Abstract
Hyperactivated ERK signaling is common in human cancer and is often the result of activating mutations in BRAF, RAS and upstream receptor tyrosine kinases. In order to characterize the MEK/ERK dependence of lung cancers harboring BRAF kinase domain mutations, we screened a large panel of human lung cancer cell lines (n= 87) and tumors (n=916) for BRAF mutations. We found that NSCLC cells with both V600E and non-V600E BRAF mutations were selectively sensitive to MEK inhibition, compared to those harboring mutations in EGFR, KRAS, or ALK and ROS kinase fusions. Supporting its classification as a “driver” mutation in the cells in which it is expressed, MEK inhibition in V600EBRAF NSCLC cells led to substantial induction of apoptosis, comparable to that seen with EGFR kinase inhibition in EGFR-mutant NSCLC models. Despite high basal ERK phosphorylation, EGFR-mutant cells were uniformly resistant to MEK inhibition. Conversely, BRAF-mutant cell lines were resistant to EGFR inhibition. These data, together with the non-overlapping pattern of EGFR and BRAF mutations in human lung cancer, suggest that these lesions define distinct clinical entities whose treatment should be guided by prospective real-time genotyping. To facilitate such an effort, we developed a mass spectrometry-based genotyping method for the detection of hot-spot mutations in BRAF, KRAS, and EGFR. Using this assay, we confirmed that BRAF mutations can be identified in a minority of NSCLC tumors, and that patients whose tumors harbor BRAF mutations have a distinct clinical profile compared to those whose tumors harbor kinase domain mutations in EGFR.
Keywords: BRAF, non-small cell lung cancer, MEK inhibitor, PD0325901, EGFR mutation, gefitinib, erlotinib
INTRODUCTION
Lung cancer is the leading cause of cancer-specific mortality worldwide, with over 160,000 deaths per year reported in the U.S. (1). Treatment options are limited for patients with advanced metastatic disease, as traditional cytotoxic chemotherapy confers only a limited survival benefit. Novel treatment strategies are therefore needed for these patients. Molecular profiling studies have shown that activating mutations in the EGFR, HER2, BRAF and KRAS genes are generally non-overlapping and identifiable in approximately 40% of non-small cell lung cancers (NSCLC). Together with the recent discovery of ALK and ROS kinase fusions, potentially targetable “driver mutations” can now be identified in approximately half of all NSCLC patients (2, 3).
In clinical studies, EGFR kinase domain mutations have been shown to strongly predict for response to EGFR tyrosine kinase inhibitors (TKIs) (4–6). Though the response of patients to these agents is often dramatic, resistance invariably develops within the first year. Mechanisms of acquired resistance include selection for the T790M mutation, which increases affinity of the receptor for ATP (7, 8), and amplification of the MET receptor tyrosine kinase (9, 10). KRAS mutation has been shown to confer primary or de novo resistance to EGFR targeted therapies in both lung and colon cancer patients (11, 12). As ERK activity is high in both EGFR and KRAS mutant tumors, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) inhibition has been proposed as a possible therapeutic strategy for patients whose tumors demonstrate resistance to EGFR tyrosine kinase inhibitors.
Although BRAF is the kinase most frequently mutated in human tumors, the reported frequency of BRAF mutations in NSCLC is low (2–3%) (13–15). In melanoma, colon and thyroid cancers, the tumor types with the highest frequency of BRAF mutation, a single nucleotide substitution resulting in a glutamic acid for valine substitution within the kinase domain at codon 600 (V600E), accounts for the majority of cases. This mutation results in elevated basal kinase activity, activation of the ERK pathway and cellular transformation. In melanoma, colon and breast cancer cells harboring the V600E BRAF mutation, cyclin D1 expression and cell cycle progression are MEK-dependent (16). Further, supporting its classification as an oncogene, lung-specific expression of V600EBRAF in mice results to the development of lung cancers with bronchioalveolar carcinoma features similar to those observed in patients (17).
In contrast to the pattern of BRAF mutations observed in most other tumor types, a substantial percentage of the BRAF mutations reported to date in lung cancer cell lines and tumors (~90%) are non-V600E (13–15). Many of these non-V600E mutations demonstrate only intermediate and low kinase activity and therefore their classification as “driver” mutations remains in doubt (18). The studies described herein were therefore designed to investigate the MEK-dependence of lung cancer cell lines harboring V600E and non-V600E BRAF mutations. We show that BRAF mutation in cell lines predicts not only for sensitivity to MEK inhibition but also resistance to EGFR inhibition. Thus the data suggest that routine testing for BRAF mutation in NSCLC may identify a subset of patients with de novo resistance to EGFR kinase inhibition and enhanced sensitivity to MEK inhibition.
MATERIALS AND METHODS
Materials
PD0325901 was obtained from Pfizer Global Research and Development. Gefitinib was obtained from AstraZeneca. Drugs for in vitro studies were dissolved in DMSO to yield 1 mM and 10 mM stock solutions, respectively, and stored at −20 C.
