 10.1001/archopht.123.11.1604. [DOI] [PubMed] [Google Scholar]
- 22.Jabbur NS, Stark WJ, Green WR. Corneal ectasia after laser-assisted in situ keratomileusis. Arch Ophthalmol. 2001;119(11):1714–1716. doi: 10.1001/archopht.119.11.1714. [DOI] [PubMed] [Google Scholar]
- 23.Ozdamar A, Aras C, Ustundag C, Bahcecioglu H, Ozkan S. Corneal iron ring associated with iatrogenic keratectasia after myopic laser in situ keratomileusis. J Cataract Refract Surg. 2000;26(11):1684–1686. doi: 10.1016/s0886-3350(00)00559-9. [DOI] [PubMed] [Google Scholar]
- 24.Philipp WE, Speicher L, Gottinger W. Histological and immunohistochemical findings after laser in situ keratomileusis in human corneas. J Cataract Refract Surg. 2003;29(4):808–820. doi: 10.1016/s0886-3350(02)01611-5. [DOI] [PubMed] [Google Scholar]
- 25.Chi H, Katzin H, Teng C. Histopathology of keratoconus. Am J Ophthalmol. 1956;42:847–860. [Google Scholar]
- 26.Teng CC. Electron microscopic study of pathology of keratoconus. Am J Ophthalmol. 1963;55:18–47. doi: 10.1016/0002-9394(63)91645-3. [DOI] [PubMed] [Google Scholar]
- 27.Sawaguchi S, Fukuchi T, Abe H, Kaiya T, Sugar J, Yue BYJT. Three-dimensional scanning electron microscopic study of keratoconus corneas. Arch Ophthalmol. 1998;116(1):62–68. doi: 10.1001/archopht.116.1.62. [DOI] [PubMed] [Google Scholar]
- 28.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Zhou L, Twining SS, Sugar J, Yue BY. Involvement of Sp1 elements in the promoter activity of the α1-proteinase inhibitor gene. J Biol Chem. 1998;273(16):9959–9965. doi: 10.1074/jbc.273.16.9959. [DOI] [PubMed] [Google Scholar]
- 30.Sawaguchi S, Twining SS, Yue BY, Wilson PM, Sugar J, Chan SK. α1 Proteinase inhibitor levels in keratoconus. Exp Eye Res. 1990;50(5):549–554. doi: 10.1016/0014-4835(90)90044-u. [DOI] [PubMed] [Google Scholar]
- 31.Whitelock RB, Li Y, Zhou L, Sugar J, Yue BY. Expression of transcription factors in keratoconus, a cornea-thinning disease. Biochem Biophys Res Commun. 1997;235(1):253–258. doi: 10.1006/bbrc.1997.6711. [DOI] [PubMed] [Google Scholar]
- 32.Maguen E, Maguen B, Regev L, Ljubimov AV. Immunohistochemical evaluation of two corneal buttons with post-LASIK keratectasia. Cornea. 2007;26(8):983–991. doi: 10.1097/ICO.0b013e3180de1d91. [DOI] [PubMed] [Google Scholar]
- 33.Seppälä HP, Maatta M, Rautia M, et al. EMMPRIN and MMP-1 in keratoconus. Cornea. 2006;25(3):325–330. doi: 10.1097/01.ico.0000183534.22522.39. [DOI] [PubMed] [Google Scholar]
- 34.Smith VA, Matthews FJ, Majid MA, Cook SD. Keratoconus: matrix matalloproteinase-2 activation and TIMP modulation. Biochim Biophys Acta. 2006;1762(4):431–439. doi: 10.1016/j.bbadis.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21(1):1–14. doi: 10.1016/s1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Song IK, Joo CK. Keratectasia after laser in situ keratomileusis: clinicopathological case report. Ophthalmologica. 2006;220(1):58–64. doi: 10.1159/000089276. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, Sawaguchi S, Twining SS, Sugar J, Feder RS, Yue BY. Expression of degradative enzymes and protease inhibitors in corneas with keratoconus. Invest Ophthalmol Vis Sci. 1998;39(7):1117–1124. [PubMed] [Google Scholar]