Cell culture
The human cancer cell lines HCC364, H1755, H1666, and H1395 were provided by Adi Gazdar, UT Southwestern. All others were obtained from ATCC. All cell lines were maintained in RPMI with 10mM HEPES supplemented with 2mM glutamine, 50 units/ml each of penicillin and streptomycin, and 10% heat inactivated fetal bovine serum (Gemini Bioproducts, Calabasa, CA) and incubated at 37 C in 5% CO2. For proliferation assays, cells were plated in 96 well plates, at a density of 2000–5000 cells per well. After 24 hours, cells were treated with the inhibitors (PD0325901 or ZD1839), at a range of concentrations prepared by serial dilution. The cells were exposed to Alamar Blue (AccuMed International, OH) three to five days following drug treatment, and plates were read using a fluorescence spectrophotometer. The dose required to inhibit growth by 50% (IC50) was calculated using the SoftMaxPro ver.5 software. For soft agar studies, 1–2 × 104 cells growing in log phase were mixed with agar (0.33%), treated with either DMSO or PD0325901 (1–50nM), and plated over a bottom layer of 0.5% agar in 60mm dishes. Cells were incubated at 37ºC for 2.5 weeks, with 200μl of media pipetted over the surface after 1 week. Colonies were then stained with crystal violet (Sigma-Aldrich, St. Louis, MO) for 1 hour and bright field images captured using a MZFL3 Stereomicroscope and Velocity 4.3 software (Improvision Inc., Waltham, MA). Images captured within a single experiment were taken at the same magnification and exposure time.
Western Blot Analysis
Treated cells were harvested, washed with PBS, and lysed in NP40 lysis buffer [50mM Tris (pH 7.4), 1% NP40, 150 mM NaCl, 40 mM NaF, 1mM, Na3VO4, 1mM phenylmethylsulfonylfluoride, and 10 μg/ml each of leupeptin, aprotinin, and soybean trypsin inhibitor] for 30 minutes on ice. Lysates were centrifuged at 13,200 rpm for 10 minutes to pellet debris, and the protein concentration of the supernatant was determined by bicinchoninic acid protein assay (Pierce). Equal amounts of total protein were resolved by SDS-PAGE and transferred onto nitrocellulose membranes by electroblotting. Membranes were blocked for one hour in 5% nonfat milk in TBS-T [0.1% Tween-20 TBS, 10 mM Tris (pH 7.4) and 150 mM NaCl] at room temperature, and subsequently probed overnight at 4 C with antibody raised against the protein of interest. Anti p42/44 MAPK, phospho p42/44 MAPK, Akt, phospho-Akt (ser473), RB, cleaved PARP, cleaved caspase-3, and GAPDH antibodies were obtained from Cell Signaling Technology. Anti- Cyclin D1, Cyclin D2, and Cyclin D3, and p27 antibodies were obtained from Santa Cruz Biotechnology. After incubation with horseradish peroxidase-conjugated secondary antibodies, proteins were detected using chemiluminescence (Amersham).
Apoptosis
To measure apoptosis, cells were seeded in 10cm dishes at a density of 1 × 106 cells/dish and the following day were treated with the indicated concentration of drug or vehicle (DMSO) for the indicated times. Both adherent and floating cells were harvested and stained with ethidium bromide using the method of Nusse (19). Detection and quantitation of apoptotic cells (sub-G1) was performed by flow cytometric analysis, with the results compared to induction of activated Caspase-3 and PARP cleavage as measured by immunoblot.
BRAF mutation analysis
We previously analyzed over 800 NSCLC tumors from patients undergoing surgical resection in four different countries (Japan, Taiwan, the United States, and Australia) to determine the EGFR and KRAS mutational status (20–22). Clinical information, including age, gender, smoking status, histology, and clinical stage, was available. Of these, DNAs from 689 NSCLC tumors [Japan (n = 375), Taiwan (n = 88), the United States (n = 125), and Australia (n = 101)] were available to perform further analyses for BRAF exon 11 and 15 mutations. Institutional Review Board permission and informed consent were obtained at each collection site. Genomic DNA was obtained from primary tumors by standard phenol-chloroform (1:1) extraction followed by ethanol precipitation or by using the DNeasy Tissue Kit (QIAGEN, Valencia, CA). For BRAF mutational analysis, the intron-based PCR primer sequences for BRAF (exons 11 and 15) were as follows (forward and reverse, respectively): exon 11 (5′-TCC CTC TCA GGC ATA AGG TAA 3′ and 5′-CGA ACA GTG AAT ATT TCC TTT GAT 3′) and exon 15 (5′-TCA TAA TGC TTG CTC TGA TAG GA 3′ and 5′-GGC CAA AAA TTT AAT CAG TGG A 3′), with PCR product lengths of 313 and 224 base pairs, respectively. All PCR reactions were carried out in 25 μl volume containing 100 ng of genomic DNA using HotStarTaq DNA polymerase (Qiagen). PCR conditions were as follows: 95°C (12 min) for 1 cycle, 94°C (30 sec), 56°C (30 sec) and 72°C (30 sec) for 36 cycles and a final extension step of 72°C (7 min). All PCR products were incubated using exonuclease I and shrimp alkaline phosphatase (Amersham Biosciences, Piscataway, NJ) and sequenced directly using Applied Biosystems PRISM dye terminator cycle sequencing method (Perkin-Elmer Corp., Foster City, CA). All the sequence variants were confirmed by independent PCR amplifications and sequenced in both directions. For all the mutant cases, corresponding non-malignant tissue DNA was available to confirm that mutations were somatic mutations.
Mass Spectrometry (Sequenom)-based mutation screens
A separate set of 227 NSCLC tumors from patients undergoing surgical resection at MSKCC1 were screened for five common point mutations in exons 11 and 15 of BRAF (G466V, G469A, L597V, L597R, and V600E) using a mass-spectrometry (Sequenom)-based genotyping assay. The Sequenom MassARRAY system is based on matrix-assisted laser desorption/ionisation-time of flight mass spectrometry (MALDI-TOF MS). In these assays, the mutant and germline alleles for a given point mutation produce single-allele base extension reaction products of different masses that are then resolved by MALDI-TOF MS. Both the amplification and extension primers were designed using Sequenom Assay Designer v3.1 software. The amplification primers were designed with a 10mer tag sequence to increase their mass so that they fall outside the range of detection of the MALDI-TOF mass spectrometer. Results were generated using the SpectroTYPER v3.4 software (Sequenom). All the positive cases were confirmed by visually reviewing the spectra. For the PCR amplification, a total of 15 ng of genomic DNA (in 1 μl) was amplified in a 5 μl reaction mixture containing 0.1 μl (0.5 U) HotStarTaq enzyme (Qiagen, Valencia, CA), 0.625 μl of 10x HotStar buffer, 0.325 μl of 25 mM (total) MgCl2, 0.25 μl of 10mM (each) deoxynucleotide triphosphate, 1 μl of 100 nM of each forward and reverse primers and 1.7 μl of water. The PCR step was initiated with a 95°C soak for 15 min, followed by 45 cycles, consisting of 95°C for 20 sec, 56°C for 30 sec, 72°C for 60 sec, and a final extension of 3 min at 72°C. After PCR, the remaining unincorporated dNTPs were dephosphorylated by adding 2 μl of the SAP cocktail, containing 1.33 μl of water, 0.17 μl of reaction buffer (Sequenom) and 0.5 μl of SAP (Sequenom). The 384-well plate was then sealed and placed in a thermal cycler with the following conditions: 37°C for 40 min, 85°C for 5 min and then held at 4°C indefinitely. After the SAP treatment, a 2 μl cocktail, consisting of 0.755 μl water; 0.2 μl iPlex 10x buffer (Sequenom), 0.2 μl iPlex terminator mix (Sequenom); 0.804 μl of 7 μM/14 μM (depending on the low vs. high mass primers) extension primer mixture and 0.041 μl iPlex enzyme (Sequenom) was added. After the iPlex cocktail addition, the plate was again sealed and placed in a thermal cycler with the following program: 94°C for 2 min followed by 40 cycles of 94°C for 5 sec, [5 cycles (52°C for 5 sec, 80°C for 5 sec) and 72°C for 5 sec]. The reaction mixture was then desalted by adding 16 μl of water and 6 mg cationic resin mixture, SpectroCLEAN (Sequenom). The plate was then sealed and placed in a rotating shaker for 20 min to desalt the iPlex solution. Completed genotyping reactions were spotted in nanoliter volumes onto a matrix arrayed silicon chip with 384 elements (Sequenom SpectroCHIP) using the MassARRAY Nanodispenser. SpectroCHIPs were analyzed using the Bruker Autoflex MALDI-TOF mass spectrometer and the spectra were processed using the SpectroTYPER v3.4 software (Sequenom).
Clinical Data Collection
MSKCC tumor specimens were obtained from an institutional tumor bank of patients who had undergone non-small cell lung cancer resections between 2002 and 2007. Retrospective review of clinical data, including smoking history, was obtained by review of a patient-completed smoking questionnaire and the medical record. Never-smokers had smoked <100 cigarettes. Former smokers had previously smoked cigarettes but quit smoking more than one year prior to diagnosis of lung cancer. Pack-years of smoking was defined as [(average number of cigarettes per day/20) × years smoking]. Smoking history and clinical information was collected independently, using similar smoking questionnaires, at each site which provided clinical DNA samples.
RESULTS
Genetic predictors of MEK-dependence in NSCLC cell lines
Activation of the classical MAP kinase cascade (RAS-RAF-MEK-ERK) is a common event in lung cancer and often results from mutations in BRAF, KRAS and upstream receptor tyrosine kinases such as EGFR. We hypothesized that the dependence of lung cancer cells on MEK-ERK pathway activity would vary as a function of the genetic alterations responsible for pathway activation. In order to compare the MEK/ERK-dependence of lung cancer cell lines with BRAF, RAS and RTK mutations, we screened a large panel (n= 87) of human lung cancer cell lines for exon 11 and 15 BRAF mutations. In total, we identified five NSCLC cell lines with BRAF mutation (See Supplemental Figure 1). Only one of these, HCC364, harbored the V600E kinase domain mutation in exon 15 that is, by far, the most frequently observed BRAF mutation in melanoma, thyroid and colon cancers (13, 23, 24). Two additional cell lines, H1755 and H1395, harbored high-activity G469ABRAF mutations, and one cell line, H1666, harbored a G466VBRAF low-activity mutation. A fifth cell line, H2087, harbored both Q61KNRAS and the L597VBRAF intermediate activity mutation (18). All of these cell lines were derived from patients with a histologic diagnosis of adenocarcinoma, and at least three of the five were former smokers (no data on smoking history were available for the patients from which the cell lines HCC364 and H1666 were derived).
To determine the dependence of these cell lines on MEK/ERK for proliferation, we used PD0325901, an allosteric inhibitor of MEK that inhibits MEK1 and MEK2 kinase activity by locking the enzyme in a closed and catalytically inactive conformation (25–27). Consistent with our prior results in melanoma and colon cancer cell lines, we found that HCC364 cells (V600EBRAF) were exquisitely MEK-dependent with an IC50 of 3.2 nM. The other four BRAF mutant cell lines were also MEK-dependent for proliferation, with IC50s for PD0325901 ranging from 2.7 to 24 nM. In contrast, all six NSCLC cell lines with EGFR kinase domain mutations were resistant to MEK inhibition (IC50 >300nM, Figure 1A). Similarly, H1703 cells, a cell line with PDGFRα overexpression, HCC78 cells, which express the SLC34A2-ROS fusion protein, and H2228 cells, which express the EML4-ALK fusion protein (2), were also resistant to MEK inhibition. Resistance was not a result of the failure of PD0325901 to inhibit MEK1/2, as in all cell lines examined, the drug was effective in inhibiting the pathway as measured by decreased expression of phosphorylated ERK following drug exposure (Figure 1B and data not shown, DNS). The sensitivity of the RAS-mutant class was more variable, with IC50s ranging from 2.7 to >300 nM. Notably, the RAS mutant tumor demonstrating the greatest MEK-dependence was the H2087 cell line, which also co-expressed the L597V BRAF mutation.
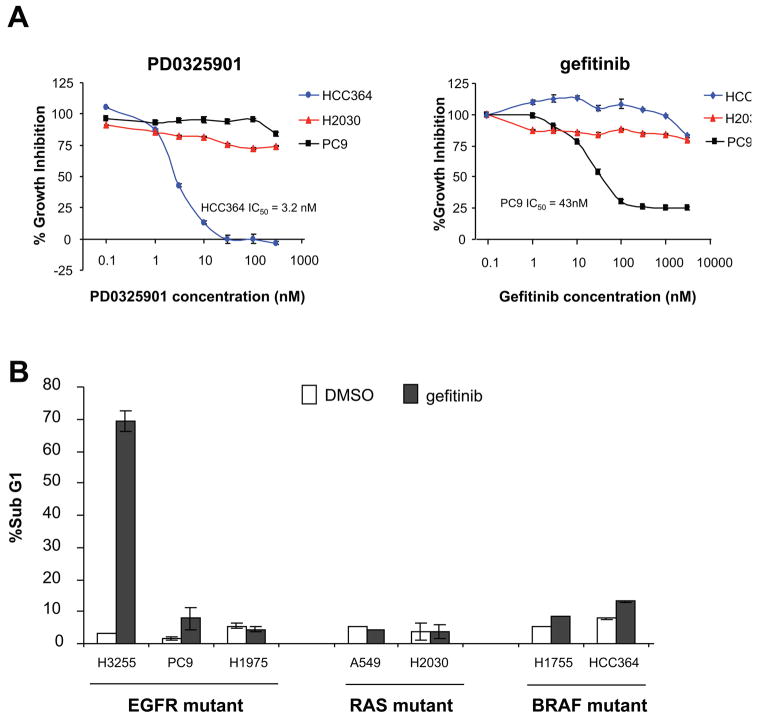
Figure 1. BRAF mutant cell lines are selectively sensitive to MEK inhibition.
A. A panel of cell lines with mutant BRAF (blue), mutant KRAS (red), concomitant BRAF and NRAS mutations (blue/red) or wild-type for RAS and BRAF (black) were grown in the presence of the MEK inhibitor PD0325901 at a range of concentrations and day 5 IC50 values were determined using the Alamar Blue assay. The BRAF and RAS wild-type NSCLC cell lines H2228 (EML4-ALK fusion), HCC78 (SLC34A2-ROS fusion), H1703 (PDGFR over-expressing) and six cell lines with EGFR mutation (H820, PC9, H3255, 11–18, H1975 and H1650) were all resistant to PD0325901. B. Immunoblot of phosphorylated ERK 1/2 (Thr202/Tyr204) and total ERK 1/2 following administration of the MEK inhibitor in selected cell lines from (A) showing that resistance to MEK inhibition was not the result of inability of the drug to inhibit ERK activity.
The difference in MEK-dependence in the EGFR and BRAF mutant cell lines could not be explained by differences in the basal expression of phosphorylated ERK, as shown in Figure 2. However, there was an inverse correlation between the level of phosphorylated AKT (pAKT, ser473) expression and MEK-dependence (p = 0.0012). In resistant cells, high pAKT was associated with EGFR or KRAS mutations, whereas the majority of BRAF mutant cell lines had low or undetectable pAKT (Figure 2, A and B).
Figure 2. The MEK-dependence of NSCLC cell lines was inversely correlated with the level of pAKT (ser473) expression.
A. Whole cell lysates from untreated cell lines harvested at 70–80% confluence were analyzed by immunoblot for phospho-AKT (Ser473), total AKT, phospho-ERK (Thr202/Tyr204) and total ERK protein expression. Band intensity was quantified using Science Lab 2003 Image Gauge software (Fujifilm, Tokyo, Japan). B. Cells were grouped as either sensitive (IC50 <50nM) or resistant (IC50 ≥ 50nM) to the MEK inhibitor PD0325901. Relative pAKT and pERK are shown for the two groups. pAKT (Ser473) levels were variable in the resistant cell lines, but low in the sensitive cell lines. Only the difference in pAKT between the groups was statistically significant (p = 0.0012, Wilcoxon test). There was no correlation between pERK levels and MEK-dependence.
To further characterize the consequences of MEK inhibition in NSCLC cell lines with BRAF, RAS and EGFR mutations, we performed immunoblot and FACS analysis as a function of both PD0325901 dose and time post-treatment. As shown in Figure 3A for the HCC364 (V600EBRAF) and H1666 (G466VBRAF) cell lines, MEK inhibition decreased the expression of phosphorylated ERK, which led to a decline in D-cyclin expression and an increase in p27 expression. This was followed by RB hypophosphorylation and accumulation of cells in G1. These changes in cell cycle related proteins were not seen in the EGFR-mutant, MEK inhibitor-resistant cell lines (See Supplemental Figure 2A, PC9). Notably, the full effects of drug on D-cyclin and p27 expression and RB phosphorylation were not observed until 24 to 48 hours post-treatment. The kinetics of the effect of MEK inhibition on RB likely explain the delayed effect of PD0325901 on cell growth, which was most prominent in cells such as H1666 that demonstrate a slow doubling time. As shown in Figure 3B, MEK inhibition had no inhibitory effect on H1666 proliferation at 48 hours; rather, there was a modest acceleration in H1666 cell growth at 48 hours, which was followed by accumulation of cells in G1 and induction of cell death at later time points (four days and beyond, Figure 3B-C and data not shown). HCC364 cells are non-adherent in 2-D culture, and therefore the effect of MEK inhibition in soft-agar was also assessed. PD0325901 treatment resulted in a dose-dependent inhibition of colony formation at the IC50 of the drug for MEK1 and MEK2. In contrast, MEK inhibition had no effect on the growth of PC9 (delE746-A750EGFR) (28) colonies (Figure 3, C and D).
Figure 3. Consequences of MEK-inhibition in BRAF mutant NSCLC cell lines.
A. Immunoblot of cell cycle related proteins in response to MEK inhibition as a function of time following MEK inhibition (50nM PD0325901). For both the high activity (HCC364) and low activity (H1666) BRAF mutant cell lines, pERK downregulation was accompanied by loss of D-cyclin protein expression, induction of p27 expression, and RB hypophosphorylation. B. Growth kinetics of the H1666 (G466VBRAF low kinase activity) NSCLC cell line following treatment with PD0325901 (1 100 nM) or control (untreated). An initial rise in cell counts was followed by decrease in the number of viable cells at later time points (day 4). C. Photomicrographs of untreated and MEK inhibitor-treated H1666 (G466VBRAF) and PC9 (delE746-A750EGFR) cells, following 72 hours of exposure. D. Soft agar colony formation assays in the presence of increasing concentrations of PD0325901 reveal inhibition of HCC364 (V600EBRAF), but not PC9 (delE746-A750EGFR), colony growth.
In the V600EBRAF HCC364 model, MEK inhibition resulted in profound apoptosis as measured by induction of PARP cleavage (Figure 3A, 4B) and accumulation of cells in the sub-G1 fraction as measured by FACS (Figure 4A). Notably, in assays of apoptosis, PD0325901 had variable effects in H1395 and H1755, two cell lines that express the same high-activity G469A BRAF mutation, suggesting that additional genetic heterogeneity within the cell lines conditions their response to MEK inhibition. Apoptosis was not observed following MEK-inhibition in EGFR mutant cell lines and was either modestly or not at all induced following MEK inhibition in the RAS mutant cell lines (Figure 4, A and B).
Figure 4. Induction of apoptosis in a panel of cell lines with EGFR, RAS and BRAF mutations in response to MEK inhibition.
A. Percent of cells in the sub-G1 population as determined by FACS analysis in the presence or absence of MEK inhibitor (50nM PD0325901 for 72 hours). Error bars represent standard deviation of replicate experiments. MEK inhibition induced a significant increase in sub-G1 fraction in four of five BRAF mutant cell lines. The variable response of H1395 and H1755 cells, which both express the G469ABRAF mutation, suggests that additional genetic heterogeneity within these lines conditions MEK-dependence in NSCLC. B. Immunoblot of activated PARP and caspase-3 in representative EGFR-, KRAS- and BRAF-mutant cell lines in the absence (−) or presence (+) of 50nM PD0325901 for 24 hours.
BRAF mutant cell lines are resistant to EGFR inhibition
We have shown that inhibition of MEK/ERK alone is insufficient to block proliferation or induce cell death in NSCLC cell lines expressing kinase domain mutations in EGFR. As the expression of activating mutations in KRAS has been shown to predict for de novo resistance to EGFR inhibitors in patients with NSCLC (11, 29–31), we examined whether cell lines expressing high-activity (V600E and G469A) BRAF mutations were also resistant to EGFR inhibition. As shown in Figure 5, PC9 (delE746-A750EGFR) cells were sensitive to gefitinib with an IC50 of 43nM whereas both HCC364 (V600EBRAF) and H2030 (G12CKRAS) cells were resistant to gefitinib at doses of up to 5 μM. Consistent with the work of others, gefitinib was variably effective in inducing apoptosis in EGFR mutant NSCLC cell lines (32, 33). Treatment of H3255 (L858REGFR) cells with gefitinib resulted in profound induction of apoptosis as measured by FACS, whereas H1975 cells were resistant to gefitinib due to the presence of the T790M mutation (7). Though both PC9 and H3255 cells have similar IC50s for gefitinib, the response of PC9 cells was primarily cytostatic (G1 increase from 72% to 95%), with gefitinib inducing only a modest induction of apoptosis (sub-G1 increase from 1.6% to 7.8%). Gefitinib had no effect on the survival of both the BRAF-mutant and RAS-mutant cell lines tested (Figure 5B). These data suggest that BRAF mutations may predict for de novo resistance to EGFR kinase inhibitors in patients with NSCLC.
Figure 5. Sensitivity of EGFR-mutant and BRAF-mutant cell lines to the selective EGFR inhibitor gefitinib (Iressa).
A. The BRAF-mutant cell line HCC364 (blue) was sensitive to PD0325901 while H2030 (KRAS-mutant, red) and PC9 (EGFR-mutant, black) cells were resistant. In contrast, only the EGFR-mutant cell lines (PC9 is shown) demonstrated sensitivity to gefitinib, whereas BRAF-mutant cell lines were resistant. Percent growth inhibition was determined using the Alamar Blue assay and five days exposure to PD0325901 or gefitinib at a range of concentrations as shown. Error bars represent standard error of replicate experiments. B. Percent of cells in sub-G1 population as determined by FACS analysis in the presence or absence of gefitinib (2 μM for 72 hours). Error bars represent standard deviation of replicate experiments. Gefitinib treatment of H3255 (L858REGFR) and PC9 (delE746-A750EGFR) NSCLC cell lines resulted in an increase in sub-G1 fraction. H1975 cells, which express both the L858R and T790M EGFR kinase domain mutations, were resistant to gefitinib as previously reported (7). Both BRAF-mutant and RAS-mutant lines were resistant to EGFR inhibition.
Frequency of BRAF mutations in NSCLC
As our preclinical models suggest that BRAF mutations may be predictive of sensitivity to MEK inhibitors and resistance to EGFR inhibitors, we studied the frequency of BRAF mutations in two large tumor repositories of patients diagnosed with NSCLC. In an initial series of 689 patients from five centers (UT MD Anderson Cancer Center, Houston, TX, USA; Okayama University, Okayama, Japan; Chiba University, Chiba, Japan; The Prince Charles Hospital, Brisbane, Australia; Chung Shan Medical University, Taichung, Taiwan), sequencing of exons 11 and 15 of BRAF identified eleven mutant cases, or 1.6%. Of note, in none of these cases were patients previously exposed to the tyrosine kinase inhibitors gefitinib or erlotinib. Six out of eleven patients were smokers, and nine out of eleven had adenocarcinoma histology (See Supplemental Table 1). Despite the availability of agents that target both RAF and MEK, this low frequency of BRAF mutation in NSCLC has discouraged widespread efforts to prospectively test NSCLC patients for mutations in this gene. With the goal of facilitating the identification of low-frequency “driver” mutations in genes such as BRAF, we developed a MALDI-TOF mass spectrometry assay to screen for mutations in EGFR, KRAS, BRAF, HER2 and PIK3CA. Using this assay, we identified an additional six patients (2.7%) harboring exon 11 or 15 BRAF mutations (Supplemental Table 1). Combining these two data sets, we observed a total incidence of 17 cases out of 916 patients, or 1.9%. The clinical characteristics of this combined data set are shown in Table 1 and Supplemental Table 1, and include a predominance of adenocarcinoma histology and female gender. Notably, in contrast to the clinical parameters associated with EGFR mutations in lung cancer, the majority of patients harboring BRAF mutations were former or current smokers.
Table 1.
Clinical characteristics of lung cancer patients with BRAF mutations. A total of 916 NSCLC patient samples were analyzed, and 17 mutations in BRAF were identified. Percentages were calculated as a function of the total number of BRAF cases (17), and as a function of the total number of cases analyzed (916).
| BRAF mutant (n=17)
|
Total (n=916)
|
|||
|---|---|---|---|---|
| n | % | % | ||
| BRAF mutation | Total | 17 | (100%) | 1.9 % |
| Exon 11 | 4 | (24%) | 0.4 % | |
| Exon 15 (non-V600) | 2 | (12%) | 0.2 % | |
| Exon 15 (V600E) | 11 | (65%) | 1.2 % | |
| Wild type | - | 98.1% | ||
|
| ||||
| Gender | Male | 6 | (35%) | 37 % |
| Female | 11 | (65%) | 63 % | |
|
| ||||
| Histology | Adenocarcinoma | 15 | (88%) | 68 % |
| Other | 2 | (12%) | 32 % | |
|
| ||||
| Smoker2,3 | Former/Current4 | 12 | (71%) | 67% |
| Never | 5 | (29%) | 33% | |
| Median Pack-years | 50 | 415 | ||
DISCUSSION
Recent advances in our understanding of the genetic alterations responsible for the development of cancer have been paralleled by an increasing capacity for the development of drugs with selectivity for specific protein targets. Such advances have led to the hope that more effective cancer therapies can be developed that work by selectively inhibiting the specific molecular alterations responsible for cancer initiation and progression. Thus far, this paradigm has been most effective with inhibitors of proteins that are mutationally activated in tumors. Examples include imatinib in patients with CML (ABL), GIST (KIT), and dermatofibrosarcoma protuberans (PDGFR) (34, 35), and gefitinib/erlotinib in non-small cell lung cancer (EGFR) (4–6).
In CML, ABL translocations, most commonly BCR-ABL, are the hallmark of this disease and therefore patient selection beyond traditional pathologic criteria is not needed in order to identify a target population predicted to be sensitive to ABL kinase inhibitors. In contrast, it is now apparent that solid tumors, traditionally classified by tissue of origin and histologic subtype, can have a diversity of mutations that confer similar selective advantage. For example, in non-small cell lung cancers, EGFR, KRAS and BRAF mutations are non-overlapping1 and all activate the MAP kinase cascade (36–38). In NSCLC, the presence or absence of mutations in the EGFR and KRAS genes has been shown to correlate strongly with response to EGFR inhibitors (4–6, 11). Whereas an activating mutation in EGFR is a positive predictor of response to gefitinib and erlotinib, KRAS mutation confers negative predictive value for the same class of agents (11). Recent data has also confirmed that KRAS mutation is sufficient to confer resistance to EGFR-targeting antibodies such as cetuximab and panitumumab (12, 39). These observations suggest that the response of patients to a particular targeted agent will depend strongly upon the complement of mutations within an individual patient’s tumor and that such predictors (both positive and negative) can be identified. The experience with gefitinib and erlotinib also suggests that it would be valuable to know the tumor genotype of the patients prospectively and to use this information to select the appropriate patients for clinical trial. This is particularly important if the frequency of mutation in the population tested is low.
BRAF missense mutations, the vast majority of which are V600E, are the most common kinase domain mutations in human tumors (13, 40). These mutations, found in approximately 8% of all tumors, are non-overlapping in distribution with RAS mutations. Supporting its classification as an oncogene, V600EBRAF stimulates ERK signaling, induces proliferation and is capable of promoting transformation and inducing tumors in transgenic mice (41, 42). To study the biology of BRAF mutation in NSCLC, we screened a large panel of cell lines for exon 11 and exon 15 BRAF mutations. We identified five cell lines with known “hot spot” mutations within the BRAF kinase domain, one of which, HCC364, harbors the V600E mutation. We observed that NSCLC with BRAF mutations were selectively sensitive to MEK inhibition, compared to cell lines expressing EGFR mutations, the SLC34A2-ROS fusion, the EML4-ALK fusion and those with amplification of PDGFRα or MET. Notably, we did not assess the MEK-dependence of cell lines in which no identifiable driver mutation has been identified. As such cell lines may contain occult mutations in genes such as NF1 or MEK1 (MAP2K1) that activate the MAPK pathway, we would expect that a subset of these “BRAF wild-type” cell lines may also be dependent upon MEK for proliferation or survival. Validating this possibility, MEK1 mutations were recently identified with low frequency in NSCLC (43).
We observed that MEK inhibition in the V600EBRAF-expressing HCC364 NSCLC cells induced levels of apoptosis comparable to those seen with EGFR inhibition in the EGFR-mutant H3255 model. However, as seen with EGFR inhibitors in EGFR-mutant models, the apoptotic response of BRAF- and KRAS-mutant cell lines to MEK inhibition was variable. These data suggest that additional genetic heterogeneity within the BRAF- and KRAS-mutant classes likely conditions the response of these cells to ERK pathway inhibition. Future studies are underway to determine which genetic and epigenetic alterations commonly co-exist with EGFR, RAS and BRAF mutations in lung cancer tumors and their impact upon MEK/ERK pathway dependence.
Using a cohort of over 900 lung cancer tumors, we identified a total of 17 patients whose lung tumors harbored BRAF exon 11 or exon 15 mutations, representing 1.9% of the total cases analyzed, an incidence consistent with previous reports (13, 14, 21). Notably, in contrast to the clinical profile of patients with EGFR kinase domain mutations, the majority of patients with BRAF mutations were current or former smokers. Our observed incidence of V600E mutations (1.2%) was also higher than would have been predicted based upon prior reports. One explanation for the higher observed frequency of V600E mutations may have been the use of mass spectrometry genotyping in the MSKCC series, a technology with greater sensitivity than Sanger sequencing.
Overall, our data suggest that BRAF is a driver mutation in patients in which it is mutated and that targeting MEK may be a useful therapeutic strategy in this subset of patients. Such a targeted strategy, however, has not been pursued to date in the clinic and no ongoing or completed Phase 1 or 2 trial of a MEK-selective inhibitor has yet enriched for NSCLC patients with BRAF mutations. The primary hurdle has been that testing for BRAF mutation is not prospectively performed in patients with NSCLC. Routine testing of NSCLC patients for KRAS mutation, on the other hand, is becoming more widespread, as the presence of a KRAS mutation predicts for de novo resistance to the EGFR inhibitors gefitinib and erlotinib. We now show that BRAF mutant cell lines are also resistant to EGFR inhibition. Based upon these data, we propose clinical studies to determine whether BRAF mutation has similar value in predicting for de novo resistance to EGFR inhibition. If confirmed, we believe that routine clinical testing of all NSCLC for BRAF mutation would be justified. Such an effort would also have the secondary benefit of accelerating the development of BRAF- and MEK-selective inhibitors by aiding in the identification of the minority of lung cancer patients likely to respond to such agents. As demonstrated by our use of the MALDI-TOF mass spectrometry assay to screen simultaneously for EGFR, KRAS and BRAF mutations, we believe that such efforts are now technically feasible. Based upon these results, we have initiated routine prospective genotyping of NSCLC patients for BRAF mutation and have proposed clinical studies of MEK inhibitors with enrollment restricted exclusively to those with an activating BRAF mutation.
Supplementary Material
Acknowledgments
The authors thank Ignacio I. Wistuba (Departments of Pathology and Thoracic/Head and Neck Medical Oncology, University of Texas, M.D. Anderson Cancer Center, Houston, TX, USA); Makoto Suzuki (Department of Thoracic Surgery, Graduate School of Medicine, Chiba University, Chiba, Japan); Kwun M. Fong (The Prince Charles Hospital, Brisbane, Australia); Huei Lee (Institute of Medical and Molecular Toxicology, Chung Shan Medical University, Taichung, Taiwan) for providing clinical DNA samples.
Funding: This study was supported by grants from the National Institutes of Health (P01-129243) (M.L., D.S. and N.R.), the Specialized Program of Research Excellence in Lung Cancer (P50CA70907) from the National Cancer Institute (A.G.), the Waxman Foundation (D.S. and N.R.), Golfers-Against-Cancer (D.S. and N.R.), and the Byrne Foundation (D.S. and N.R.).
Footnotes
Chitale D, Gong Y, Taylor BS, Broderick S, Brennan C, Somwar R, Golas B, Wang L, Motoi N, Szoke J, Reinersmann JM, Major J, Sander C, Seshan EV, Zakowski MF, Rusch V, Pao W, Gerald W, Ladanyi M. Integrated genomic characterization of lung adenocarcinoma reveals loss of the negative regulator of mitogenic signaling, DUSP4, in EGFR-mutant tumors. (submitted)
Smoking history is defined as: Current smoker, Former smoker (previously smoked, but quit more than one year prior to diagnosis of lung cancer), or Never-smoker (has smoked <100 cigarettes in a lifetime).
Smoking history was unknown for two patients: 1 of 227 at MSKCC; 1 of 375 from Okayama University, Japan.
MSKCC series of 227 patients includes 29 current smokers, 154 former smokers, and 43 never smokers.
Median pack-years is based upon 179 patients of 183 former or current smokers from the MSKCC series of 227.
References
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 2.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Perner S, Wagner PL, Demichelis F, et al. EML4-ALK fusion lung cancer: a rare acquired event. Neoplasia (New York, NY. 2008;10:298–302. doi: 10.1593/neo.07878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS medicine. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–5. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 10.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS medicine. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 13.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 14.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 15.Naoki K, Chen TH, Richards WG, Sugarbaker DJ, Meyerson M. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res. 2002;62:7001–3. [PubMed] [Google Scholar]
- 16.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji H, Wang Z, Perera SA, et al. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res. 2007;67:4933–9. doi: 10.1158/0008-5472.CAN-06-4592. [DOI] [PubMed] [Google Scholar]
- 18.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 19.Nusse M, Beisker W, Hoffmann C, Tarnok A. Flow cytometric analysis of G1- and G2/M-phase subpopulations in mammalian cell nuclei using side scatter and DNA content measurements. Cytometry. 1990;11:813–21. doi: 10.1002/cyto.990110707. [DOI] [PubMed] [Google Scholar]
- 20.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 21.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. International journal of cancer. 2006;118:257–62. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 22.Nomura M, Shigematsu H, Li L, et al. Polymorphisms, mutations, and amplification of the EGFR gene in non-small cell lung cancers. PLoS medicine. 2007;4:e125. doi: 10.1371/journal.pmed.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikenoue T, Hikiba Y, Kanai F, et al. Functional analysis of mutations within the kinase activation segment of B-Raf in human colorectal tumors. Cancer Res. 2003;63:8132–7. [PubMed] [Google Scholar]
- 24.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–7. [PubMed] [Google Scholar]
- 25.Ohren JF, Chen H, Pavlovsky A, et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat Struct Mol Biol. 2004;11:1192–7. doi: 10.1038/nsmb859. [DOI] [PubMed] [Google Scholar]
- 26.Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006;441:457–62. doi: 10.1038/nature04874. [DOI] [PubMed] [Google Scholar]
- 27.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–47. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 28.Arao T, Fukumoto H, Takeda M, Tamura T, Saijo N, Nishio K. Small in-frame deletion in the epidermal growth factor receptor as a target for ZD6474. Cancer Res. 2004;64:9101–4. doi: 10.1158/0008-5472.CAN-04-2360. [DOI] [PubMed] [Google Scholar]
- 29.Massarelli E, Varella-Garcia M, Tang X, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13:2890–6. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 30.Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol. 2008;3:111–6. doi: 10.1097/JTO.0b013e318160c607. [DOI] [PubMed] [Google Scholar]
- 31.Baselga J, Rosen N. Determinants of RASistance to anti-epidermal growth factor receptor agents. J Clin Oncol. 2008;26:1582–4. doi: 10.1200/JCO.2007.15.3700. [DOI] [PubMed] [Google Scholar]
- 32.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–7. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 33.Tracy S, Mukohara T, Hansen M, Meyerson M, Johnson BE, Janne PA. Gefitinib induces apoptosis in the EGFRL858R non-small-cell lung cancer cell line H3255. Cancer Res. 2004;64:7241–4. doi: 10.1158/0008-5472.CAN-04-1905. [DOI] [PubMed] [Google Scholar]
- 34.Sjoblom T, Shimizu A, O’Brien KP, et al. Growth inhibition of dermatofibrosarcoma protuberans tumors by the platelet-derived growth factor receptor antagonist STI571 through induction of apoptosis. Cancer Res. 2001;61:5778–83. [PubMed] [Google Scholar]
- 35.Sawyers CL. Imatinib GIST keeps finding new indications: successful treatment of dermatofibrosarcoma protuberans by targeted inhibition of the platelet-derived growth factor receptor. J Clin Oncol. 2002;20:3568–9. doi: 10.1200/JCO.2002.20.17.3568. [DOI] [PubMed] [Google Scholar]
- 36.Shibata T, Hanada S, Kokubu A, et al. Gene expression profiling of epidermal growth factor receptor/KRAS pathway activation in lung adenocarcinoma. Cancer Sci. 2007;98:985–91. doi: 10.1111/j.1349-7006.2007.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki M, Shigematsu H, Iizasa T, et al. Exclusive mutation in epidermal growth factor receptor gene, HER-2, and KRAS, and synchronous methylation of nonsmall cell lung cancer. Cancer. 2006;106:2200–7. doi: 10.1002/cncr.21853. [DOI] [PubMed] [Google Scholar]
- 38.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–51. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 39.Lievre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 40.Ikediobi ON, Davies H, Bignell G, et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Molecular cancer therapeutics. 2006;5:2606–12. doi: 10.1158/1535-7163.MCT-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wellbrock C, Ogilvie L, Hedley D, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64:2338–42. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- 42.Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198–202. [PubMed] [Google Scholar]
- 43.Marks JL, Gong Y, Chitale D, et al. Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res. 2008;68:5524–8. doi: 10.1158/0008-5472.CAN-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.